Abstract
Wastewater effluent from aquaculture ponds can affect aquatic ecosystems. To mitigate this problem, we designed 2 sets (southern and northern) of land-based and farm-scale sequential integrated multi-trophic aquaculture (IMTA) systems in order to reduce water pollution and to diversify and optimize aquaculture products in coastal southern Taiwan. In each system, the 1st pond cultivated milkfish as the main aquaculture product, the 2nd pond cultivated Portuguese oysters as the product to reduce suspended particles, and the 3rd pond cultivated the seaweed Gracilaria sp. as feed and to absorb nutrients. Photosynthetic bacteria (PSB) were added to the southern system in order to reduce nutrients. The objective of this study was to evaluate and compare performance parameters of the compartments and the overall IMTA systems preliminarily. Our results showed that the southern system with the addition of PSB had lower PO4−3-P, slightly higher turbidity, and higher brown algal biomass than the northern system. In the southern system, PO4−3-P and cyanobacteria levels were lowest at the end of the seaweed pond. In the northern system, NO2−-N and phytoplankton levels were lowest at the end of the seaweed pond. Turbidity was reduced in the oyster pond and further reduced in the Gracilaria pond in both systems. The high seaweed yield in the northern system indicated substantial nutrient absorption. Advantages and limitations in terms of water purification and aquaculture production of these IMTA systems are evaluated in the present paper.
1. Introduction
The global production of aquaculture products has increased over time following the increasing demands of aquatic products like fish, shellfish, seaweed, and seafood in general [1,2]. However, the wastewater effluents of aquaculture facilities have also enhanced nutrient loads and turbidity both in the aquaculture facilities and in ambient waters [3,4,5,6]. Effluents can cause dramatic changes in ecosystem structure and function outside aquaculture facilities. These changes include increasing phytoplankton biomass and production rates and changing bottom sediments and their benthic macroinvertebrates, seaweed, and fish assemblages in ambient receiving waters [7,8,9,10,11]. To mitigate such environmental deteriorations in coastal environments, a land-based sequential integrated multitrophic aquaculture (IMTA) system was designed [12,13,14,15,16,17]. Besides environmental improvement, an IMTA system can increase income and economic stability of aquaculture farmers by providing diversified products [18,19,20,21]. A land-based shellfish and seaweed production has the benefit of being isolated from coastal waters. This way it is protected from environmental harshness, such as tidal fluctuations [22,23], wave damage during storms [24,25,26], red tides [27,28], and serious water pollution from other anthropogenic sources [21].
A land-based sequential IMTA system commonly cultivates a fed species in the main pond and extractive species in several sequential ponds to recycle inorganic and organic waste [16,20]. Shellfish, such as the bivalves Crassostrea sp. and Chlamys sp., are often used to reduce particulate organic matter and phytoplankton by their suspension feeding activity in a sequential pond system [20,29]. Seaweeds, such as Gracilaria and Ulva, are often used to extract nutrients, stabilize water quality, and provide additional production [30,31,32,33]. Seaweeds can also release allelopathic chemicals to inhibit harmful microalgal blooms [34,35]. In addition, photosynthetic bacteria (PSB) can be added to reduce nitrogen (N) and phosphorous (P) in the system and to provide a food source for suspension feeders at the same time [36,37]. The design of an IMTA is commonly adjusted to the cultivated organisms [20], which may change with different regions [20]. Only a few farm-scale land-based sequential IMTA systems have been constructed and tested in the East Asia region [19].
Aquaculture is a major industry in the coastal regions of southern Taiwan [38]. However, regulations for the treatment of aquaculture effluents are not established here as yet. As a consequence, algal blooms occur occasionally, partly caused by high nutrient contents in the water [39,40,41]. This caused the Mariculture Research Center of the Fisheries Research Institute, located in the coastal regions of southern Taiwan (Tainan City), to construct 2 sets of land-based sequential IMTAs in order to study their performance in terms of water purification and aquaculture production. These IMTA systems were semi-intensive.
The goal of this study was to evaluate the performance of water purification of these sequential units preliminarily and to compare the performance of these 2 sets of farm-scale IMTAs. The 2 systems differed in the amount of Gracilaria and the addition of Proteobacteria Rhodovulum sulfidophilum (PSB). Only a few studies have evaluated the overall effectiveness of farm-scale land-based sequential and tropical IMTA systems [42,43]. The present IMTA system is one of the rare systems tested in the East Asia region.
2. Materials and Methods
2.1. Study Area
The study area is located at 23.1215° N and 120.08° E. It is close to the Cigu lagoon of Tainan City in southwest Taiwan. The area is situated on a coastal plain, which was gradually formed by fluvial processes and land filling. The weather is subtropical. The average monthly temperature is generally highest in July (mean monthly temperature was 27.8 °C in 2012 according to the Central Weather Bureau of Taiwan) and lowest in January (mean monthly temperature 16.4 °C in 2012 according to the Central Weather Bureau of Taiwan). The raining season occurs commonly from May through September, whereas the dry season is from October to April in this part of Taiwan. The dominant land use type is aquaculture ponds, where primarily common orient clam (Meretrix lusoria), tilapia (Oreochromis niloticus), and milkfish (Chanos chanos) are cultivated. This area is one of the major land-based and off-shore aquaculture regions in Taiwan [38].
2.2. The Studied IMTA Systems
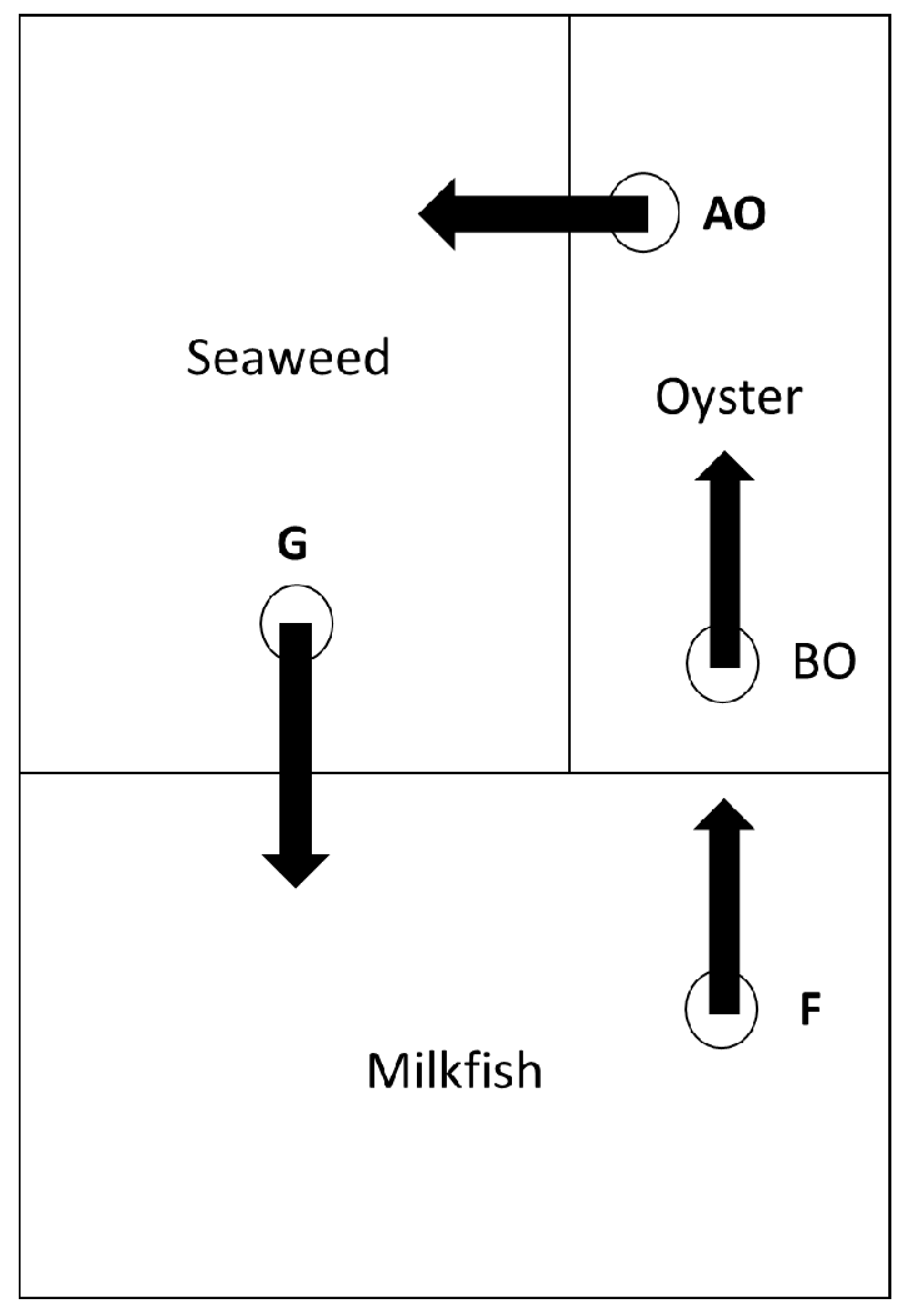
The 2 IMTA systems of the Mariculture Research Center of the Fisheries Research Institute were composed of 3 sequential ponds (Figure 1). The 1st pond was a milk fish pond with the white shrimp (Litopenaeus vannamei) as a co-cultured aquaculture product with the main function of reducing leftover feed. Portuguese oysters (Crassostrea angulata) were cultivated in the 2nd pond to reduce phytoplankton and suspended organic matter. Line-tied oysters were hung vertically from bamboo racks into the pond. The seaweed Gracilaria verrucosa was cultivated in the 3rd pond as an aquaculture product to reduce the nutrient load (Figure 1). Portuguese oysters and Gracilaria verrucosa are commonly cultivated in this region [38] and were bought from local suppliers. The 3 ponds were 2500, 800 and 1700 m2 in area and were 1.8, 1.5 and 0.5 m in depth, respectively. Water was recirculated in the direction depicted in Figure 1 and had no inflow and neither outflow once cultivation began. A tidal channel connected to the Cigu lagoon was the water source. The water in the recirculated IMTA system flowed daily from 12 a.m. to 6 a.m. at a rate of about 84 m3/h, which was made possible by an underwater pump.
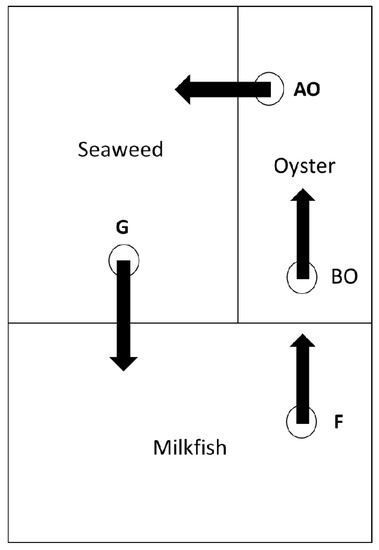
Figure 1.
Diagram of an integrated multi-trophic aquaculture system (IMTA). Sampling points of water quality: F (end of fish pond), BO (before oyster pond), AO (after oysters), and G (end of seaweed pond).
The two studied sets of IMTA systems were adjacent to each other and named the northern and southern systems. The northern system was stocked with 15,000 milkfish Chanos chanos in the 1st pond, 10,000 oysters Crassostrea angulata in the 2nd pond, and 12,000 kg of seaweed Gracilaria verrucosa (0.71 kg/m2) in the 3rd pond. The 1st and 2nd ponds of the southern system had the same stocking densities as the northern system, but the 3rd pond of the southern system was stocked with 3000 kg of Gracilaria verrucosa (0.29 kg/m2). Additionally, 1000 L/week of PSB (108 cells/L) were added to the fish pond of the southern system. At the start of the experiment, the stocked milkfish were 15.2 ± 3.2 cm and 22.13 ± 2.17 g (mean ± 1 SD; n = 30) in size for both systems, while oysters were 5.81 ± 1.02 cm (n = 30) wide. The white shrimp Litopenaeus vanamei population, pre-stocked in the first pond, was decimated by white spot virus syndrome before this experiment and only about 4000 shrimps were left for the stocking in each system. The experiment began on 20 August 2012 and ended on 5 November 2012, covering the major growing season of the cultivated organisms. The additional pellet feed used in this experiment was primarily herbaceous and was composed of 80% rice bran, 18.5% wheat middlings, and 1.5% calcium powder, and had only 14.3% crude protein. This feed did not contain protein from the ocean in order to reduce the demand of bycatch and small-size marine organisms for protein-feed production. The milkfish were fed twice: between 8 a.m. and 12 p.m., and between 13 p.m. and 17 p.m. When the fish were 15–18 cm in length, the daily amount of feed was 8% of the weight of the milkfish. When the fish were 18–21 cm in length, the daily amount of feed was 7% of the fish weight.
Cultivated organisms were sampled on 20 August, 20 September, and 20 October, and again on 5 November. The length and weight of 30 milk fishes of each system were measured every month. At the end of the experiment, the milkfish, oysters, and seaweeds were harvested and their numbers and biomass were measured. The condition index of oysters was measured as the percentage of dry meat mass to dry shell weight.
2.3. Water Sampling
Water quality parameters were recorded once per week, which resulted in 8 readings during the entire experiment. The 4 sampling points were at the end of the milk fish pond (F), before the oyster pond (BO), behind the oyster and in front of the Gracilaria pond (AO), and at the end of the Gracilaria pond (G). The water sample at F represented the water quality of the fish pond, the differences in the water quality between BO and AO indicated the effectiveness of the oysters, and the differences between AO and G showed the effectiveness of Gracilaria verrucosa in reducing nutrients and suspended particles including phytoplankton. Field water quality parameters including salinity, pH, water temperature, and DO were measured in the field by YSITM multiprobes (YSITM, Yellow Springs, OH, USA). A MERCKTM NOVA 60 was used to measure PO4−3-P, NH4+-N, NO3−-N and NO2−-N. A five-day biochemical oxygen demand (BOD5) was analyzed with the WTWTM BOD 701 low temperature incubator (WTWTM, Weilheim, Germany). Calibration lines were established for each chemical before analysis. Turbidity was measured with the HANNATM instrument HI 98703-11 (HANNATM, Woonsocket, RI, USA) in the lab, immediately after samples were collected.
Phytoplankton was measured in situ with a submersible fluorescence probe (FluoroProbeTM, bbe-Moldaenke, Kiel, Germany). This device can differentiate between spectral groups of microalgae in situ on the basis of their relative fluorescence intensity of Chl a (Chlorophyll a) at 680 nm [44,45]. The spectral fluorescence measured by this instrument is based on the selective excitation of the different accessory pigments in the photosystem II among algal taxonomic groups. It is applicable to aquaculture ponds when Chl a is <250 μg/L [46]. This fluorescence probe can separate among green algae (chlorophytes and euglenophytes; at 450 nm), brown algae (diatoms, chrysophytes, and dinoflagellates, or the heterokontophytes and pyrrhophytes of the dinoflagellates; at 525 nm), a phycoerythrin-containing (PE-rich) group (cryptophytes and red cyanobacteria; at 570 nm), and a blue-green group (cyanobacteria; at 590 and 610 nm) [44].
2.4. Statistical Analysis
To compare the treatment effects on the specific water-quality variables between the 2 IMTA systems, we used paired t-test to analyze the difference between values at F and G sampling points among 8 samples. The higher the difference is, the better is the treatment effect. With paired t-test, we can test which IMTA set is better at treatment of certain variables.
To compare the water quality variables and algal measurements among the 4 sampling points in each IMTA system, repeated-measures ANOVA (analysis of variance) and pair-wise multiple comparisons were used to compare water variables and separate groups among the sampling points. In this case, water variables were the independent variables, and the sampling point was the within-subject variable [46]. The repeated-measures ANOVA compared data of 4 sampling points through 8 times of measurements in each IMTA set. In most cases, data violated the sphericity assumption, so the degrees of freedom were corrected using the Greenhouse–Geisser estimates of sphericity. The weight and length of the harvested fish were compared between the 2 IMTA systems using a t-test.
3. Results
The northern IMTA set treated turbidity better than the southern set (Paired t-test; Table 1, Figure 2). The 2 IMTA sets had the same treatment effects on other water and phytoplankton variables.

Table 1.
Comparisons of treatment effects of water quality and algal variables between the 2 IMTA sets with paired t-tests. The mean is the average of variable value in the northern set minus the value in the southern set. The number in bold indicates a significant difference.
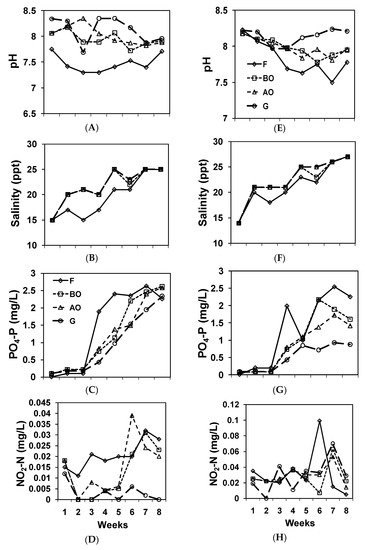
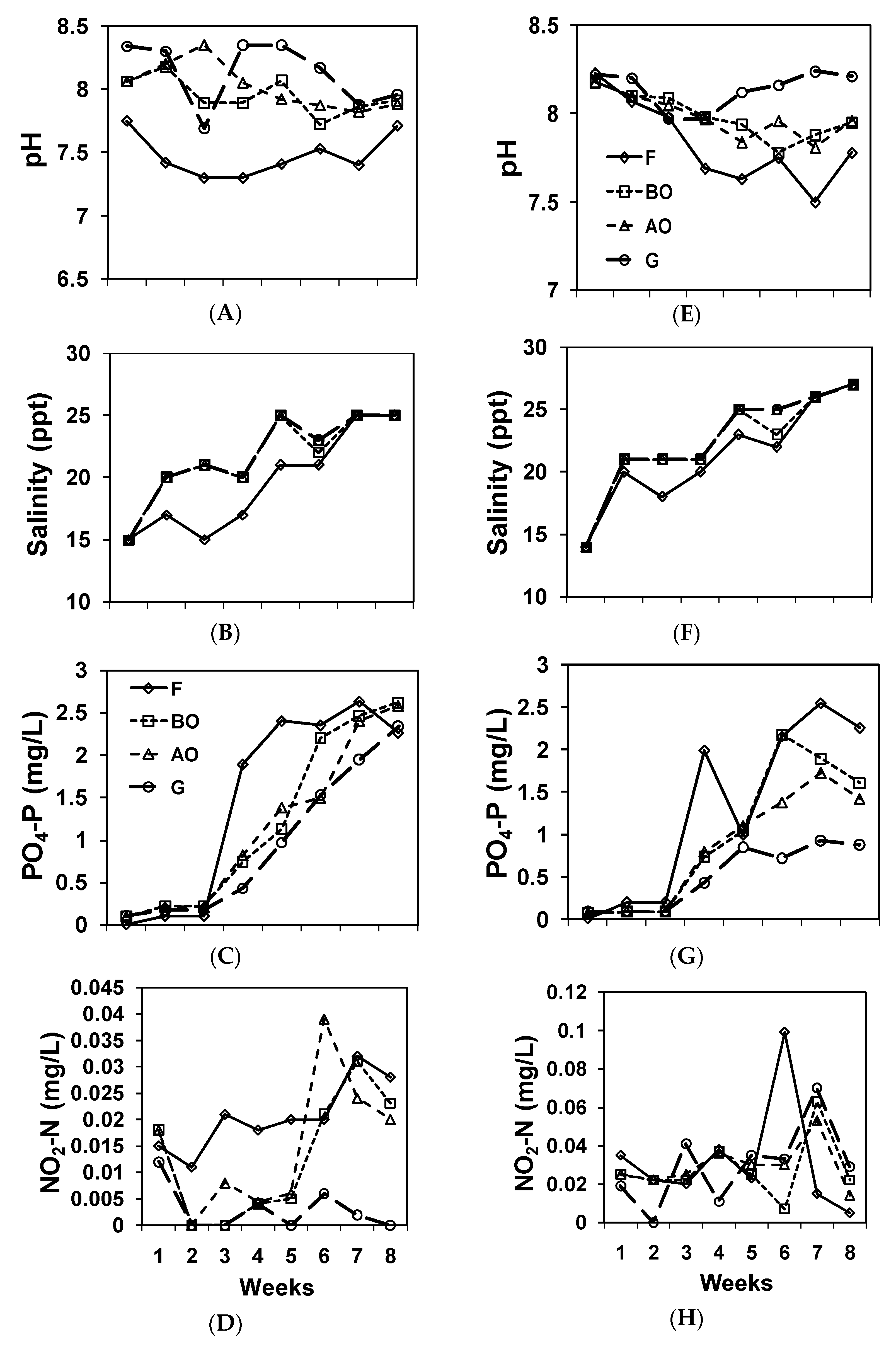
Figure 2.
Water quality of sampling points through time. (A) pH, (B) Salinity, (C) PO4−3-P, and (D) NO2−-N of the northern set, (E) pH, (F). Salinity, (G) PO4−3-P, and (H) NO2−-N of the southern set.
Salinity differed among the sampling points in both systems (repeated-measures ANOVA; Table 2). In both systems, salinity was higher in the BO, AO, and G group and lower at the F group based on the results of the pair-wise comparisons. The pH was lower in the F and higher in the BO, AO, and G group. DO and water temperature were the same within each IMTA set.

Table 2.
Statistical results of differences among sampling points in each IMTA set. The numbers under each sampling point are means ± 1SE, and characters, a, b, and c, are grouping results of pairwise comparisons. Numbers in bold indicate significant differences.
PO4−3-P levels differed within the southern systems (Table 2). In the southern system, PO4−3-P levels were highest in the F and BO group, second highest in the BO and AO group, and lowest at the G sampling point, indicating a reduction in the Gracilaria pond. NO2−-N levels did not differ within the southern system, but differed within the northern system. NO2−-N levels were highest in the F and AO group, followed by the BO and AO group, and then by the G group in the northern system, indicating a reduction of NO2−-N in the Gracilaria pond. NH4+-N levels did not differ within each IMTA set. BOD5 levels only differed in the northern system, where the water sampled at F had higher BOD5 than that of the others. Turbidity only differed within the northern system. The southern system had a higher turbidity than the northern system. Water sampled at F and BO had the highest turbidity, AO the 2nd, and G was the lowest group in the northern set. Most NO3−-N concentrations were below the detection level, and, therefore, not reported in this paper.
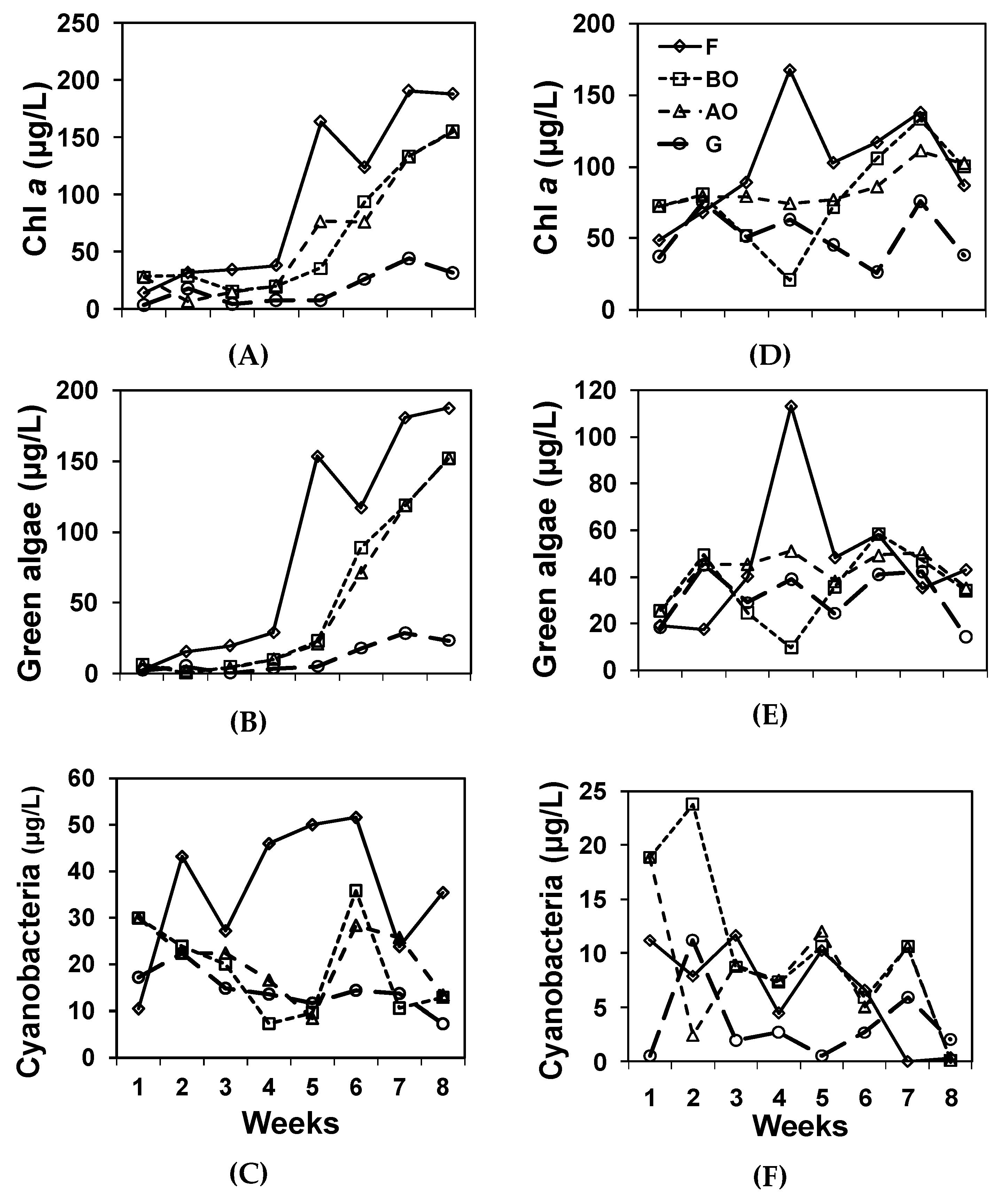
Phytoplankton density as indicated by Chl a differed within both sets (Table 2, Figure 3). In the northern set, F and BO were in the highest phytoplankton group, BO and AO were in the 2nd group, and G represented the lowest group. In the southern set, F, BO, AO were in the higher group, and BO and G were in the lower group. Green algal density only differed within the northern set. F had a higher level of green algae, and BO, AO, and G had lower levels in the northern system. Cyanobacteria only differed within the southern set. F and AO were in the highest cyanobacteria group, BO and AO were in the 2nd group, and BO and G were in the lowest group in the southern system. The Brown and PE-rich Groups did not differ within each IMTA set.
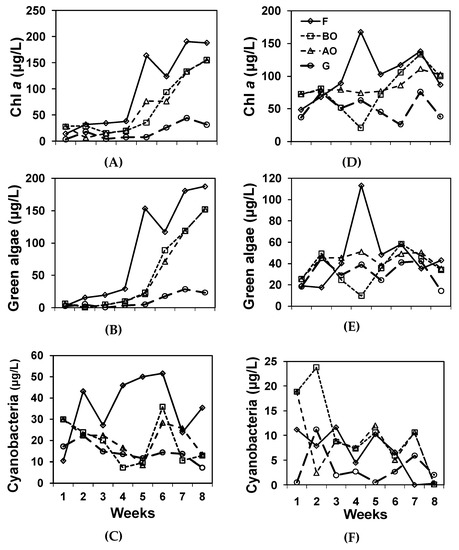
Figure 3.
Phytoplankton measurements of sampling points through time. (A) Overall Chl a, (B) Green algae, (C) Cyanobacteria of the northern system. (D) Chl a, (E). Green algae, (F) Cyanobacteria of the southern system.
The length and weight of the harvested fish in the northern system were higher than those in the southern system (t-test; t = 9.64, p < 0.001; t = 8.09, p < 0.001; Table 3). The feed conversion ratio was 1.96 and 2.13 for the northern and southern system, respectively. The survival rate of the oysters was 52.4% in the northern system and 47.6% in the southern system. In the northern system, the condition index of the oysters increased from 6.42% on 20 September to 10.51% at complete harvest on 5 November. In the southern system, the condition index of the oysters increased from 8.60% on 20 September to 10.90% at harvest. The weight of the harvested Gracilaria verrucosa was 20,088 kg in the northern system (equivalent to 11,816 kg per 1000 m2) and 1620 kg (equivalent to 953 kg per 1000 m2) in the southern system. The amount of Gracilaria in the northern system increased by 67%, whereas it decreased by 46% in the southern system.

Table 3.
Production parameters of milkfish in the 2 IMTA systems.
4. Discussion
These 2 farm-scale IMTA systems demonstrated the effects of oysters Crassostrea angulata and seaweed Gracilaria verrucosa on various water quality variables. The oysters in the northern system reduced the turbidity. The seaweed reduced NO2−-N and phytoplankton in the northern system, and reduced PO4−3-P, phytoplankton, and cyanobacteria in the southern system. Overall, the southern system with PSB had lower PO4−3-P than the northern system. However, the addition of PSB may have resulted in higher turbidity in the southern system. In addition, milk fish grew larger and seaweed grew better in the northern than in the southern system. The water quality conditions were not harmful for the cultivated organisms.
Weather conditions, especially strong winds inducing turbulence, affected both IMTA systems. Strong winds occur frequently in the coastal regions of Taiwan [47,48]. Winds can cause turbulence or mixing in shallow ponds, which can re-suspend particles and nutrients from the bottom of the ponds, thus mask the effects of the different treatments. Martínez-Porchas et al. [16] proposed to reduce water turbulence in polyculture ponds using bamboo sticks, which could be tested in future experiments.
Oysters reduced turbidity in the northern system, but not in the southern system. The effects of filtering shellfish on suspended solids and turbidity have been observed in laboratory-scale studies [49,50] and in a larger tank study [51]. Reduced turbidity was probably caused by feeding on organic particles. Phytoplankton was not reduced through the oyster pond, probably because the suspension feeding of oysters could not even exploit the overproduction of phytoplankton, which was indicated by pseudofeces produced by oysters (personal observations by Y.-J.C.). The oysters in the southern system did not reduce the turbidity probably because the added PSB increased the turbidity beyond the suspension feeding capability of oysters.
The Gracilaria pond had multiple effects on both IMTA systems, where the northern system was stocked with 3 times more Gracilaria than the southern system. In the northern system, the Gracilaria pond reduced NO2−-N, turbidity, and phytoplankton. Given less density and reduced populations of Gracilaria in the southern system, the Gracilaria pond had more effects on PO4−3-P and phytoplankton. With a higher seaweed stocking density, the functions of the Gracilaria pond in the northern system included absorbing nutrients and increasing sedimentation. Despite the fact that the reductions of PO4−3-P and NH4+-N in the Gracilaria pond were not significant in the northern set, the growth of 12,088 kg of Gracilaria verrucosa is expected to provide a substantial assimilation of nutrients [52,53].
The addition of PSB showed an effect on the overall PO4−3-P in the southern set compared to the northern set, but no effects on N appeared here. Wang and He [54], and Luo et al. [37] reported that PSB reduced dissolved inorganic nitrogen and PO4−3-P in PSB-enriched ponds compared to control ponds. However, McIntosh et al. [55] and Zhou et al. [56] reported a lack of effects on various nutrients when treated with PSB. In this experiment, the PSB concentration was quite low (222 CFU/mL) compared to other experiments (105 or 106 CFU/mL) [36,37]. Our experimental IMTA systems may require higher PSB concentrations to test for their respective effects in the future.
The lower salinity and pH at the fish pond originated from the properties and operation of the system. A reduced pH probably resulted from rice bran deposition in the fish pond. The lowest pH was within the range of tolerance of growth of milk fish, shrimp, and phytoplankton. The higher water surface to volume ratio and subsequent higher evaporation of ponds commonly increased the salinity content, particularly in the oyster and Gracilaria ponds. To the contrary, the lowest water surface to volume ratio in the fish pond provided a reduced evaporation rate.
Besides those aforementioned adjustments, our 2 IMTA systems may have been improved in the following ways. Firstly, the performance of extractive species in each compartment could be calculated when different densities were stocked for the optimization of each compartment. Secondly, alternatives of harvested species may be considered. For example, the clams Septifer bilocularis and Meretrix lusoria may replace Portuguese oysters as suspension feeders. However, their performance in this IMTA needs to be tested. Seaweed may not have a proper alternative for cultivation in this time period and in this region. For example, Ulva lactuca only grows from February through April, which is not suitable for cultivation in summer and fall. Thirdly, more replicated IMTAs may help improve our understanding of how environmental variations may affect performance of each compartment and the whole system. Space and financial constraints limited the number of replicated IMTA in this farm-scaled study. Many IMTA studies were at the tank or aquarium scale, which is much smaller in size and requires less resources. Under the current study design, we preliminarily tested the effects of each compartment on water quality and algal variables in each system using temporally repeated-measured data. In the future, more repeats through time can increase the robustness of our results.
5. Conclusions
This IMTA system study provided an initial understanding of the interactions and effectiveness in terms of water purification of each unit of harvested species in combination with the addition of PSB. This study showed reduction of turbidity, nutrients, and phytoplankton in variable degree. In the future, an increase of the Gracilaria verrucosa seedling density may be used to increase the absorption of nutrients in the 3rd pond of the IMTA system. Effects of diverse available clam species may be further tested. A detailed economic analysis of IMTA systems may provide the basis of feasibility and profitability for promoting integrated systems to aquaculture farmers.
Acknowledgments
This study was supported by the Fisheries Research Institute of the Fisheries Center of the Council of Agriculture of The Republic of China (101 Agriculture Science-11.3.1-Water-A4(2).
Author Contributions
This study was designed by Shinn-Lih Yeh, Yi-Kuang Wang, and Ying-Jer Chiu. Ying-Jer Chiu and Shinn-Lih Yeh conducted field work and sample processing. Yi-Kuang Wang, Ying-Jer Chiu, and Su-Jung Chang analyzed the data. Yi-Kuang Wang and Hans-Uwe Dahms wrote this paper. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bostock, J.; McAndrew, B.; Richards, R.; Jauncey, K.; Telfer, T.; Lorenzen, K.; Little, D.; Ross, L.; Handisyde, N.; Gatward, I.; et al. Aquaculture: Global status and trends. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2010, 365, 2897–2912. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). The State of World Fisheries and Aquaculture; Food and Agriculture Organization: Rome, Italy, 2014. [Google Scholar]
- Pillay, T.V.R. Aquaculture and the Environment; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Diana, J.S. Aquaculture Production and Biodiversity Conservation. BioScience 2009, 59, 27–38. [Google Scholar] [CrossRef]
- Martinez-Porchas, M.; Martinez-Cordova, L.R. World Aquaculture: Environmental Impacts and Troubleshooting Alternatives. Sci. World J. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Oettinger, M.; Clauss, K.; Kuenzer, C. Aquaculture: Relevance, distribution, impacts and spatial assessments—A review. Ocean Coast. Manag. 2016, 119, 244–266. [Google Scholar] [CrossRef]
- Stephens, W.W.; Farris, J.L. Instream community assessment of aquaculture effluents. Aquaculture 2004, 231, 149–162. [Google Scholar] [CrossRef]
- Peterson, M.; Slack, W.T.; Woodley, C.M.; Springs, O. The occurrence of non-indigenous nile tilapia, Oreochromins niloticus (Linnaeus) in coastal Mississippi, USA: Ties to aquaculture and thermal effluents. Wetlands 2005, 25, 112–121. [Google Scholar] [CrossRef]
- Holmer, M.; Argyrou, M.; Dalsgaard, T.; Danovaro, R.; Diaz-Almela, E.; Duarte, C.M.; Frederiksen, M.; Grau, A.; Karakassis, I.; Marbà, N.; et al. Effects of fish farm waste on Posidonia oceanica meadows: Synthesis and provision of monitoring and management tools. Mar. Pollut. Bull. 2008, 56, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Thomas, Y.; Courties, C.; El Helwe, Y.; Herbland, A.; Lemonnier, H. Spatial and temporal extension of eutrophication associated with shrimp farm wastewater discharges in the New Caledonia lagoon. Mar. Pollut. Bull. 2010, 61, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Ansah, Y.B.; Frimpong, E.A.; Amisah, S. Biological assessment of aquaculture effects on effluent-receiving streams in Ghana using structural and functional composition of fish and macroinvertebrate assemblages. Environ. Manag. 2012, 50, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Shpigel, M.; Neori, A.; Popper, D.M.; Gordin, H. A proposed model for “environmentally clean” land-based culture of fish, bivalves and seaweeds. Aquaculture 1993, 117, 115–128. [Google Scholar] [CrossRef]
- Shpigel, M.; Neori, A. The integrated culture of seaweed, abalone, fish and clams in modular intensive land-based systems: I. Proportions of size and projected revenues. Aquac. Eng. 1996, 15, 313–326. [Google Scholar] [CrossRef]
- Neori, A.; Ragg, N.; Shpigel, M. The integrated culture of seaweed, abalone, fish and clams in modular intensive land-based systems: II. Performance and nitrogen partitioning within an abalone (Haliotis tuberculata) and macroalgae culture system. Aquac. Eng. 1998, 17, 215–239. [Google Scholar] [CrossRef]
- Neori, A.; Shpigel, M.; Ben-Ezra, D. A sustainable integrated system for culture of fish, seaweed and abalone. Aquaculture 2000, 186, 279–291. [Google Scholar] [CrossRef]
- Martínez-Porchas, M.; Martínez-Córdova, L.R.; Porchas-Cornejo, M.A.; López-Elías, J.A. Shrimp polyculture: A potentially profitable, sustainable, but uncommon aquacultural practice. Rev. Aquac. 2010, 2, 73–85. [Google Scholar] [CrossRef]
- Sri-uam, P.; Donnuea, S.; Powtongsook, S.; Pavasant, P. Integrated multi-trophic recirculating aquaculture system for Nile tilapia (Oreochromis niloticus). Sustainability 2016, 8, 1–15. [Google Scholar] [CrossRef]
- Chopin, T.; Buschmann, A.H.; Halling, C.; Troell, M.; Kautsky, N.; Neori, A.; Kraemer, G.P.; Zertuche-gonzález, J.; Yarish, C.; Neefus, C. Integrating seaweeds into marine aquaculture systems: A key towards sustainability. J. Phycol. 2001, 37, 975–986. [Google Scholar] [CrossRef]
- Ridler, N.; Wowchuk, M.; Robinson, B.; Barrington, K.; Chopin, T.; Robinson, S.; Page, F.; Reid, G.; Szemerda, M.; Sewuster, J. Integrated Multi-Trophic Aquaculture (IMTA): A Potential Strategic Choice for Farmers. Aquac. Econ. Manag. 2007, 11, 99–110. [Google Scholar] [CrossRef]
- Troell, M. Integrated marine and brackishwater aquaculture in tropical regions. In Integrated Mariculture—A Global Review; FAO Fisheries and Aquaculture Technical. Paper No. 529; Soto, D., Ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009; pp. 47–132. [Google Scholar]
- Nobre, A.M.; Robertson-Andersson, D.; Neori, A.; Sankar, K. Ecological-economic assessment of aquaculture options: Comparison between abalone monoculture and integrated multi-trophic aquaculture of abalone and seaweeds. Aquaculture 2010, 306, 116–126. [Google Scholar] [CrossRef]
- Bartol, I.K.; Mann, R.; Luckenbach, M. Growth and mortality of oysters (Crassostrea virginica) on constructed intertidal reefs: Effects of tidal height and substrate level. J. Exp. Mar. Biol. Ecol. 1999, 237, 157–184. [Google Scholar] [CrossRef]
- La Peyre, M.K.; Eberline, B.S.; Soniat, T.M.; La Peyre, J.F. Differences in extreme low salinity timing and duration differentially affect eastern oyster (Crassostrea virginica) size class growth and mortality in Breton Sound, LA. Estuar. Coast. Shelf Sci. 2013, 135, 146–157. [Google Scholar] [CrossRef]
- Munroe, D.; Tabatabai, A.; Burt, I.; Bushek, D.; Powell, E.N.; Wilkin, J. Oyster mortality in Delaware Bay: Impacts and recovery from Hurricane Irene and Tropical Storm Lee. Estuar. Coast. Shelf Sci. 2013, 135, 209–219. [Google Scholar] [CrossRef]
- Solís, D.; Perruso, L.; del Corral, J.; Stoffle, B.; Letson, D. Measuring the initial economic effects of hurricanes on commercial fish production: The US Gulf of Mexico grouper (Serranidae) fishery. Nat. Hazards 2013, 66, 271–289. [Google Scholar] [CrossRef]
- Spalding, M.D.; Ruffo, S.; Lacambra, C.; Meliane, I.; Hale, L.Z.; Shepard, C.C.; Beck, M.W. The role of ecosystems in coastal protection: Adapting to climate change and coastal hazards. Ocean Coast. Manag. 2014, 90, 50–57. [Google Scholar] [CrossRef]
- Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002, 10, 113–390. [Google Scholar] [CrossRef]
- Lewitus, A.J.; Horner, R.A.; Caron, D.A.; Garcia-Mendoza, E.; Hickey, B.M.; Hunter, M.; Huppert, D.D.; Kudela, R.M.; Langlois, G.W.; Largier, J.L. Harmful algal blooms along the North American west coast region: History, trends, causes, and impacts. Harmful Algae 2012, 19, 133–159. [Google Scholar] [CrossRef]
- Martinez-Cordova, L.R.; Martinez-Porchas, M. Polyculture of Pacific white shrimp, Litopenaeus vannamei, giant oyster, Crassostrea gigas and black clam, Chione fluctifraga in ponds in Sonora, Mexico. Aquaculture 2006, 258, 321–326. [Google Scholar] [CrossRef]
- Neori, A.; Krom, M.D.; Ellner, S.P.; Boyd, C.E.; Popper, D.; Rabinovitch, R.; Davison, P.J.; Dvir, O.; Zuber, D.; Ucko, M.; et al. Seaweed biofilters as regulators of water quality in integrated fish-seaweed culture units. Aquaculture 1996, 141, 183–199. [Google Scholar] [CrossRef]
- Schuenhoff, A.; Shpigel, M.; Lupatsch, I.; Ashkenazi, A.; Msuya, F.E.; Neori, A. A semi-recirculating, integrated system for the culture of fish and seaweed. Aquaculture 2003, 221, 167–181. [Google Scholar] [CrossRef]
- Abreu, M.H.; Pereira, R.; Yarish, C.; Buschmann, A.H.; Sousa-Pinto, I. IMTA with Gracilaria vermiculophylla: Productivity and nutrient removal performance of the seaweed in a land-based pilot scale system. Aquaculture 2011, 312, 77–87. [Google Scholar] [CrossRef]
- Samocha, T.M.; Fricker, J.; Ali, A.M.; Shpigel, M.; Neori, A. Growth and nutrient uptake of the macroalga Gracilaria tikvahiae cultured with the shrimp Litopenaeus vannamei in an Integrated Multi-Trophic Aquaculture (IMTA) system. Aquaculture 2015, 446, 263–271. [Google Scholar] [CrossRef]
- Granéli, E.; Pavia, H. Allelopathy in marine ecosystems. In Allelopathy: A Physiological Process with Ecological Implications; Reigosa, M.J., Pedrol, N., González, L., Eds.; Springer: New York, NY, USA, 2006; pp. 415–431. [Google Scholar]
- Tang, Y.Z.; Gobler, C.J. The green macroalga, Ulva lactuca, inhibits the growth of seven common harmful algal bloom species via allelopathy. Harmful Algae 2011, 10, 480–488. [Google Scholar] [CrossRef]
- Wang, Y.B.; Xu, Z.R.; Xia, M.S. The effectiveness of commercial probiotics in northern white shrimp Penaeus vannamei ponds. Fish. Sci. 2005, 71, 1036–1041. [Google Scholar] [CrossRef]
- Luo, W.; Deng, X.; Zeng, W.; Zheng, D. Treatment of wastewater from shrimp farms using a combination of fish, photosynthetic bacteria, and vegetation. Desalin. Water Treat. 2012, 47, 221–227. [Google Scholar] [CrossRef]
- Fisheries Agency. Fisheries Statistical Yearbook of Taiwan, Kinmen, and Matsu Areas; Fisheries Agency, Council of Agriculture: Taipei City, Taiwan, 2011.
- Hwang, D. Research on marine toxins in Taiwan. A festschrift in honor of Professor Anthony T. Tu. J. Toxicol. Toxin Rev. 2003, 22, 663–678. [Google Scholar] [CrossRef]
- Hung, J.J.; Hung, C.S.; Su, H.M. Biogeochemical responses to the removal of maricultural structures from an eutrophic lagoon (Tapong Bay) in Taiwan. Mar. Environ. Res. 2008, 65, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tew, K.S.; Meng, P.J.; Lee, H.J.; Ye, Y.X.; Kuo, J.; Fang, L.S.; Chou, W.R. Dynamics of phytoplankton and picoplankton over a tidal cycle in a subtropical lagoon. Chin. Sci. Bull. 2010, 55, 2522–2528. [Google Scholar] [CrossRef]
- Neori, A.; Shpigel, M. An Integrated System for Farming Fish, Seaweed and Abalone; CAB International Aquaculture Compendium: Wallingford, UK, 2006. [Google Scholar]
- Abreu, M.H.; Varela, D.A.; Henríquez, L.; Villarroel, A.; Yarish, C.; Sousa-Pinto, I.; Buschmann, A.H. Traditional vs. Integrated Multi-Trophic Aquaculture of Gracilaria chilensis C.J. Bird, J. McLachlan & E.C. Oliveira: Productivity and physiological performance. Aquaculture 2009, 293, 211–220. [Google Scholar]
- Beutler, M.; Wiltshire, K.H.; Meyer, B.; Moldaenke, C.; Lüning, C.; Meyerhöfer, M.; Hansen, U.P.; Dau, H. A fluorometric method for the differentiation of algal populations in vivo and in situ. Photosynth. Res. 2002, 72, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.K.; Chen, P.Y.; Dahms, H.U.; Yeh, S.L.; Chiu, Y.J. Comparing methods for measuring phytoplankton biomass in aquaculture ponds. Aquac. Environ. Interact. 2016, 8, 665–673. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using SPSS; SAGE Publications: Thousand Oaks, CA, USA, 2005; p. 816. [Google Scholar]
- Chang, T.J.; Wu, Y.T.; Hsu, H.Y.; Liao, C.M.; Chu, C.R. Assessment of wind characteristics and wind turbine characteristics in Taiwan. Renew. Energy 2003, 28, 851–871. [Google Scholar] [CrossRef]
- Lai, C.; Lin, T. Technical assessment of the use of a small-scale wind power system to meet the demand for electricity in a land aquafarm in Taiwan. Renew. Energy 2006, 31, 877–892. [Google Scholar] [CrossRef]
- Jones, A.B.; Dennison, W.C.; Preston, N.P. Integrated treatment of shrimp effluent by sedimentation, oyster filtration and macroalgal absorption: A laboratory scale study. Aquaculture 2001, 193, 155–178. [Google Scholar] [CrossRef]
- Jones, A.B.; Preston, N.P.; Dennison, W.C. The efficiency and condition of oysters and macroalgae used as biological filters of shrimp pond effluent. Aquac. Res. 2002, 33, 1–19. [Google Scholar] [CrossRef]
- Ramos, R.; Vinatea, L.; Seiffert, W.; Beltrame, E.; Silva, J.S.; da Costa, R.H.R. Treatment of shrimp effluent by sedimentation and oyster filtration using Crassostrea gigas and C. rhizophorae. Braz. Arch. Biol. Technol. 2009, 52, 775–783. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, Y.; Mao, Y.; Li, X.; Liu, Y.; Zhang, F. Growth characters and photosynthetic capacity of Gracilaria lemaneiformis as a biofilter in a shellfish farming area in Sanggou Bay, China. J. Appl. Phycol. 2005, 17, 199–206. [Google Scholar] [CrossRef]
- Yokoyama, H.; Ishihi, Y. Bioindicator and biofilter function of Ulva spp. (Chlorophyta) for dissolved inorganic nitrogen discharged from a coastal fish farm—Potential role in integrated multi-trophic aquaculture. Aquaculture 2010, 310, 74–83. [Google Scholar] [CrossRef]
- Wang, Y.; He, Z. Effect of probiotics on alkaline phosphatase activity and nutrient level in sediment of shrimp, Penaeus vannamei, ponds. Aquaculture 2009, 287, 94–97. [Google Scholar] [CrossRef]
- McIntosh, D.; Samocha, T.M.; Jones, E.R.; Lawrence, A.L.; McKee, D.A.; Horowitz, S.; Horowitz, A. The effect of a commercial bacterial supplement on the high-density culturing of Litopenaeus vannamei with a low-protein diet in an outdoor tank system and no water exchange. Aquac. Eng. 2000, 21, 215–227. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Li, W. Effect of probiotics on larvae shrimp (Penaeus vannamei) based on water quality, survival rate and digestive enzyme activities. Aquaculture 2009, 287, 349–353. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).