Co-Culture of Gracilariopsis longissima Seaweed and Penaeus monodon Shrimp for Environmental and Economic Resilience in Poor South-East Asian Coastal Aquaculture Communities
Abstract
:1. Introduction
2. Materials and Methods
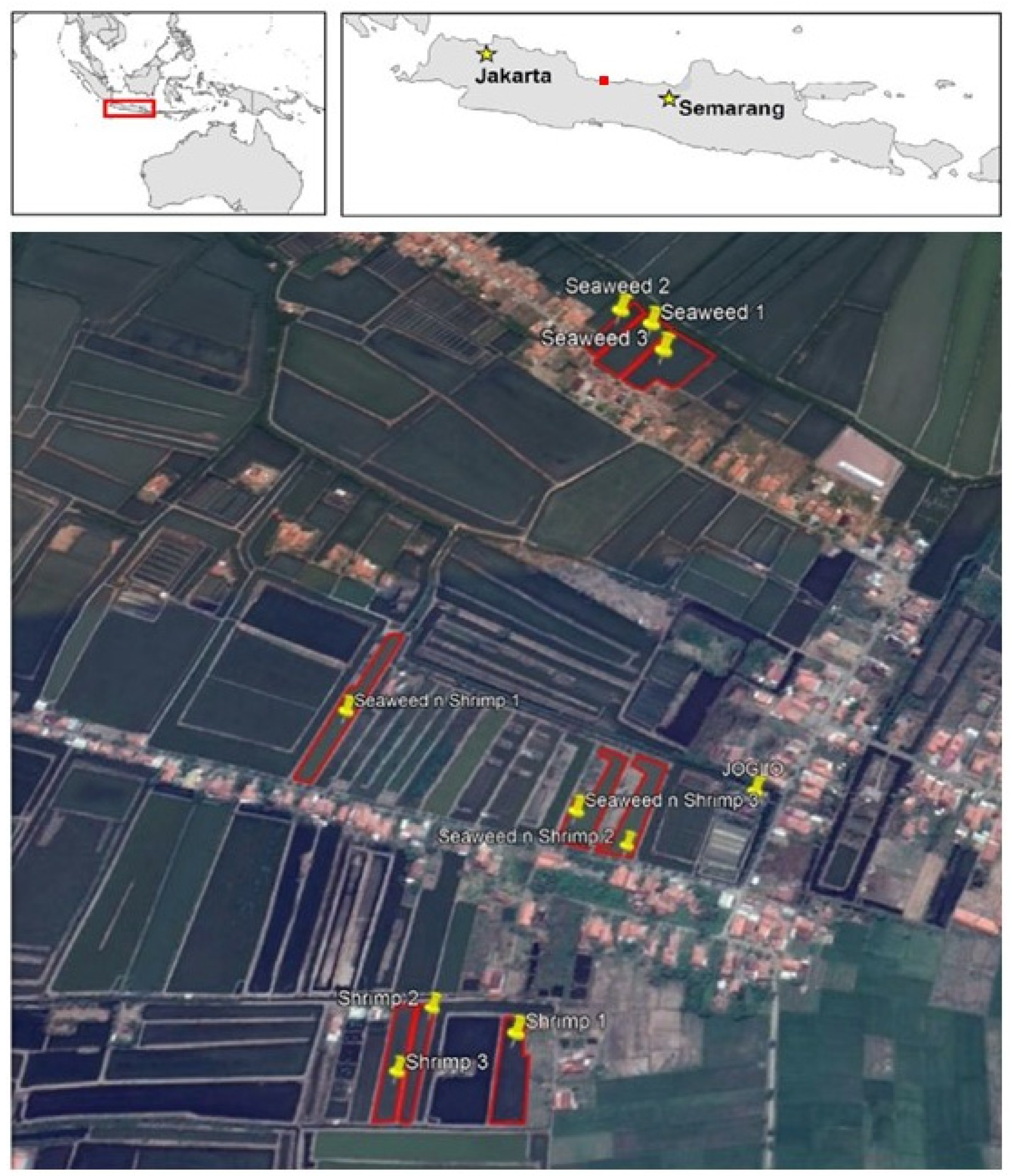
2.1. Study Site and Pond Selection
2.2. Physical Parameters and Nutrients of Pond Water and Sediment
2.3. Experimental Design for Growth and Yield
2.4. Calculation of Biological and Economic Yield
2.5. Product Quality and Food Safety Assessment
2.6. Statistical Analysis
3. Results
3.1. Water and Sediment Parameters
3.2. Production
3.3. Product Quality Aspects
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chopin, T.; Cooper, J.A.; Reid, G.; Cross, S.; Moore, C. Open-water integrated multi-trophic aquaculture: Environmental bio-mitigation and economic diversification of fed aquaculture by extractive aquaculture. Rev. Aquac. 2012, 4, 209–220. [Google Scholar] [CrossRef]
- Huo, Y.Z.; Xu, S.N.; Wang, Y.Y.; Zhang, J.H.; Zhang, Y.J.; Wu, W.N.; Chen, Y.Q.; He, P.M. Bioremediation efficiencies of Gracilaria verrucosa cultivated in an enclosed sea area of Hangzhou Bay, China. J. Appl. Phycol. 2011, 23, 173–182. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Panucci, R.A.; Carneiro, M.-A.; Pereira, D.C. Evaluation of Gracilaria caudata. J. Agardh. for bioremediation of nutrients from shrimp farming waste water. Bioresour. Technol. 2009, 100, 6192–6198. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Nunes, S.O.; Carneiro, M.-A.; Pereira, D.C. Nutrients′ removal from aquaculture wastewater using the macroalgae Gracilaria birdiae. Biomass Bioenergy 2009, 33, 327–331. [Google Scholar] [CrossRef]
- Neori, A.; Chopin, T.; Troell, M.; Buschmann, A.H.; Kraemer, G.P.; Halling, C.; Shpigel, M.; Yarish, C. Integrated aquaculture: Rational, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 2004, 231, 361–391. [Google Scholar] [CrossRef]
- Andayani, S.; Yuwanita, R.; Izzah, N. Biofilter application using seaweed (Gracilaria verrucosa) to increase production of Vannamei shrimp in traditional pond district Bangil-Pasuruan. Res. J. Life Sci. 2016, 3, 16–22. [Google Scholar] [CrossRef]
- Raj, R.; Manju, N.K.; Fazil, T.S.; Chatterji, N.S.; Anandan, R.; Mathew, S. Seaweed and its role in bioremediation—A review. Fish Technol. 2022, 59, 147–153. [Google Scholar]
- Roleda, M.Y.; Hurd, C.L. Seaweed nutrient physiology: Application of concepts to aquaculture and bioremediation. Phycologia 2019, 58, 552–562. [Google Scholar] [CrossRef]
- Susilowati, T.; Hutabarat, J.; Anggoro, S.; Zainuri, M. The improvement of the survival, growth of vannamei shrimp (Litopenaeus vannamei) and seaweed (Gracilaria verrucosa) based on polyculture cultivation. Int. J. Mar. Aquat. Resour. Conserv. Co-Exist. 2014, 1, 6–11. [Google Scholar]
- Widowati, L.L.; Ariyati, R.W.; Rejeki, S.; Bosma, R.H. Organic matter reduction using four densities of seaweed (Gracilaria verrucosa) and green mussel (Perna viridis) to improve water quality for aquaculture in Java, Indonesia. Aquat. Living Resour. 2021, 34, 5. [Google Scholar] [CrossRef]
- Znad, H.; Awual, M.R.; Martini, S. The utilization of algae and seaweed biomass for bioremediation of heavy metal-contaminated wastewater. Molecules 2022, 27, 1275. [Google Scholar] [CrossRef]
- Bashir, M.A.; Liu, J.; Geng, Y.; Wang, H.; Pan, J.; Zhang, D.; Rehim, A.; Aon, M.; Liu, H. Co-culture of rice and aquatic animals: An integrated system to achieve production and environmental sustainability. J. Clean. Prod. 2020, 249, 119310. [Google Scholar] [CrossRef]
- Fierro-Sañudo, J.F.; Rodríguez-Montes de Oca, G.A.; Páez-Osuna, F. Co-culture of shrimp with commercially important plants: A review. Rev. Aquac. 2020, 12, 2411–2428. [Google Scholar] [CrossRef]
- Alam, M.I.; Ahmed, M.U.; Yeasmin, S.; Debrot, A.O.; Ahsan, M.N.; Verdegem, M.-J. Effect of mixed leaf litter of four mangrove species on shrimp post larvae (Penaeus monodon, Fabricius, 1798) performance in tank and mesocosm conditions in Bangladesh. Aquaculture 2022, 551, 737968. [Google Scholar] [CrossRef]
- Anh, N.-N.; An, B.-T.; Lan, L.M.; Hai, T.N. Integrating different densities of white leg shrimp Lithopenaeus vannamei and red seaweed Gracilaria tenuistipitata in the nursery phase: Effects on water quality and shrimp performance. J. Appl. Phycol. 2019, 31, 3223–3234. [Google Scholar] [CrossRef]
- Cruz-Suárez, L.E.; León, A.; Peña-Rodríguez, A.; Rodríguez-Peña, G.; Moll, B.; Ricque-Marie, D. Shrimp/Ulva co-culture: A sustainable alternative to diminish the need for artificial feed and improve shrimp quality. Aquaculture 2010, 301, 64–68. [Google Scholar] [CrossRef]
- Omont, A.; Elizondo-González, R.; Quiroz-Guzmán, E.; Escobedo-Fregoso, C.; Hernández-Herrera, R.; Peña-Rodríguez, A. Digestive microbiota of shrimp Penaeus vannamei and oyster Crassostrea gigas co-cultured in integrated multi-trophic aquaculture system. Aquaculture 2020, 521, 735059. [Google Scholar] [CrossRef]
- Anh, N.-N.; Ngan, L.-H.; Vinh, N.H.; Hai, T.N. Co-culture of red seaweed (Gracilaria tenuistipitata) and black tiger shrimp (Penaeus monodon) with different feeding rations. Int. J. Sci. Res. Publ. 2018, 8, 269–277. [Google Scholar]
- Anaya-Rosas, R.E.; Rivas-Vega, M.E.; Miranda-Baeza, A.; Piña-Valdez, P.; Nieves-Soto, M. Effects of a co-culture of marine algae and shrimp (Litopenaeus vannamei) on the growth, survival and immune response of shrimp infected with Vibrio parahaemolyticus and white spot virus (WSSV). Fish Shellfish Immunol. 2019, 87, 136–143. [Google Scholar] [CrossRef]
- An, B.-T.; Anh, N.-N. Co-culture of Nile tilapia (Oreochromis niloticus) and red seaweed (Gracilaria tenuistipitata) under different feeding rates: Effects on water quality, fish growth and feed efficiency. J. Appl. Phycol. 2020, 32, 2031–2040. [Google Scholar] [CrossRef]
- Das, R.R.; Sarkar, S.; Saranya, C.; Esakkiraj, P.; Aravind, R.; Saraswathy, R.; Rekha, P.N.; Muralidhar, M.; Panigrahi, A. Co-culture of Indian white shrimp, Penaeus indicus and seaweed, Gracilaria tenuistipitata in amended biofloc and recirculating aquaculture system (RAS). Aquaculture 2022, 548, 737432. [Google Scholar] [CrossRef]
- Fourooghifard, H.; Matinfar, A.; Mortazavi, M.S.; Ghadikolaee, K.R.; Mirbakhsh, M. Growth parameters of whiteleg shrimp Litopenaeus vannamei and red seaweed Gracilaria corticata in integrated culturing method under zero water exchange system. Aquac. Res. 2017, 48, 5235–5242. [Google Scholar] [CrossRef]
- Izzati, M. The role of seaweeds Sargassum polycistum and Gracilaria verrucosa on growth performance and biomass production of tiger shrimp (Penaeus monodon Fabr). J. Coast. Dev. 2011, 14, 235–241. [Google Scholar]
- Porchas-Cornejo, M.A.; Martínez-Córdova, L.R.; Magallón-Barajas, F.; Naranjo-Páramo, J.; Portillo-Clark, G. Efecto de la macroalga Caulerpa sertularioides en el desarrollo del camarón Farfantepenaeus californiensis (Decapoda: Peneidae). Rev. Biol. Trop. 1999, 47, 437–442. [Google Scholar]
- Alam, M.I.; Yeasmin, S.; Khatun, M.M.; Rahman, M.M.; Ahmed, M.U.; Debrot, A.O.; Ahsan, M.N.; Verdegem, M.-J. Effect of mangrove leaf litter on shrimp (Penaeus monodon, Fabricius, 1798) growth and color. Aquac. Rep. 2022, 25, 101185. [Google Scholar] [CrossRef]
- Ihsan, Y.N.; Pribadi, T.D.; Schulz, C. Nitrogen assimilation potential of seaweed (Gracilaria verrucosa) in polyculture with Pacific white shrimp (Penaeus vannamei). Aquac. Aquar. Conserv. Legis. 2019, 12, 51–62. [Google Scholar]
- Yang, Y.; Chai, Z.; Wang, Q.; Chen, W.; He, Z.; Jiang, S. Cultivation of seaweed Gracilaria in Chinese coastal waters and its contribution to environmental improvements. Algal Res. 2015, 9, 236–244. [Google Scholar] [CrossRef]
- Ajjabi, C.L.; Abaab, M.; Segni, R. The red macroalga Gracilaria verrucosa in co-culture with the Mediterranean mussels Mytilus galloprovincialis: Productivity and nutrient removal performance. Aquac. Int. 2018, 26, 253–266. [Google Scholar] [CrossRef]
- Hargrave, M.S.; Nylund, G.M.; Enge, S.; Pavia, H. Co-cultivation with blue mussels increases yield and biomass quality of kelp. Aquaculture 2022, 550, 737832. [Google Scholar] [CrossRef]
- Gao, G.; Gao, L.; Fu, Q.; Li, X.; Xu, J. Coculture of the Pacific white shrimp Litopenaeus vannamei and the macroalga Ulva linza enhances their growth rates and functional properties. J. Clean. Prod. 2022, 349, 131407. [Google Scholar] [CrossRef]
- Castilho-Barros, L.; Almeida, F.B.; Henriques, M.B.; Seiffert, W.Q. Economic evaluation of the commercial production between Brazilian samphire and whiteleg shrimp in an aquaponics system. Aquac. Int. 2018, 26, 1187–1206. [Google Scholar] [CrossRef]
- Naufal, I.; Nurhayati, A.; Rizal, A.; Maulina, I.; Handaka Suryana, A.A. Feasibility analysis of seaweed, Gracilaria sp., cultivation in polyculture system in ponds: A case study from Domas Village, Pontang Serang Banten, Indonesia. Asian J. Fish. Aquat. Res. 2022, 16, 1–11. [Google Scholar] [CrossRef]
- Anh, N.-N.; Vinh, N.H.; Doan, D.T.; Lan, L.M.; Kurihara, A.; Hai, T.N. Coculture of red seaweed Gracilaria tenuistipitata and black tiger shrimp Penaeus monodon in an improved extensive pond at various stocking densities with partially reduced feed rations: A pilot-scale study. J. Appl. Phycol. 2022, 34, 1109–1121. [Google Scholar] [CrossRef]
- Laapo, A.; Howara, D. Coastal community welfare improvement through optimization of integrated pond farming management in Indonesia. Int. J. Agric. Syst. 2016, 4, 73–84. [Google Scholar]
- Tran, N.; Le Cao, Q.; Shikuku, K.M.; Phan, T.P.; Banks, L.K. Profitability and perceived resilience benefits of integrated shrimp-tilapia-seaweed aquaculture in North Central Coast, Vietnam. Mar. Policy 2020, 120, 104153. [Google Scholar] [CrossRef]
- FAO. Aquaculture Production. State of the World Fisheries and Aquaculture 2022; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2022; Available online: https://openknowledge.fao.org/server/api/core/bitstreams/9df19f53-b931-4d04-acd3-58a71c6b1a5b/content/sofia/2022/aquaculture-production.html (accessed on 21 April 2025).
- Ahmed, M.U.; Alam, M.I.; Debnath, S.; Debrot, A.O.; Rahman, M.M.; Ahsan, M.N.; Verdegem, M.-J. The impact of mangroves in small-holder shrimp ponds in south-west Bangladesh on productivity and economic and environmental resilience. Aquaculture 2023, 571, 739464. [Google Scholar] [CrossRef]
- Alam, S.N. Advancing quality and health management practices in extensive shrimp (Penaeus monodon) farming in Bangladesh. Aquac. Int. 2023, 31, 1–13. [Google Scholar] [CrossRef]
- Yi, D.; Reardon, T.; Stringer, R. Shrimp aquaculture technology change in Indonesia: Are small farmers included? Aquaculture 2018, 493, 436–445. [Google Scholar] [CrossRef]
- Gusmawati, N.; Soulard, B.; Selmaoui-Folcher, N.; Proisy, C.; Mustafa, A.; Le Gendre, R.; Laugier, T.; Lemonnier, H. Surveying shrimp aquaculture pond activity using multitemporal VHSR satellite images-case study from the Perancak estuary, Bali, Indonesia. Mar. Pollut. Bull. 2018, 131, 49–60. [Google Scholar] [CrossRef]
- Ilman, M.; Dargusch, P.; Dart, P.J.; Onrizal, O. A historical analysis of the drivers of loss and degradation of Indonesia′s mangroves. Land Use Policy 2016, 54, 448–459. [Google Scholar] [CrossRef]
- Amelia, F.; Yustiati, A.; Andriani, Y. Review of shrimp (Litopenaeus vannamei (Boone, 1931)) farming in Indonesia: Management operating and development. World Sci. News 2021, 158, 145–158. [Google Scholar]
- Primavera, J.H.; Yap, W.G.; Savaris, J.P.; Loma, R.-A.; Moscoso, A.-E.; Coching, J.D.; Montilijao, C.L.; Poingan, R.P.; Tayo, I.D. Manual on Mangrove Reversion of Abandoned and Illegal Brackishwater Fishponds; Mangrove Manual Series No. 2; Zoological Society of London: London, UK, 2014; pp. 1–108. [Google Scholar]
- Lestariadi, R.A.; Yamao, M. Where do risks in shrimp farming come from? Empirical results from small farmers in East Java, Indonesia. J. Agribus. Rural Dev. 2018, 1, 39–47. [Google Scholar] [CrossRef]
- Feng, L.; Qiao, Y.; Xiao, C.; Chen, D. Interaction between live seaweed and various Vibrio species by co-culture: Antibacterial activity and seaweed microenvironment. Algal Res. 2022, 65, 102741. [Google Scholar] [CrossRef]
- Anshary, H.; Baxa, D.V. Multiple viral pathogens occurrence in tiger shrimp (Penaeus monodon) broodstock from Sulawesi coastal waters. Aquac. Aquar. Conserv. Legis. 2017, 10, 936–950. [Google Scholar]
- Hargiyatno, I.T.; Sumiono, B. Catch rate, stock density and some biological aspect (Penaeus merguiensis) in Dolak waters, Arafura seas. Res. Cent. Fish. Manag. Conserv. 2013, 5, 123–129. [Google Scholar]
- Wiradana, P.A.; Nur, A.; Sani, M.D.; Affandi, M.; Putranto, T.-C. A short review on status, trends and prospects of jerbung shrimp (Fenneropenaeus merguiensis de Man) in Indonesia. Ecol. Environ. Conserv. 2020, 26, 1657–1664. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2019; Available online: https://www.algaebase.org (accessed on 4 July 2024).
- Neish, I.C. Social and economic dimensions of carrageenan seaweed farming in Indonesia. In Social and Economic Dimensions of Carrageenan Seaweed Farming; FAO Fish. Tech. Pap. 580; Valderrama, D., Cai, J., Hishamunda, N., Ridler, N., Eds.; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2013; pp. 61–89. [Google Scholar]
- WWF Indonesia Aquaculture Team. Seaweed Farming—Cottonii (Kappaphycus alvarezii), Sacol (Kappaphycus striatum) and Spinosum (Eucheuma denticulatum): Better Management Practices Manual for the Small-Scale Fishery; WWF Indonesia: Jakarta, Indonesia, 2014; pp. 1–45. [Google Scholar]
- Strickland, J.-H.; Parsons, T.R. A Practical Handbook of Seawater Analysis; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1972; pp. 1–311. [Google Scholar]
- Beveridge, M.-M. Cage and Pen Fish Farming. Carrying Capacity Models and Environment Impact; FAO Fish. Tech. Pap. 255; Food and Agriculture Organisation of the United Nations: Rome, Italy, 1984; pp. 1–124. [Google Scholar]
- Hariati, A.M.; Wiadnya, D.-R.; Sukkel, M.; Boon, J.H.; Verdegem, M.-J. Pond production of Penaeus monodon in relation to stocking density, survival rate, and mean weight at harvest in East Java, Indonesia. Aquac. Res. 1996, 27, 277–282. [Google Scholar] [CrossRef]
- Hariati, A.M.; Wiadnya, D.-R.; Fadjar, M.; Muhammad, S.; Faber, R.; Verdegem, M.-J.; Boon, J.H. The impact of tiger shrimp, Penaeus monodon Fabricius, postlarvae stocking density on production in traditional tambak systems in East Java, Indonesia. Aquac. Res. 1998, 29, 229–236. [Google Scholar] [CrossRef]
- Gallo, A. Contribution margin: What it is, how to calculate it and why you need it. Harv. Bus. Rev. 2017, 2, 1–3. Available online: https://hbr.org/2017/10/contribution-margin-what-it-is-how-to-calculate-it-and-why-you-need-it (accessed on 21 April 2025).
- Alam, M.I.; Debrot, A.O.; Ahmed, M.U.; Ahsan, M.N.; Verdegem, M.-J. Synergistic effects of mangrove leaf litter and supplemental feed on water quality, growth and survival of shrimp (Penaeus monodon, Fabricius, 1798) post larvae. Aquaculture 2021, 545, 737237. [Google Scholar] [CrossRef]
- Rejeki, S.; Ariyati, R.W.; Widowati, L.L.; Bosma, R.H. The effect of three cultivation methods and two seedling types on growth, agar content and gel strength of Gracilaria verrucosa. Egypt. J. Aquat. Res. 2018, 44, 65–70. [Google Scholar] [CrossRef]
- Bono, A.; Anisuzzaman, S.M.; Ding, O.W. Effect of process conditions on the gel viscosity and gel strength of semi-refined carrageenan (SRC) produced from seaweed (Kappaphycus alvarenzii). J. King Saud Univ.-Eng. Sci. 2014, 26, 3–9. [Google Scholar] [CrossRef]
- SNI 2332.3:2015; Cara Uji Mikrobiologi—Bagian 3: Penentuan Angka Lempeng Total (ALT) Pada Produk Perikanan. BSN (Badan Standardisasi Nasional): Jakarta, Indonesia, 2015.
- SNI 2332.1:2015; Cara Uji Mikrobiologi—Bagian 1: Penentuan Koliform dan Escherichia coli Pada Roduk Perikanan. BSN (Badan Standardisasi Nasional): Jakarta, Indonesia, 2015.
- SNI 01-2332.4-2006; Cara Uji Mikrobiologi—Bagian 4: Penentuan Vibrio Cholerae Pada Produk Perikanan. BSN (Badan Standardisasi Nasional): Jakarta, Indonesia, 2006.
- Ye, L.; Jiang, S.; Zhu, X.; Yang, Q.; Wen, W.; Wu, K. Effects of salinity on growth and energy budget of juvenile Penaeus monodon. Aquaculture 2009, 290, 140–144. [Google Scholar] [CrossRef]
- SNI 2711.1:2009; Batas Maksimum Cemaran Logam Berat Dalam Pangan. BSN (Badan Standardisasi Nasional): Jakarta, Indonesia, 2009.
- European Union. Commission regulation (EC) No 1881/2006. Setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. Available online: https://eur-lex.europa.eu/eli/reg/2006/1881/oj/eng (accessed on 21 April 2025).
- KKP. Indonesia Marine and Fisheries Book 2017; Ministry of Marine Affairs and Fisheries and Japan International Cooperation Agency: Tokyo, Japan, 2017. [Google Scholar]
- Bombeo-Tuburan, I.; Guanzon, N.G., Jr.; Schroeder, G.L. Production of Penaeus monodon (Fabricius) using four natural food types in an extensive system. Aquaculture 1993, 112, 57–65. [Google Scholar] [CrossRef]
- Campos, C.-S.; Moraes, L.-D.; Farias, R.-S.; Severi, W.; Brito, L.O.; Gálvez, A.O. Phytoplankton communities in aquaculture system (integration of shrimp and seaweed). Chem. Ecol. 2019, 35, 903–921. [Google Scholar] [CrossRef]
- Liao, Z.; Chuang, H.; Huang, H.; Wang, P.; Chen, B.; Lee, P.; Wu, Y.; Nan, F. Bioaccumulation of arsenic and immunotoxic effect in white shrimp (Penaeus vannamei) exposed to trivalent arsenic. Fish Shellfish Immunol. 2022, 122, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Romera, E.; González, F.; Ballester, A.; Blázquez, M.L.; Muñoz, J.A. Comparative study of biosorption of heavy metals using different types of algae. Bioresour. Technol. 2007, 98, 3344–3353. [Google Scholar] [CrossRef]
- Brinza, L.; Dring, M.J.; Gavrilescu, M. Marine micro and macro algal species as biosorbents for heavy metals. Environ. Eng. Manag. J. 2007, 6, 237–251. [Google Scholar] [CrossRef]
- Ra, W.; Yoo, H.; Kim, Y.; Yun, T.; Soh, B.; Cho, S.; Joo, Y.; Lee, K. Heavy metal concentration according to shrimp species and organ specificity: Monitoring and human risk assessment. Mar. Pollut. Bull. 2023, 197, 115761. [Google Scholar] [CrossRef]
- Emerenciano, M.G.; Rombenso, A.N.; Vieira, F.-N.; Martins, M.A.; Coman, G.J.; Truong, H.H.; Noble, T.H.; Simon, C.J. Intensification of penaeid shrimp culture: An applied review of advances in production systems, nutrition and breeding. Animals 2022, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Xiang, L.; Chen, F.; Zhu, W. The diffusion path and influencing factors of shrimp farming technology. Israeli J. Aquac. 2024, 76, 22–35. [Google Scholar] [CrossRef]
- Xuan, B.B.; Sandorf, E.D.; Ngoc, Q.-K. Potential for sustainable aquaculture: Insights from discrete Choice experiments. Environ. Resour. Econ. 2020, 77, 401–421. [Google Scholar] [CrossRef]
- Saha, G.S.; De, H.K.; Mahapatra, A.S.; Panda, N. Factors contributing to the success of aquaculture field schools. J. Ext. Educ. 2020, 32, 1. [Google Scholar] [CrossRef]
- Widowati, L.L.; Ariyati, R.W.; Rejeki, S.; Bosma, R.H. The impact of aquaculture field school on the shrimp and milkfish yield and income of farmers in Demak, Central Java. J. World Aquac. Soc. 2021, 52, 362–377. [Google Scholar] [CrossRef]
- Rimmer, M.A.; Larson, S.; Lapong, I.; Purnomo, A.H.; Pong-Masak, P.R.; Swanepoel, L.; Paul, N.A. Seaweed aquaculture in Indonesia contributes to social and economic aspects of livelihoods and community wellbeing. Sustainability 2021, 13, 10946. [Google Scholar] [CrossRef]
- Saleh, H.; Sebastian, E. Seaweed Nation: Indonesia′s New Growth Sector; Backgrounder April 2020, No. 02/2020; Australia—Indonesia Centre: Caulfield East, VIC, Australia, 2020; p. 18. [Google Scholar]
- Ahmed, Z.U.; Hasan, O.; Rahman, M.M.; Akter, M.; Rahman, M.S.; Sarker, S. Seaweeds for the sustainable blue economy development: A study from the south east coast of Bangladesh. Heliyon 2022, 8, e09079. [Google Scholar] [CrossRef]
- Chowdhury, M.-N.; Hossain, M.S.; AftabUddin, S.; Alamgir, M.; Sharifuzzaman, S.M. Seaweed aquaculture in Bangladesh: Present status, challenges and future prospects. Ocean. Coast. Manag. 2022, 228, 106309. [Google Scholar] [CrossRef]
- Islam, M.M.; Khan, M.-K.; Hasan, J.; Mallick, D.; Hoq, M.E. Seaweed Hypnea sp. culture in Cox s Bazar coast, Bangladesh. Bangladesh J. Zool. 2017, 45, 37–46. [Google Scholar] [CrossRef]
- Khan, A.-S.; Islam, M.A.; Bosu, A.; Ullah, M.R.; Haque, A.-T.; Islam, M.M.; Rahman, M.A.; Mahmud, Y. Potentialities of seaweed culture in Kuakata Coast of Patuakhali, Bangladesh. Bangladesh J. Fish. Res. 2021, 20, 35–43. [Google Scholar]
- Ullah, M.R.; Islam, M.A.; Khan, A.-S.; Bosu, A.; Yasmin, F.; Hasan, M.M.; Islam, M.M.; Rahman, M.A.; Mahmud, Y. Effect of stocking density and water depth on the growth and production of red seaweed, Gracilaria tenuistipitata in the Kuakata coast of Bangladesh. Aquac. Rep. 2023, 29, 101509. [Google Scholar] [CrossRef]

| Combined | Shrimp | Seaweed | Significance ¹ | |
|---|---|---|---|---|
| Total diss. sol. (ppt) | 19.29 ± 2.20 | 21.67 ± 2.63 | 23.79 ± 1.51 | *** |
| Salinity (‰) | 24.94 ± 2.91 | 28.37 ± 3.73 | 31.42 ± 2.05 | *** |
| pH | 7.53 ± 0.59 | 7.77 ± 0.63 | 7.41 ± 0.50 | NS |
| Temperature (°C) | 31.61 ± 2.08 | 31.26 ± 2.30 | 31.38 ± 2.11 | NS |
| Dissolved oxygen (%) | 4.8 ± 1.11 | 5.08 ± 1.36 | 4.77 ± 4.36 | *** |
| Turbidity (NTU) | 18.89 ± 12.23 | 23.04 ± 12.20 | 17.21 ± 8.31 | *** |
| Chl-a (mg·m−3) | 1.71 ± 0.94 | 2.06 ± 1.41 | 2.12 ± 0.99 | NS |
| Primary prod. (gC·m−2·d−1) | 0.18 ± 0.07 | 0.20 ± 0.08 | 0.21 ± 0.06 | NS |
| Water | Sediment | ||||
|---|---|---|---|---|---|
| Treatment | Start (T0) | End (T6) | Start (T0) | End (T6) | |
| Nitrate (mg·L−1) | Seaweed | 0.97 ± 0.14 | 0.37 ± 0.05 | 2.93 ± 0.50 | 3.03 ± 1.69 |
| Shrimp | 1.29 ± 0.19 | 0.73 ± 0.12 | 2.54 ± 2.50 | 2.71 ± 0.99 | |
| Seaweed + Shrimp | 0.78 ± 0.07 | 0.47 ± 0.38 | 2.73 ± 1.88 | 2.86 ± 0.76 | |
| Phosphate (mg·L−1) | Seaweed | 0.03 ± 0.03 | 0.02 ± 0.00 | 0.32 ± 0.03 | 0.04 ± 0.02 |
| Shrimp | 0.07 ± 0.05 | 0.02 ± 0.00 | 0.34 ± 0.05 | 0.02 ± 0.00 | |
| Seaweed + Shrimp | 0.04 ± 0.01 | 0.02 ± 0.00 | 0.32 ± 0.04 | 0.02 ± 0.00 | |
| Carbon (mg·L−1) | Seaweed | 0.48 ± 0.02 | 0.53 ± 0.11 | 3.89 ± 0.24 | 3.70 ± 0.32 |
| Shrimp | 0.76 ± 0.12 | 0.67 ± 0.22 | 1.08 ± 0.21 | 1.43 ± 0.61 | |
| Seaweed + Shrimp | 0.66 ± 0.11 | 0.68 ± 0.14 | 1.29 ± 0.24 | 1.59 ± 0.32 | |
| Source | Treatment | Replicate. | Arsenic (As) | Lead (Pb) | Mercury (Hg) | Cadmium (Cd) |
|---|---|---|---|---|---|---|
| Shrimp | Combined | 1 | 0.011 ± 0.002 | 0.008 ± 0.000 | 0.051 ± 0.002 | |
| 2 | 0.020 ± 0.001 | 0.008 ± 0.000 | 0.008 | 0.047 ± 0.006 | ||
| 3 | 0.008 ± 0.000 | 0.043 ± 0.006 | 0.143 ± 0.021 | |||
| Mean | 0.013 ± 0.005 | 0.020 ± 0.018 | 0.008 ± 0.000 | 0.080 ± 0.048 | ||
| Shrimp | 1 | 0.008 ± 0.000 | 0.012 ± 0.001 | 0.017 ± 0.002 | 0.056 ± 0.002 | |
| 2 | 0.034 ± 0.005 | 0.008 ± 0.000 | 0.008 ± 0.000 | 0.023 ± 0.006 | ||
| 3 | 0.008 ± 0.000 | 0.024 ± 0.004 | 0.008 ± 0.000 | 0.027 ± 0.003 | ||
| Mean | 0.017 ± 0.013 | 0.015 ± 0.008 | 0.011 ± 0.004 | 0.036 ± 0.016 | ||
| Sediment | Combined | 1 | 0.005 ± 0.001 | 0.008 ± 0.000 | 0.008 | 0.008 |
| 2 | 0.038 ± 0.005 | 0.008 ± 0.000 | ||||
| 3 | 0.008 ± 0.000 | 0.018 ± 0.002 | ||||
| Mean | 0.017 ± 0.016 | 0.011 ± 0.005 | 0.008 ± 0.000 | 0.008 ± 0.000 | ||
| Shrimp | 1 | 0.018 ± 0.003 | 0.008 ± 0.000 | 0.008 | 0.008 | |
| 2 | 0.021 ± 0.004 | 0.018 ± 0.003 | ||||
| 3 | 0.008 ± 0.000 | 0.064 ± 0.044 | ||||
| Mean | 0.016 ± 0.006 | 0.030 ± 0.034 | 0.008 ± 0.000 | 0.008 ± 0.000 | ||
| Seaweed | 1 | 0.008 ± 0.000 | 0.008 ± 0.000 | 0.008 | 0.008 | |
| 2 | 0.008 ± 0.000 | 0.048 ± 0.009 | ||||
| 3 | 0.008 ± 0.000 | 0.008 ± 0.000 | ||||
| Mean | 0.008 ± 0.000 | 0.021 ± 0.021 | 0.008 ± 0.000 | 0.008 ± 0.000 |
| SGR (% Day−1) | Treatment | ||
|---|---|---|---|
| Shrimp | Seaweed | Combined | |
| Shrimp | 4.4 ± 0.06 * | - | 4.79 ± 0.16 * |
| Seaweed | - | 3.30 ± 0.08 * | 3.62 ± 0.02 * |
| Shrimp SR (%): | 61 ± 2.45 * | - | 80 ± 3.76 * |
| Seaweed | Shrimp | Combined | ||
|---|---|---|---|---|
| Revenues | ||||
| Seaweed Yield (kg·ha−1) | 1st harvest (Mean ± SD) | 1923.3 ± 25.2 | - | 2161.0 ± 348.0 |
| 2nd harvest (Mean ± SD) | 1858.3 ± 150.7 | - | 2361.0 ± 127.3 | |
| Shrimp yield (kg·ha−1) | - | 171.7 ± 10.4 | 264.4 ± 47.6 | |
| Mean Revenue ($) | 1st harvest | 680.61 | 800.14 | |
| 2nd harvest | 657.61 | 835.53 | ||
| Shrimp | 1093.46 | 1680.88 | ||
| Subtotal | 1338.22 | 1093.46 | 3316.54 | |
| Fixed costs ($) | pm | pm | pm | |
| Variable costs | ||||
| ($·ha−1) | Pond prep. labor | 28.31 | 28.31 | 28.31 |
| Pond prep. pump rent | 21.23 | 21.23 | 21.23 | |
| Pond prep. consumables | 7.08 | 7.08 | 7.08 | |
| Stocking material | 159.24 | 212.32 | 371.56 | |
| Seeding–labor | 14.15 | 7.08 | 21.23 | |
| Harvest–labor | 70.77 | 70.77 | 141.55 | |
| Drying–labor | 14.15 | 14.15 | ||
| Drying–materials | 17.69 | 17.69 | ||
| Subtotal | 332.64 | 346.79 | 622.81 | |
| Contribution Margin ($·ha−1) | 1005.58 | 746.66 | 2693.73 | |
| Treatment | Time in d and (Cycle) | Gel Strength | Agar Content | SGR %·d−1 | ||
|---|---|---|---|---|---|---|
| g·cm−2 | n | (%) | n | |||
| Stocking material | 0 (1) | 20.2 ± 4.1 | 6 | 13.59 ± 0.75 * | 3 | - |
| Seaweed | 30 (1) | 35.7 ± 8.1 | 9 | 21.15 ± 0.06 | 9 | 2.4 ± 0.15 |
| Combined | 30 (1) | 19.3 ± 13.1 | 9 | 21.62 ± 0.10 | 9 | 2.1 ± 0.55 |
| Restocking material | 0 (2) | 41.3 ± 5.8 | 3 | 25.54 ± 0.16 | 9 | - |
| Seaweed | 30 (2) | 43.8 ± 18.0 | 9 | 22.24 ± 0.20 | 9 | 3.2 ± 0.03 |
| Combined (hanging) | 30 (2) | 16.2 ± 9.2 | 9 | 24.70 ± 0.26 | 9 | 3.7± 0.2 ** |
| Combined (broadcast) | 30 (2) | 16.7 ± 11.5 | 9 | 22.43 ± 0.20 | 9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nauta, R.W.; Lansbergen, R.A.; Ariyati, R.W.; Widowati, L.L.; Rejeki, S.; Debrot, A.O. Co-Culture of Gracilariopsis longissima Seaweed and Penaeus monodon Shrimp for Environmental and Economic Resilience in Poor South-East Asian Coastal Aquaculture Communities. Sustainability 2025, 17, 3910. https://doi.org/10.3390/su17093910
Nauta RW, Lansbergen RA, Ariyati RW, Widowati LL, Rejeki S, Debrot AO. Co-Culture of Gracilariopsis longissima Seaweed and Penaeus monodon Shrimp for Environmental and Economic Resilience in Poor South-East Asian Coastal Aquaculture Communities. Sustainability. 2025; 17(9):3910. https://doi.org/10.3390/su17093910
Chicago/Turabian StyleNauta, Reindert W., Romy A. Lansbergen, Restiana W. Ariyati, Lestari L. Widowati, Sri Rejeki, and Adolphe O. Debrot. 2025. "Co-Culture of Gracilariopsis longissima Seaweed and Penaeus monodon Shrimp for Environmental and Economic Resilience in Poor South-East Asian Coastal Aquaculture Communities" Sustainability 17, no. 9: 3910. https://doi.org/10.3390/su17093910
APA StyleNauta, R. W., Lansbergen, R. A., Ariyati, R. W., Widowati, L. L., Rejeki, S., & Debrot, A. O. (2025). Co-Culture of Gracilariopsis longissima Seaweed and Penaeus monodon Shrimp for Environmental and Economic Resilience in Poor South-East Asian Coastal Aquaculture Communities. Sustainability, 17(9), 3910. https://doi.org/10.3390/su17093910






