Use of Fluorescence Sensing to Detect Nitrogen and Potassium Variability in Maize
Abstract
1. Introduction
2. Materials and Methods
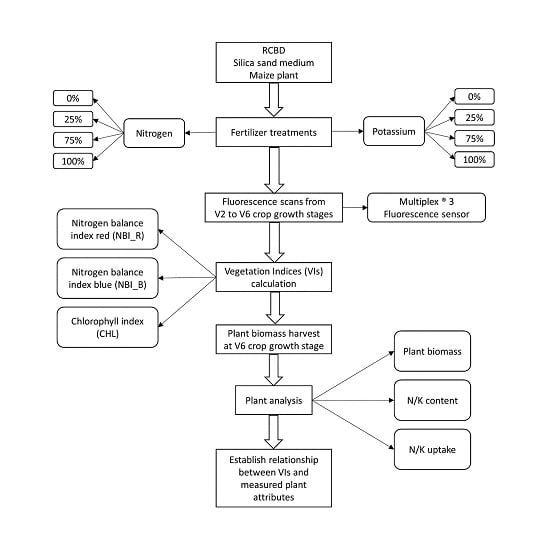
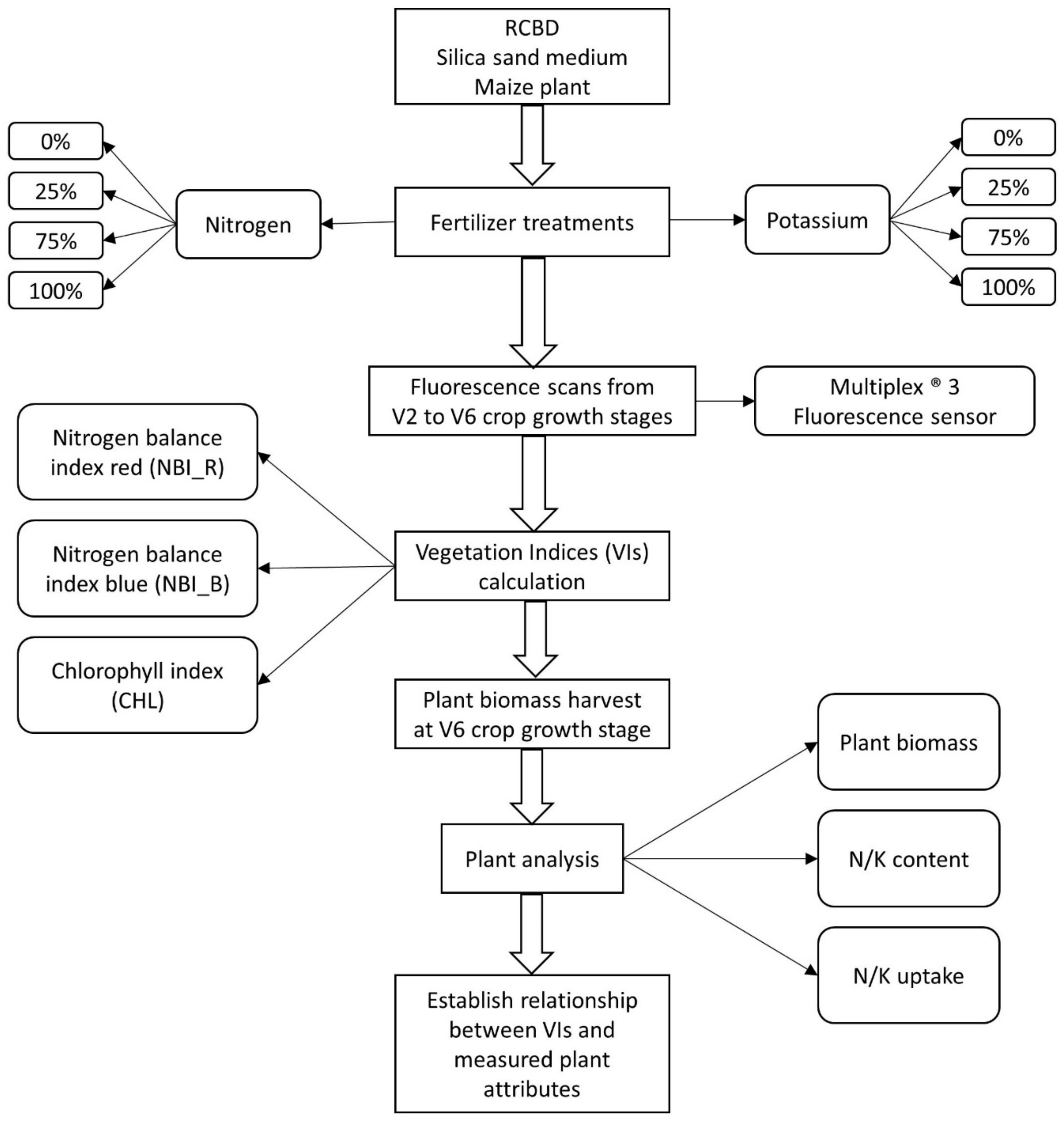
2.1. Study Site and Crop Management Scenario
2.2. Experimental Setup
2.3. Fluorescence Sensor and Indices
2.4. Fluorescence Data Acquisition
2.5. Biomass Sampling and Tissue Analysis
2.6. Statistical Analysis
3. Results
3.1. Biomass and Nitrogen Variability across Treatments in the Nitrogen Experiment
3.2. Biomass and Potassium Variability across Treatments in the Potassium Experiment
3.3. Characterizing Nitrogen Uptake using Fluoro-Sensing
3.4. Characterizing Potassium Uptake using Fluoro-Sensing
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Koch, B.; Khosla, R.; Frasier, W.M.; Westfall, D.G.; Inman, D. Economic feasibility of variable-rate nitrogen application utilizing site-specific management zones. Agron. J. 2004, 96, 1572–1580. [Google Scholar] [CrossRef]
- Moran, M.S.; Inoue, Y.; Barnes, E.M. Opportunities and limitations for image-based remote sensing in precision crop management. Remote Sens. Environ. 1997, 61, 319–346. [Google Scholar] [CrossRef]
- Cordero, E.; Longchamps, L.; Khosla, R.; Sacco, D. Spatial management strategies for nitrogen in maize production based on soil and crop data. Sci. Total Environ. 2019, 697. [Google Scholar] [CrossRef] [PubMed]
- Lowenberg-DeBoer, J. The management time economics of on-the-go sensing for nitrogen application. SSMC Newsl. 2004, 5, 1–5. [Google Scholar]
- Yao, Y.; Miao, Y.; Cao, Q.; Wang, H.; Gnyp, M.L.; Bareth, G.; Khosla, R.; Yang, W.; Liu, F.; Liu, C. In-season estimation of rice nitrogen status with an active crop canopy sensor. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 4403–4413. [Google Scholar]
- Bender, R.R.; Haegele, J.W.; Ruffo, M.L.; Below, F.E. Modern corn hybrids’ nutrient uptake patterns. Better Crop. 2013, 97, 7–10. [Google Scholar]
- Fageria, N.K.; Baligar, V.C. Enhancing nitrogen use efficiency in crop plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar] [CrossRef]
- Khosla, R.; Fleming, K.; Delgado, J.A.; Shaver, T.M.; Westfall, D.G. Use of site-specific management zones to improve nitrogen management for precision agriculture. J. Soil Water Conserv. 2002, 57, 513–518. [Google Scholar]
- Khosla, R.; Shaver, T. Zoning in on nitrogen needs. Colo. State Univ. Agron. Newsl. 2001, 21, 24–26. [Google Scholar]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Huang, J.; Xu, C.-C.; Ridoutt, B.G.; Wang, X.-C.; Ren, P.-A. Nitrogen and phosphorus losses and eutrophication potential associated with fertilizer application to cropland in China. J. Clean. Prod. 2017, 159, 171–179. [Google Scholar]
- Sun, M.; Huo, Z.; Zheng, Y.; Dai, X.; Feng, S.; Mao, X. Quantifying long-term responses of crop yield and nitrate leaching in an intensive farmland using agro-eco-environmental model. Sci. Total Environ. 2018, 613, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Baligar, V.; Fageria, N.; He, Z. Nutrient use efficiency in plants. Commun. Soil Sci. Plan. 2001, 32, 921–950. [Google Scholar] [CrossRef]
- Carlson, K.M.; Gerber, J.S.; Mueller, N.D.; Herrero, M.; MacDonald, G.K.; Brauman, K.A.; Havlik, P.; O’Connell, C.S.; Johnson, J.A.; Saatchi, S. Greenhouse gas emissions intensity of global croplands. Nat. Clim. Chang. 2017, 7, 63–68. [Google Scholar]
- Cassman, K.G.; Dobermann, A.; Walters, D.T. Agroecosystems, nitrogen-use efficiency, and nitrogen management. AMBIO 2002, 31, 132–140. [Google Scholar] [CrossRef]
- Gupta, M.; Khosla, R. Precision nitrogen management and global nitrogen use efficiency. In Proceedings of the 11th International Conference on Precision Agriculture, Indianapolis, IN, USA, 15–18 July 2015. [Google Scholar]
- Hawkesford, M.J.; Griffiths, S. Exploiting genetic variation in nitrogen use efficiency for cereal crop improvement. Curr. Opin. Plant Biol. 2019, 49, 35–42. [Google Scholar] [CrossRef]
- Fixen, P.E.; Williams, R.; Rund, Q.B. NUGIS: A Nutrient Use Geographic Information System for the US; International Plant Nutrition Institute: Brookings, SD, USA, 2012. [Google Scholar]
- Agati, G.; Foschi, L.; Grossi, N.; Guglielminetti, L.; Cerovic, Z.G.; Volterrani, M. Fluorescence-based versus reflectance proximal sensing of nitrogen content in Paspalum vaginatum and Zoysia matrella turfgrasses. Eur. J. Agron. 2013, 45, 39–51. [Google Scholar] [CrossRef]
- Bilger, W.; Veit, M.; Schreiber, L.; Schreiber, U. Measurement of leaf epidermal transmittance of UV radiation by chlorophyll fluorescence. Physiol. Plant. 1997, 101, 754–763. [Google Scholar] [CrossRef]
- Blackmer, T.M.; Schepers, J.S.; Varvel, G.E.; Walter-Shea, E.A. Nitrogen deficiency detection using reflected shortwave radiation from irrigated corn canopies. Agron. J. 1996, 88, 1–5. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Cerovic, Z.G. Sensing crop nitrogen status with fluorescence indicators. A review. Agron. Sustain. Dev. 2012, 32, 451–464. [Google Scholar]
- Herrmann, I.; Bdolach, E.; Montekyo, Y.; Rachmilevitch, S.; Townsend, P.A.; Karnieli, A. Assessment of maize yield and phenology by drone-mounted superspectral camera. Precis. Agric. 2020, 21, 51–76. [Google Scholar] [CrossRef]
- Ma, B.; Morrison, M.J.; Dwyer, L.M. Canopy light reflectance and field greenness to assess nitrogen fertilization and yield of maize. Agron. J. 1996, 88, 915–920. [Google Scholar] [CrossRef]
- Naser, M.A.; Khosla, R.; Longchamps, L.; Dahal, S. Using NDVI to Differentiate Wheat Genotypes Productivity Under Dryland and Irrigated Conditions. Remote Sens. 2020, 12, 824. [Google Scholar] [CrossRef]
- Solari, F.; Shanahan, J.; Ferguson, R.; Schepers, J.; Gitelson, A. Active sensor reflectance measurements of corn nitrogen status and yield potential. Agron. J. 2008, 100, 571–579. [Google Scholar] [CrossRef]
- Rouse, J.; Haas, R.; Schell, J.; Deering, D. Monitoring vegetation systems in the Great Plains with ERTS. NASA Spec. Publ. 1974, 351, 309. [Google Scholar]
- Haboudane, D.; Miller, J.; Tremblay, N.; Zarco-Tejada, P.; Dextraze, L.; Viau, A. Heterogeneity of CASI-estimated leaf chlorophyll in corn: Assessment and comparison with ground truth from L’Acadie GEOIDE experimental site. In Proceedings of the 23rd Canadian Symposium on Remote Sensing and 10th Congress of the Quebec Remote Sensing Association, Université Laval University Sainte-Foy, Quebec, QC, Canada, 21–24 August 2001. [Google Scholar]
- Martin-Neto, L.; Milori, D.; Da Silva, W.; Simões, M. EPR, FT-IR, Raman, UV–visible absorption, and fluorescence spectroscopies in studies of NOM. In Biophysico-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems Biophys; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 651–727. [Google Scholar]
- Shaver, T.; Khosla, R.; Westfall, D. Evaluation of two ground-based active crop canopy sensors in maize: Growth stage, row spacing, and sensor movement speed. Soil Sci. Soc. Am. J. 2010, 74, 2101–2108. [Google Scholar] [CrossRef]
- Shaver, T.; Khosla, R.; Westfall, D. Evaluation of two crop canopy sensors for nitrogen variability determination in irrigated maize. Precis. Agric. 2011, 12, 892–904. [Google Scholar] [CrossRef]
- Shaver, T.; Khosla, R.; Westfall, D. Evaluation of two crop canopy sensors for nitrogen recommendations in irrigated maize. J. Plant Nutr. 2014, 37, 406–419. [Google Scholar] [CrossRef]
- Longchamps, L.; Khosla, R. Early detection of nitrogen variability in maize using fluorescence. Agron. J. 2014, 106, 511–518. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Barnes, E.M.; Sudduth, K.A.; Hummel, J.W.; Lesch, S.M.; Corwin, D.L.; Yang, C.; Daughtry, C.S.; Bausch, W.C. Remote-and ground-based sensor techniques to map soil properties. Photogramm. Eng. Remote Sens. 2003, 69, 619–630. [Google Scholar] [CrossRef]
- Nørbæk, R.; Aaboer, D.; Bleeg, I.; Christensen, B.T.; Kondo, T.; Brandt, K. Flavone C-glycoside, phenolic acid, and nitrogen contents in leaves of barley subject to organic fertilization treatments. J. Agric. Food Chem. 2003, 51, 809–813. [Google Scholar] [PubMed]
- Agati, G.; Cerovic, Z.G.; Pinelli, P.; Tattini, M. Light-induced accumulation of ortho-dihydroxylated flavonoids as non-destructively monitored by chlorophyll fluorescence excitation techniques. Environ. Exp. Bot. 2011, 73, 3–9. [Google Scholar] [CrossRef]
- Agati, G.; Meyer, S.; Matteini, P.; Cerovic, Z.G. Assessment of anthocyanins in grape (Vitis vinifera L.) berries using a noninvasive chlorophyll fluorescence method. J. Agric. Food Chem. 2007, 55, 1053–1061. [Google Scholar] [CrossRef]
- Cerovic, Z.; Ounis, A.; Cartelat, A.; Latouche, G.; Goulas, Y.; Meyer, S.; Moya, I. The use of chlorophyll fluorescence excitation spectra for the non-destructive in situ assessment of UV-absorbing compounds in leaves. Plant Cell Environ. 2002, 25, 1663–1676. [Google Scholar] [CrossRef]
- Cartelat, A.; Cerovic, Z.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.-L.; Barbottin, A.; Jeuffroy, M.-H.; Gate, P.; Agati, G. Optically assessed contents of leaf polyphenolics and chlorophyll as indicators of nitrogen deficiency in wheat (Triticum aestivum L.). Field Crop. Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Holland, J.E.; Cammarano, D.; Fitzgerald, G.J.; Perry, E.M.; Poile, G.; Conyers, M.K. Proximal fluorescence sensing of potassium responsive crops to develop improved predictions of biomass, yield and grain quality of wheat and barley. Precis. Agric. 2019, 20, 379–397. [Google Scholar] [CrossRef]
- Muñoz-Huerta, R.F.; Guevara-Gonzalez, R.G.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Prado-Olivarez, J.; Ocampo-Velazquez, R.V. A review of methods for sensing the nitrogen status in plants: Advantages, disadvantages and recent advances. Sensors 2013, 13, 10823–10843. [Google Scholar] [CrossRef]
- Srinivasarao, C.; Shanker, A.K.; Kundu, S.; Reddy, S. Chlorophyll fluorescence induction kinetics and yield responses in rainfed crops with variable potassium nutrition in K deficient semi-arid alfisols. J. Photochem. Photobiol. B Biol. 2016, 160, 86–95. [Google Scholar] [CrossRef]
- Zhao, X.; Du, Q.; Zhao, Y.; Wang, H.; Li, Y.; Wang, X.; Yu, H. Effects of different potassium stress on leaf photosynthesis and chlorophyll fluorescence in maize (Zea mays L.) at seedling stage. Agric. Sci. 2016, 7, 44–53. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Cerovic, Z.G.; Goutouly, J.-P.; Hilbert, G.; Destrac-Irvine, A.; Martinon, V.; Moise, N. Mapping winegrape quality attributes using portable fluorescence-based sensors. Frutic 2009, 9, 301–310. [Google Scholar]
- Helvich, K. Official Methods of Analysis; Association of Official Analytical Chemists: Rockville, MD, USA, 1990. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; Team, R.C.: Austrial, Vienna, 2013. [Google Scholar]
- Xiong, H.; Xiong, Y.; Zhang, G.; Peng, Z.; He, S.; Xu, D.; Liu, W. Effects of nitrogen, phosphorus and potassium on yield of sweet corn. In Proceedings of the International Conference on Material Science, Energy and Environmental Engineering (MSEEE 2017), Suzhou, China, 22–24 March 2017. [Google Scholar]
- Sayre, J. Mineral accumulation in corn. Plant Physiol. 1948, 23, 267. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Ma, B.; Luan, L.; Li, C. Nitrogen, phosphorus, and potassium nutrient effects on grain filling and yield of high-yielding summer corn. J. Plant Nutr. 2011, 34, 1516–1531. [Google Scholar] [CrossRef]
- Kolb, C.A.; Käser, M.A.; Kopecký, J.; Zotz, G.; Riederer, M.; Pfündel, E.E. Effects of natural intensities of visible and ultraviolet radiation on epidermal ultraviolet screening and photosynthesis in grape leaves. Plant Physiol. 2001, 127, 863–875. [Google Scholar] [CrossRef]



| Ingredients | Stock | Hoagland | Nitrogen | Potassium | ||||
|---|---|---|---|---|---|---|---|---|
| Solution | 100% | 50% | 25% | 0% | 50% | 25% | 0% | |
| (g/L water) | ------------------------mL/L------------------------ | |||||||
| KH2PO4 (pH to 6.0 with 3 M KOH) | 136.09 | 1 | 1 | 1 | 1 | - | - | - |
| KNO3 | 101.11 | 5 | 2.5 | 1.25 | - | - | - | - |
| Ca(NO3)2 × 4H2O | 236.16 | 5 | 2.5 | 1.25 | - | 5 | 5 | 5 |
| MgSO4 × 7H2O | 247.47 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| KCl | 74.56 | - | 5 | 5 | 5 | 2.5 | 1.25 | 0 |
| CaCl2 × 2H2O | 147.02 | - | 5 | 5 | 5 | - | - | - |
| NH4H2PO4 | 115.31 | - | - | - | - | 1 | 1 | 1 |
| NH4NO3 | 80.04 | - | - | - | - | 2 | 2 | 2 |
| NaH2PO4 | 119.98 | - | - | - | - | 1 | 1 | 1 |
| Minors: | * | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Fe-EDTA: | ** | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY-NC-ND) license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Share and Cite
Siqueira, R.; Longchamps, L.; Dahal, S.; Khosla, R. Use of Fluorescence Sensing to Detect Nitrogen and Potassium Variability in Maize. Remote Sens. 2020, 12, 1752. https://doi.org/10.3390/rs12111752
Siqueira R, Longchamps L, Dahal S, Khosla R. Use of Fluorescence Sensing to Detect Nitrogen and Potassium Variability in Maize. Remote Sensing. 2020; 12(11):1752. https://doi.org/10.3390/rs12111752
Chicago/Turabian StyleSiqueira, Rafael, Louis Longchamps, Subash Dahal, and Raj Khosla. 2020. "Use of Fluorescence Sensing to Detect Nitrogen and Potassium Variability in Maize" Remote Sensing 12, no. 11: 1752. https://doi.org/10.3390/rs12111752
APA StyleSiqueira, R., Longchamps, L., Dahal, S., & Khosla, R. (2020). Use of Fluorescence Sensing to Detect Nitrogen and Potassium Variability in Maize. Remote Sensing, 12(11), 1752. https://doi.org/10.3390/rs12111752