Characterization and Potential Use of Biochar for the Remediation of Coal Mine Waste Containing Efflorescent Salts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Location and Description of Study Area and Sampling
2.2. Biochar Origin
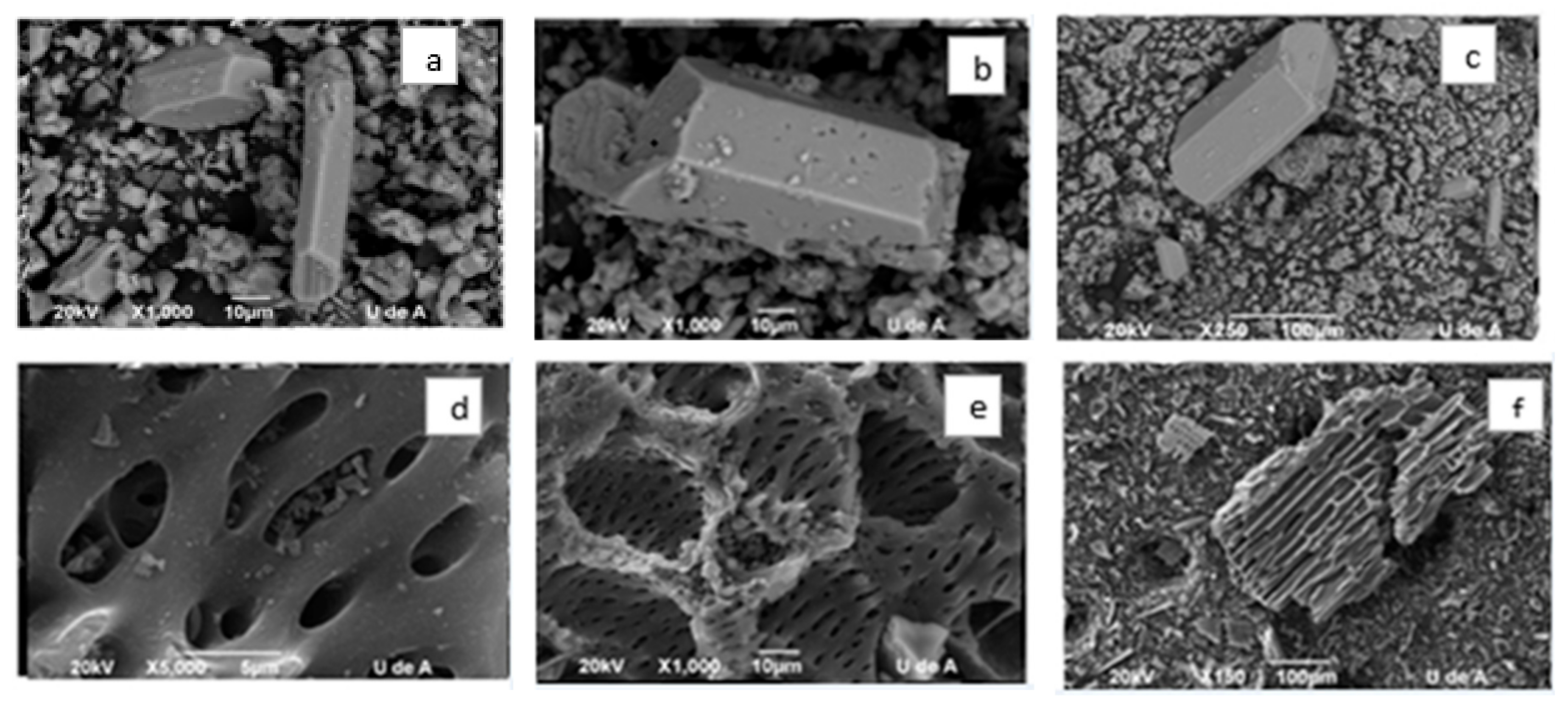
2.3. Characterization of Efflorescent Salts and Biochar
2.4. Petri Dish Bioassays
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zielinski, R.A.; Otton, J.K.; Johnson, C.A. Source of salinity near a coal mine spoil mine pile, North-Central Colorado. J. Environ. Qual. 2001, 30, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Ghose, M.; Kundu, N.K. Deterioration of soil quality due to stockpiling in coal mining areas. Int. J. Environ. Stud. 2003, 61, 327–335. [Google Scholar] [CrossRef]
- Arranz-González, J.C. Caracterización geoambiental de lugares alterados por minería de carbón en la provincia de León (España). Bull. Geol. Min. 2006, 117, 171–186. [Google Scholar]
- Arranz-González, J.C. Mine soils associated with open-cast coal mining in Spain: A review. Newslett. Geol. Min. 2011, 122, 171–186. [Google Scholar]
- Park, J.H.; Li, X.; Edraki, M.; Baumgartl, K.B. Geochemical assessments and classification of coal mine spoils for better understanding of potential salinity issues at closure. Environ. Sci. Process. Impacts 2013, 15, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, W.; Huttl, R. Direct and indirect effects of soil pollution by lignite mining. Water Air Soil Pollut. 2006, 6, 353–364. [Google Scholar] [CrossRef]
- Daniels, W.; Zipper, C. Creation and Management of Productive Mine Soils; Reclamation Guidelines; Publication No. 460-121; Virginia Cooperative Extension Publication: Blacksburg, VA, USA, 1997; pp. 1–13. [Google Scholar]
- Almansouri, M.; Kinet, J.; Lutts, S. Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum). Plant Soil 2001, 231, 243–254. [Google Scholar] [CrossRef]
- Khajeh-Hosseini, M.; Powell, A.; Bimgham, I. The interaction between salinity stress and force during seed germination of soybean seeds. Seed Sci. Technol. 2003, 31, 715–725. [Google Scholar] [CrossRef]
- Cook, S.; Shu, A.; Goodman, S. Leachability and toxicity of hydrocarbons, metals and salt contamination from flare pit soil. Water Air Soil Pollut. 2002, 133, 297–314. [Google Scholar] [CrossRef]
- Hammarstrom, J.; Seal, R.; Meier, A.; Kornfeld, J. Secondary sulfate minerals associated with acid drainage in the eastern US: Recycling of metals and acidity in surficial environments. Chem. Geol. 2005, 215, 407–431. [Google Scholar] [CrossRef]
- O’Day, A.; Vlassopoulos, D. Mineral-Based amendments for remediation. Elements 2010, 6, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Hudson, B.E. Soil organic matter and available water capacity. J. Soil Water Conserv. 1994, 49, 189–194. [Google Scholar]
- Tryon, E.H. Effect of charcoal on certain physical, chemical, and biological properties of forest soils. Ecol. Monogr. 1948, 18, 81–115. [Google Scholar] [CrossRef]
- Briggs, C.M.; Breiner, J.; Graham, R.C. Contributions of Pinus Ponderosa charcoal to soil chemical and physical properties. In Proceedings of the ASA-CSSA-SSSA International Annual Meetings, Salt Lake City, UT, USA, 6–10 November 2005. [Google Scholar]
- Gundale, M.J.; Deluca, T.H. Temperature and source material influence ecological attributes of Ponderosa pine and Douglas-fir charcoal. For. Ecol. Manag. 2006, 231, 86–93. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizao, F.J.; Petersen, J.; et al. Black coal increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly libre soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Diaz-Muegue, L.; Pinto, N.; Peñuela, G. Characterization of biochar from palm oil waste and remediation of mine waste and land affected by opencast coal mining. In Proceedings of the Annual World Conference on Carbon, Rio de Janeiro, Brazil, 14–19 July 2013. [Google Scholar]
- Diaz-Muegue, L.; Pinto, N.; Peñuela, G. Biochar from oil plan waste as an amendment for the remetiation of soil disturbed by open-cast coal mining. Glob. Adv. Res. J. Eng. 2016, 5, 17–22. [Google Scholar]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Earthscan: Sterling, VA, USA, 2009; ISBN 978-1-84407-65-1. [Google Scholar]
- Masulili, A.; Utomo, W.H.; MS, S. Rice Husk Biochar for Rice Based Cropping System in Acid Soil 1. The Characteristics of Rice Husk Biochar and Its Influence on the Properties of Acid Sulfate Soils and Rice Growth in West Kalimantan, Indonesia. J. Agric. Sci. 2010, 2, 39–47. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. J. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Solaiman, Z.; Murphy, D.; Abbott, L.K. Biochars influence seed germination and early growth of seedlings. Plant Soil 2010, 353, 273–287. [Google Scholar] [CrossRef]
- Free, H.F.; McGill, C.R.; Rowarth, J.S.; Hedley, M.J. The effect of biochars on maize (Zea mays) germination. N. Z. J. Agric. Res. 2010, 53, 1–4. [Google Scholar] [CrossRef]
- Uchimiya, M.; Klasson, K.T.; Wartelle, L.H.; Lima, I.M. Influence of soil properties on heavy metal sequestration by biochar amendment: 1. Copper sorption isotherms and the release of cations. Chemosphere 2011, 82, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Bartell, F.E.; Miller, E.J. Adsorption by activated sugar charcoal. II. J. Am. Chem. Soc. 1923, 45, 1106–1115. [Google Scholar] [CrossRef]
- Zou, L.; Morris, G.; Qi, D. Using activated carbon electrode in electrosorptive deionization of brackish water. Desalination 2008, 225, 329–340. [Google Scholar] [CrossRef]
- Thomas, S.; Frye, S.; Gale, N.; Garmon, M.; Launchbury, R.; Machado, N.; Melamed, S.; Murray, J.; Petroff, A.; Winsborough, C. Biochar mitigates negative effects of salt additions on two herbaceous plant species. J. Environ. Manag. 2013, 129, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, S.; Zhao, Y.; Jing, M.; Xu, Y.; Chen, J. Field Experiment on Enhancement of Crop Yield by Rice Straw and Corn Stalk-Derived Biochar in Northern China. Sustainability 2015, 7, 13713–13725. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Yao, Y.; Xue, Y.; Mandu, I. Synthesis, characterization, and environmental implications of graphene-coated biochar. Sci. Total Environ. 2012, 435, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Sheoran, V.; Sheoran, A.; Poonia, P. Soil reclamation of abandoned mine land by revegetation: A Review. Int. J. Soil Sediment Water 2010, 3, 1–20. [Google Scholar]
- Deenik, J.; Cooney, M. The Potential Benefits and Limitations of Corn Coband Sewage Sludge Biochars in an Infertile Oxisol. Sustainability 2016, 8, 131. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perez, M.; Kruger, C.; Fuchs, M.; Sokhansanj, S.; Badger, P.; Garcia-Nunez, J.; Lewis, T.; Kantor, S. Methods for Producing Biochar and Advanced Biofuels in Washington State Part 2: Literature Review of the Biomass Supply Chain and Preprocessing Technologies from Field to Pyrolysis Reactor; Publication No. 12-07-033; Department of Biological Systems Engineering, Center for Sustaining Agriculture Natural Resources: Pullman, WA, USA, 2012. [Google Scholar]
- Sobek, A.A.; Schuller, W.A.; Freeman, J.R.; Smith, R.M. Field and Laboratory Methods Applicable to Overburdens and Minesoils; EPA-600r2-78-054; United States Environmental Protection Agency (U.S. EPA): Cincinnati, OH, USA, 1978.
- Warncke, D.D. Analyzing greenhouse growth media by the saturation extraction method. Hortic. Sci. 1986, 21, 223–225. [Google Scholar]
- Instituto Colombiano de Normas Técnicas y Certificación (ICONTEC). Standard for Agricultural Industry Products, Organics Products Used as Fertilizers and Soil Amendments NTC 5167; Instituto Colombiano de Normas Técnicas y Certificación: Bogotá, Colombia, 2004; pp. 1–32. [Google Scholar]
- United States Environmental Protection Agency (US EPA). SW-846 Reference Methodology: Method 3050B. Standard Operating Procedure for the Digestion of Soil/Sediment Samples Using a Hotplate/Beaker Digestion Technique; U.S. Environmental Protection Agency: Chicago, IL, USA, 1999; pp. 1–12.
- Singh, B.; Fang, Y.; Johnston, C.T. A Fourier-Transform Infrared Study of Biochar Aging in Soils. Soil Sci. Soc. Am. J. 2016, 80, 613–622. [Google Scholar] [CrossRef]
- Office of Surface Mining Reclamation and Enforcement (OSMRE). Overburden Sampling and Analytical Quality Assurance and Quality Control (QA/QC) Requirements for Soils, Overburden, and Regraded Spoil Characterization and Monitoring Programs for Federal Lands in the Southwestern United States; Office of Surface Mining Reclamation and Enforcement, U.S. Department of the Interior, Western Regional Coordinating Center: Denver, CO, USA, 1998.
- Murad, E.; Rojik, P. Iron-rich precipitates in a mine drainage environment: Influence of pH on mineralogy mapper. Am. Mineral. 2003, 88, 1915–1918. [Google Scholar] [CrossRef]
- Saikia, B.; Boruah, R.; Gogoi, P. A X-ray diffraction analysis on graphene layers of Assam coal. J. Chem. Sci. 2009, 121, 103–106. [Google Scholar] [CrossRef]
- Cantrell, K.; Hunt, P.; Uchimiya, M.; Novak, J. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, M. Role of gypsum and compost in reclaiming saline-sodic soils. J. Agric. Vet. Sci 2012, 30–38. [Google Scholar] [CrossRef]
- Organization for Economic Co-operation and Development (OECD). Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test; OECD Guidelines for Testing of Chemicals 208; Organization for Economic Co-operation and Development: Paris, France, 1984; pp. 1–6. [Google Scholar]
- Oleszczuk, P.; Malara, A.; Jośko, I.; Lesiuk, A. The Phytotoxicity changes of sewage sludge-amended soils. Water Air Soil Pollut. 2012, 223, 4937–4948. [Google Scholar] [CrossRef] [PubMed]
- Abari, A.; Nasr, M.H.; Hojjati, M.; Bayat, D. Salt effects on seed germination and seedling emergence of two Acacia species. Afr. J. Plant Sci. 2011, 5, 52–56. [Google Scholar]
- Wang, X.; Sung, C.; Gao, S.; Wang, L. Variation of germination rate and root elongation as indicator to assess phytotoxicity with Cucumis sativus. Chemosphere 2001, 44, 1711–1721. [Google Scholar] [CrossRef]






| References | Units | Salt Mine Spoil | Biochar |
|---|---|---|---|
| pH | 8.85 | 8.92 | |
| EC | dS/m | 13.37 | 0.24 |
| Sand | % | 53 | - |
| Silt | % | 33.5 | - |
| Clay | % | 13.5 | - |
| WHC | % | - | 241.6 |
| Soil texture | - | Sandy loam | - |
| Munsell color (dry) | - | 10YR 9.5/1 | 2.5Gley1/N |
| SOC | % | 1.06 | 0.68 |
| P | mg/kg | 37.7 | - |
| N Total | % | 0.13 | - |
| CEC | (cmol(+) kg−1) | 36 | 42.80 |
| Ca | mg/kg | 9480.0 | 129.80 |
| Na | mg/kg | 3591.2 | 1620 |
| Mg | mg/kg | 302.6 | 34.70 |
| K | mg/kg | 288.5 | 109.76 |
| Sulfates | mg/kg | 11,814.62 | - |
| Total copper | mg/kg | 37.88 | 3.20 |
| Total zinc | mg/kg | 314.46 | 35.13 |
| Total iron | mg/kg | 37,208.37 | 211.36 |
| Total aluminum | mg/kg | 8702.29 | 284.82 |
| Total manganese | mg/kg | 490.43 | 47.44 |
| S | % | 2.20 | 0.15 |
| C | % | - | 70.10 |
| H | % | - | 2.81 |
| N | % | - | 0.86 |
| O | % | - | 25.16 |
| Factor | df | Brachiaria | Brachiaria |
|---|---|---|---|
| Germination | Root Length | ||
| Mine waste with salt dose | 2 | 0.4527 | 0.0066 * |
| Biochar dose | 3 | 0.0001 * | 0.0000 * |
| Biochar dose × soil type | 6 | 0.7068 | 0.1172 |
| Brachiaria Root Length (cm) | ||
|---|---|---|
| Biochar percent | Mean | Homogeneous groups |
| 0 | 4.8 | a |
| 1 | 4.07 | a |
| 2.5 | 4.39 | a |
| 5 | 14,7 | b |
| Brachiaria Germination (%) | ||
| Biochar percent | Mean | Homogeneous groups |
| 0 | 4.78 | a |
| 1 | 5.33 | a |
| 2.5 | 4.22 | a |
| 5 | 11.9 | b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muegue, L.C.D.; González, J.C.A.; Mesa, G.P. Characterization and Potential Use of Biochar for the Remediation of Coal Mine Waste Containing Efflorescent Salts. Sustainability 2017, 9, 2100. https://doi.org/10.3390/su9112100
Muegue LCD, González JCA, Mesa GP. Characterization and Potential Use of Biochar for the Remediation of Coal Mine Waste Containing Efflorescent Salts. Sustainability. 2017; 9(11):2100. https://doi.org/10.3390/su9112100
Chicago/Turabian StyleMuegue, Luis Carlos Díaz, Julio César Arranz González, and Gustavo Peñuela Mesa. 2017. "Characterization and Potential Use of Biochar for the Remediation of Coal Mine Waste Containing Efflorescent Salts" Sustainability 9, no. 11: 2100. https://doi.org/10.3390/su9112100
APA StyleMuegue, L. C. D., González, J. C. A., & Mesa, G. P. (2017). Characterization and Potential Use of Biochar for the Remediation of Coal Mine Waste Containing Efflorescent Salts. Sustainability, 9(11), 2100. https://doi.org/10.3390/su9112100




