1. Introduction
Traditional practices of land preparation involve soil tillage through moldboard ploughing to soften the seedbed, ensure uniform germination, remove weed plants, and release soil nutrients through mineralization and oxidation. However, this mechanical disturbance is leading to a decline in organic matter, an increase of the loss of water by runoff, and, finally, to soil erosion [
1]. Together with increasing threats of climate change, the loss of soil and its fertility is expected to become more critical for global agricultural production [
2]. Over the next century, Sub-Saharan Africa (SSA) is expected to be particularly vulnerable due to the range of projected impacts: e.g., the multiple stresses and low adaptive capacity of current cropping systems, as well as population increase [
3]. Maize (
Zea mays L.) is the principal staple food crop in large parts of SSA and is usually grown in small-holder farming systems under rainfed conditions. The limited availability of inputs is a leading factor that contributes to low yields that in turn are not able to keep pace with the food demand [
4]. Hence, one of the most effective pathways for adaptation is to focus on breeding new varieties and also on changing crop management [
5,
6,
7,
8].
In light of soil degradation, conservation agriculture (CA) practices have been proposed as an alternative to tillage-based agriculture in SSA as a pragmatic solution to increasing production while conserving the natural resource base [
9]. CA is a set of core principles, including minimum soil disturbance, permanent soil cover, and diversified crop rotations supported by integrated soil, crop, and water management, which aims to reduce and/or revert many negative effects of conventional farming practices [
10]. Besides the control of soil erosion, CA has become increasingly popular as the crop management system conserves soil moisture, reduces fossil fuel use, lowers costs and, once established, increases yield permanently [
11]. Recent literature has shown the potential of CA to improve resilience against seasonal drought events and thereby reduce the risk of crop failure in SSA [
8,
12,
13,
14,
15,
16]. However, most crop cultivars currently grown under CA have been developed under conventional or full tillage conditions, and it is likely that relevant genetic adaptations of CA conditions may have been removed during previous breeding efforts. Furthermore, large scale phenotyping studies under zero-tillage conditions are missing.
For the selection of genotypes with high-performing yield components under CA, accurate phenotyping tools capable of estimating yield at early crop stages are needed. During early growth stages, environmental factors such as temperature or soil moisture have a vital influence on germination rate, seedling vigor, and, consequently, on yield [
17]. However, assessing those traits usually requires destructive laboratory measurements and/or visual scoring assessments that are laborious under field conditions. They are also prone to be subjective and incur additional associated costs.
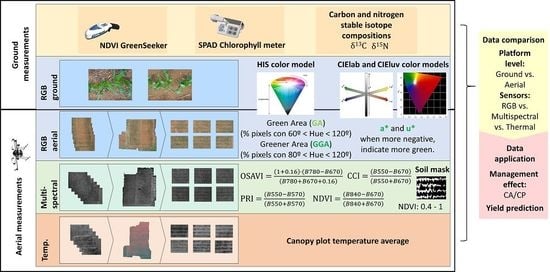
Specialized sensors have become an important component for crop monitoring, particularly for improving precision, efficiency, and throughput in phenotyping [
18]. Remote sensing indexes have largely demonstrated their various applications in agriculture, including yield prediction, stress detection, and control of plant diseases under a wide range of growing and environmental conditions [
19]. The classical approach has involved the use of multispectral data for the development of numerous vegetation indexes to measure biomass (e.g., Normalized Difference Vegetation Index, NDVI), water content (e.g., Water Band Index, WBI), or pigment composition (e.g., Modified Chlorophyll Absorption Ratio Index, MCARI) in yield studies. At present, the use of information derived from RGB images (using red, green, and blue color bands) acquired with conventional digital cameras represents a low-cost alternative. The images can be processed to convert RGB values into indexes based on the models of Hue-Intensity-Saturation (HIS), CIELab, and CIELuv cylindrical coordinate representations of colors [
20]. Moreover, recent technological advances have led the incorporation of these sensors into aerial based platforms, enabling the simultaneous characterization of specific crop physiological traits for a larger number of plots, which may help to minimize the effect of changing environmental conditions during critical sampling moments [
18,
21,
22,
23,
24].
Alternative applications of remote sensing techniques include the measurement of canopy temperature [
25]. This can provide high-value information of the crop water status, since transpiration is a principal factor reducing the leaf’s temperature. The use of thermal cameras has been proposed as an easy approach in crop management, and in breeding it can replace other more laborious techniques like the use of stable isotopes, which are costly, time-consuming, and require extensive laboratory work [
26]. Moreover, in case of C4 crops like maize, the usefulness of stable isotopes for breeding is questionable [
27]. The possibility of applying these methodologies in CA systems could be critical in improving our knowledge and supporting the full implementation of crop phenotyping for CA in developing countries.
The aim of the present study was to evaluate the efficiency of a set of remote sensing indexes in assessing the yield differences of different maize hybrids at early growth stages under conventionally ploughed (CP) and zero-tillage (CA) conditions. Different categories of sensors were tested, including RGB cameras (placed on an aerial platform as well as at ground level), alongside multispectral and thermal cameras (both installed on the aerial platform) and an active sensor portable field spectrometer designed to measure the NDVI at ground level. Additionally, canopy temperature, leaf chlorophyll content, and dry matter isotopic composition were evaluated.
2. Materials and Methods
2.1. Site Description
The experiment was conducted at Domboshawa Training Centre (17°37’S, 31°10’E and 1560 m.a.s.l.), situated at the north-east of Harare (Zimbabwe), during the 2015/2016 crop season (
Figure 1). This site is characterized by moderately deep Arenosols and Luvisols under FAO classification [
28]. It has approximately 5% clay content and is derived from granite parent material [
29]. The climate conditions correspond to the Zimbabwean agro-ecological region II [
30], with generally long dry periods (April to October), in which April to July is cool and August to October is warm. This region receives an average rainfall of between 700 and 1000 mm and mean maximum daily temperatures of 32 °C during summer.
2.2. Plant Materials and Experimental Design
Seven maize drought tolerant commercial hybrids (SC621, Pan53, 30G19, Zap55, Pristine601, PGS61, and Zap61) and one drought-sensitive commercial control variety (SC513) were manually planted on 14 December 2015 in plots of 23 m
2 (5 × 4.6 m) with four lines per plot. Two differential plot management regiments have been applied to the field since 2009 (
Figure 2). One half was managed using no-tillage and the application of 2.5–3.0 Mg ha
−1 of maize stover to all the plots. Rotation, a critical component for CA, was not practiced in this trial. Weed control was done by applying a combination of 2.5 L ha
−1 of glyphosate, 3 L ha
−1 of atrazine, and 1 L ha
−1 of dual immediately after planting, if there were weeds present. The other half was conventionally ploughed and without any residue management.
Both cropping systems were fertilized twice equally with 200 kg ha−1 using Compound D (7:14:7) as a basal NPK dressing and top dressed with ammonium nitrate (200 kg ha−1, 46% N) in a split application four weeks and seven weeks after seeding. Maize plants seeded at different density sub-treatments were applied: one low-planting density (44,444 plants ha−1) and one high-planting density (53,333 plants ha−1). Each sub-treatment was repeated in three replicates in which varieties were ordered in a completely randomized block design. A total of 96 plots were studied (2 agricultural practices × 2 density conditions × 8 varieties × 3 replicates, 24 plots per growing conditions).
2.3. Agronomical Traits and Proximal (Ground) Data Collection
The crop was harvested at physiological maturity. Grain yield (Mg ha−1) and total above-ground biomass (Mg ha−1) were determined as corresponding to the central 3.6 m of the two central rows of each plot (6.48 m2), omitting the border plants. The harvest index was calculated as grain yield as a portion of total biomass.
Proximal (ground) data was collected 45 days after sowing on 28 January 2016 when the hybrids reached the stage of 4 to 6 leaves. Leaf chlorophyll content (LCC) was measured using a Minolta SPAD-502 portable chlorophyll meter (Spectrum Technologies Inc., Plainfield, IL, USA). For each plot, five leaves were selected randomly (being the last fully expanded leave within a plant) and were measured in the middle portion of the lamina and averaged. The Normalized Difference Vegetation Index was determined at ground level using a portable spectrometer (GreenSeeker handheld crop sensor, Trimble, Sunnyvale, CA, USA) by passing the sensor over the middle of each plot at a constant height of 0.5 m above and perpendicular to the canopy (NDVI.g).
One RGB picture was taken per plot, holding the camera at 80 cm above the plant canopy in a zenithal plane and focusing near the center of each plot. The conventional digital camera used was an Olympus OM-D (Olympus, Tokyo, Japan), a digital single lens mirrorless camera with an image sensor size of 17.3 × 13.0 mm of 16-megapixel (MP) resolution. The images were saved in JPEG format with a resolution of 4608 × 3072 pixels. As the plots were too big for a single photograph, three different images samples were taken of each central row.
RGB images were subsequently analyzed using a version of the Breedpix 2.0 software (Jaume Casadesús,
https://bio-protocol.org/e1488, IRTA, Lleida, Spain) adapted to JAVA8 and other RGB image analyses together integrated as freely available CIMMYT MaizeScanner plugin within FIJI (
https://imagej.net/Fiji and
https://github.com/George-haddad/CIMMYT). This software enables the extraction of RGB vegetation indexes in relation to different color properties [
20]. Essentially, the indexes are based on either the average color of the entire image, in diverse units related to its “greenness”, or on the fraction of pixels classified as green canopy relative to the total number of pixels in the image. In HIS color space, the Hue (H) component describes the color value itself by traversing the visible spectrum in the form of an angle between 0° and 360°. Derived from the Hue portion of HIS color space, Green Area (GA) and Greener Area (GGA) analyze the proportion of green/yellow and green pixels in the image. GA is the percentage of pixels in the image in the hue range from 60° to 180°, that is, from yellow to bluish green. Meanwhile, GGA is somewhat more restrictive, because the range of hue considered by the index is from 80° to 180°, thus excluding the yellowish-green tones. Additionally, those two indexes are used to formulate the Crop Senescence Index (CSI), which provides a scaled ratio between yellow and green vegetation pixels [
31], as follows:
In the CIELab color space model, dimension L* represents lightness, and the green to red range is expressed by the a* component, with a more positive value representing a purer red, and conversely a more negative value indicating a greener color. Meanwhile, blue to yellow is expressed by the b* component, in which the more positive the value the closer it is to a pure yellow, whereas the more negative the value the closer it is to blue. Similarly, in the CIELuv color space model, dimensions u* and v* are perceptually uniform coordinates, in which the visible spectrum starts with blue at the bottom of the space, moving through green in the upper left (mostly scaled by v*) and out to red in the upper right (mostly scaled by u*) [
32].
2.4. Aerial Data Collection
Furthermore, aerial measurements were acquired during the same visit as the ground data using an unmanned aerial vehicle (UAV) (Mikrokopter OktoXL 6S12, Moormerland, Germany) flying under manual remote control at an altitude of 30 m (
Figure 3). Two flights were performed; on one flight only, the RGB digital camera was mounted, and the other included both the multispectral and thermal cameras.
The RGB aerial images were obtained using a Lumix GX7 (Panasonic, Osaka, Japan) digital mirrorless camera with an image sensor size of 17.3 × 13.0 mm using a 20 mm lens. Images were taken at 16-MP and were saved in JPEG format, on this occasion with a resolution of 4592 × 3448 pixels. For the multispectral data, a Tetracam micro-MCA (Tetracam Inc., Chatsworth, CA, USA) was used. The camera consists of twelve independent image sensors and optics each with user configurable filters of center wavelengths and full-width half-max band-with (450 ± 40, 550 ± 10, 570 ± 10, 670 ± 10, 700 ± 10, 720 ± 10, 780 ± 10, 780 ± 10, 840 ± 10, 860 ± 10, 900 ± 20, 950 ± 40 nm), and one sensor dedicated to calibration (Light Incident Sensor, ILS). That sensor uses micro-filters housed in the ILS module behind a diffusor plate that corresponds to the same spectral characteristic of the 11 downwards looking sensors, thus providing an accurate band-by-band reflectance calibration in real-time. It captures 15.6-MP of image data as 12 × 1.3-MP images transferred to twelve separate flash memory cards. The multispectral images acquired were aligned and calibrated to reflectance using PixelWrench II version 1.2.2.2 (Tetracam, Chatsworth, CA, USA). Canopy temperature was measured using a FLIR Tau2 640 (FLIR Systems, Nashua, NH, USA) with a VOx uncooled microbolometer equipped with a TEAX Thermal Capture model (TEAX Tecnology, Wilnsdorf, Germany) for recording of full resolution thermal video (640 × 520 pixels at 20 frames per second). The thermal images were first exported using the TeAx ThermoViewer v1.3.12 (TeAx Technology, Wilnsdorf, Germany) to raw 16-bit TIFF format as Kelvin × 10,000 and converted to 32-bit temperature in Celsius using a custom batch processing macro function in FIJI [
33].
To obtain an accurate orthomosaic of the pre-processed aerial images from each sensor, a 3D reconstruction was produced using Agisoft PhotoScan Professional (Agisoft LLC, St. Petersburg, Russia,
www.agisoft.com) [
34]. A total of 30 overlapped images were needed for each orthomosaic. Then, the procedure of cropping the plots was done using the open source image analysis platform FIJI (Fiji is Just ImageJ;
http://fiji.sc/Fiji), in which regions of interest were established at each plot and then exported, taking care that exactly the same ground area was segmented for each plot across all treatments. The RGB aerial exported plots were processed the same way as the ground images as described previously in
Section 2.3. For the formulation of the different multispectral indexes, as detailed in
Table 1, we developed a customized FIJI macro code. This macro code enabled the calculation of the multispectral indexes through two different approaches: on the one hand, measuring the mean value of the plot image of each band (plot measurements), and on the other hand, in order to make more accurate measurements, we applied a threshold of NDVI values of 0.4–1 to focus the measurements of all the indexes on only the pixels corresponding to maize and lower NDVI values (<0.4) corresponding to bare-ground; other background image components were discarded. Therewith, percentage of vegetation cover was estimated by the inverse of the implementation of the soil mask. Finally, the thermal average temperature of the whole exported plots was also measured using FIJI.
2.5. Carbon and Nitrogen Stable Isotope Compositions
Similar leaves were sampled for LCC, carbon, and nitrogen measurements, and were subsequently oven dried at 70 °C for 24 h and ground to a fine powder. Samples of approximately 0.7 mg of dry matter and reference materials were weighed into tin capsules, sealed, and then loaded into an elemental analyzer (Flash 1112 EA; ThermoFinnigan, Schwerte, Germany) coupled with an isotope ratio mass spectrometer (Delta C IRMS, ThermoFinnigan), operating in continuous flow mode. Measurements were carried out at the Scientific Facilities of the University of Barcelona. The
13C/
12C ratios (R) of plant material were expressed in composition (δ
13C) notation [
47] as follows:
in which sample refers to plant material and standard to Pee Dee Belemmite (PDB) calcium carbonate. International isotope secondary standards of a known
13C/
12C ratio (IAEA CH7, polyethylene foil, IAEA CH6 sucrose and USGS 40 l-glutamic acid) were calibrated against Vienna Pee Dee Belemnite calcium carbonate (VPDB) with an analytical precision of 0.1‰. The
15N/
14N ratios of plant material were also expressed in δ notation (δ
15N) using international secondary standards of known
15N/
14N ratios (IAEA N1 and IAEA N2 ammonium sulfate and IAEA NO
3 potassium nitrate), with analytical precision of about 0.2‰. Furthermore, total carbon and nitrogen (%) were analyzed in the same samples, and the C/N ratio was calculated.
2.6. Statistical Analysis
Statistical analyses were conducted using the open source software, R and RStudio 1.0.44 (R Foundation for Statistical Computing, Vienna, Austria). Data for the set of physiological traits were subjected to factorial completely randomized analyses of variance (ANOVAs) to test the effects of growing conditions on the different traits studied. A bivariate correlation procedure was used to calculate the Pearson correlation coefficients of the different remote sensing indexes against the grain yield. Multiple linear regressions were calculated via a forward stepwise method with GY as the dependent variable and the different indexes as independent parameters. The figures were also drawn using the RStudio software.
5. Conclusions
Conservation agriculture management practices had a positive effect on increasing yields as compared to conventional ploughed system. These results may help support the adoption of CA to combat declining yields that affect SSA agriculture. Henceforth, in order to fully exploit the yield potential, future efforts should focus on the study of the impact of the genotype selection for a particular management system (e.g., Genotype x Environment x Management interaction). The main point of field phenotyping is to understand the genotypic responses and dissect that traits associated with a better performance under CA as a management system. Thus, further work is required before breeding programs invest resources into a whole new management system.
The use of remote sensing technologies, as presented here, would be increasingly useful for large-scale phenotyping studies. The results suggest, even at early crop growth stages, that the different RGB and multispectral indexes have the potential to effectively assess yield differences under CA conditions, even if their performance is lower than under CP conditions. This is assumed to be mainly due to the residue cover effect on the measurements; however, applying a soil (and stover) mask to the images could help in overcoming this technical problem, which may be best accomplished by the fusion of high resolution RGB with multispectral and/or thermal data or by employing advanced image segmentation algorithms not explored in this study. Nevertheless, the performance of the RGB indexes in predicting yield was less affected by tillage conditions than the multispectral indexes. The indexes that best correlated with yield were mostly related to the greenness of the canopy vegetation, such as the RGB indexes GA and a*, and the multispectral indexes NDVI and RDVI. Finally, the platform proximity effect on the image resolution did not have a negative impact on the performance of the indexes, reinforcing the usefulness of UAV and its associated image processing for high throughput plant phenotyping studies under field conditions.