Hygroscopicity of Different Types of Aerosol Particles: Case Studies Using Multi-Instrument Data in Megacity Beijing, China
Abstract
:1. Introduction
2. Instruments and Methodology
2.1. Experiment Site
2.2. Instruments
2.2.1. Lidar Systems
2.2.2. Aerosol Chemical Speciation Monitor
2.2.3. Particle Sizers
2.2.4. Radiosondes and PM Measurement Instruments
2.3. Methodology
2.3.1. Retrieval of Aerosol Optical Depth, Water Vapor, and RH Profiles
2.3.2. The selection of Hygroscopic Growth Cases
2.3.3. Aerosol Chemical Ion-Pairing Scheme
2.3.4. POLIPHON Method
3. Results and Discussion
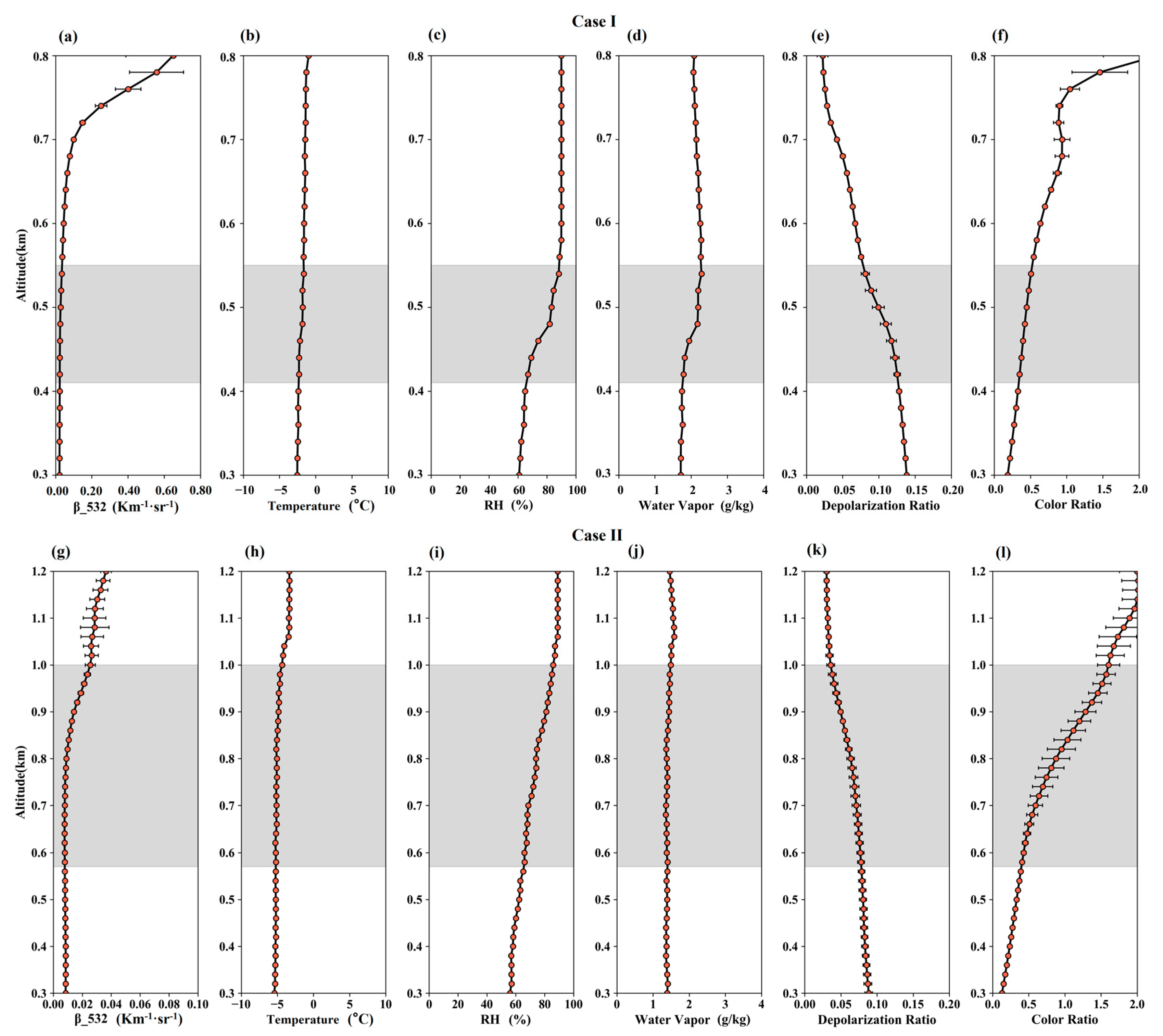
3.1. Selection of Dust and Non-Dust Cases and Their General Properties
3.2. Case Studies
3.2.1. Lidar-Estimated Hygroscopicity
3.2.2. Isolating Non-Dust Fine-Mode Aerosol Hygroscopic Properties
3.3. The Influence of Chemical Composition Inferred from ACSM Measurements
4. Conclusions
Data Availability
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M.M.B., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Kaufman, Y.J.; Tanré, D.; Boucher, O. A satellite view of aerosols in the climate system. Nature 2002, 419, 215–223. [Google Scholar] [CrossRef]
- Li, Z.; Lau, W.K.-M.; Ramanathan, V.; Wu, G.; Ding, Y.; Manoj, M.G.; Liu, J.; Qian, Y.; Li, J.; Zhou, T.; et al. Aerosol and monsoon climate interactions over Asia. Rev. Geophys. 2016, 54, 866–929. [Google Scholar] [CrossRef]
- Petters, M.D.; Kreidenweis, S.M. A single parameter representation of hygroscopic growth and cloud condensation nucleus activity. Atmos. Chem. Phys. 2007, 7, 1961–1971. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Wang, Y.; Li, Z.; Zhang, F.; Xu, W.; Sun, Y.; Fan, X.; Chen, G.; Wu, H.; Ren, J.; et al. Significant contribution of organics to aerosol liquid water content in winter in Beijing, China. Atmos. Chem. Phys. Discuss. 2020, 20, 901–914. [Google Scholar] [CrossRef] [Green Version]
- Zieger, P.; Weingartner, E.; Henzing, J.; Moerman, M.; de Leeuw, G.; Mikkilä, J.; Ehn, M.; Petäjä, T.; Clémer, K.; van Roozendael, M.; et al. Comparison of ambient aerosol extinction coefficients obtained from in-situ, MAX-DOAS and LIDAR measurements at Cabauw. Atmos. Chem. Phys. 2011, 11, 2603–2624. [Google Scholar] [CrossRef] [Green Version]
- Kuang, Y.; Zhao, C.S.; Ma, N.; Liu, H.J.; Bian, Y.X.; Tao, J.C.; Hu, M. Deliquescent phenomena of ambient aerosols on the North China Plain: Deliquescence of Ambient Aerosols. Geophys. Res. Lett. 2016, 43, 8744–8750. [Google Scholar] [CrossRef]
- Meier, J.; Wehner, B.; Massling, A.; Birmili, W.; Nowak, A.; Gnauk, T.; Wiedensohler, A. Hygroscopic growth of urban aerosol particles in Beijing (China) during wintertime: a comparison of three experimental methods. Atmos. Chem. Phys. 2009, 16. [Google Scholar]
- Wang, Y.; Zhang, F.; Li, Z.; Tan, H.; Xu, H.; Ren, J.; Zhao, J.; Du, W.; Sun, Y. Enhanced hydrophobicity and volatility of submicron aerosols under severe emission control conditions in Beijing. Atmos. Chem. Phys. 2017, 17, 5239–5251. [Google Scholar] [CrossRef] [Green Version]
- Gysel, M.; Crosier, J.; Topping, D.O.; Whitehead, J.D.; Bower, K.N.; Cubison, M.J.; Williams, P.I.; Flynn, M.J.; McFiggans, G.B.; Coe, H. Closure study between chemical composition and hygroscopic growth of aerosol particles during TORCH2. Atmos. Chem. Phys. 2007, 7, 6131–6144. [Google Scholar] [CrossRef] [Green Version]
- Svenningsson, B.; Rissler, J.; Swietlicki, E.; Mircea, M.; Bilde, M.; Facchini, M.C.; Decesari, S.; Fuzzi, S.; Zhou, J.; Mønster, J.; et al. Hygroscopic growth and critical supersaturations for mixed aerosol particles of inorganic and organic compounds of atmospheric relevance. Atmos. Chem. Phys. 2006, 16. [Google Scholar] [CrossRef] [Green Version]
- Chang, R.Y.-W.; Slowik, J.G.; Shantz, N.C.; Vlasenko, A.; Liggio, J.; Sjostedt, S.J.; Leaitch, W.R.; Abbatt, J.P.D. The hygroscopicity parameter (κ) of ambient organic aerosol at a field site subject to biogenic and anthropogenic influences: Relationship to degree of aerosol oxidation. Atmos. Chem. Phys. 2010, 10, 5047–5064. [Google Scholar] [CrossRef] [Green Version]
- Petters, M.D.; Kreidenweis, S.M. A single parameter representation of hygroscopic growth and cloud condensation nucleus activity—Part 3: Including surfactant partitioning. Atmos. Chem. Phys. Discuss. 2012, 12, 22687–22712. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhao, C.; Tao, J.; Bian, Y.; Ma, N.; Zhao, G. A novel method for deriving the aerosol hygroscopicity parameter based only on measurements from a humidified nephelometer system. Atmos. Chem. Phys. 2017, 17, 6651–6662. [Google Scholar] [CrossRef] [Green Version]
- Sheridan, P.J.; Delene, D.J.; Ogren, J.A. Four years of continuous surface aerosol measurements from the Department of Energy’s Atmospheric Radiation Measurement Program Southern Great Plains Cloud and Radiation Testbed site. J. Geophys. Res. Atmos. 2001, 106, 20735–20747. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Yoon, S.-C.; Jefferson, A.; Kim, S.-W. Aerosol hygroscopic properties during Asian dust, pollution, and biomass burning episodes at Gosan, Korea in April 2001. Atmos. Environ. 2006, 40, 1550–1560. [Google Scholar] [CrossRef]
- Tang, M.; Cziczo, D.J.; Grassian, V.H. Interactions of Water with Mineral Dust Aerosol: Water Adsorption, Hygroscopicity, Cloud Condensation, and Ice Nucleation. Chem. Rev. 2016, 116, 4205–4259. [Google Scholar] [CrossRef] [Green Version]
- Krueger, B.J. The transformation of solid atmospheric particles into liquid droplets through heterogeneous chemistry: Laboratory insights into the processing of calcium containing mineral dust aerosol in the troposphere. Geophys. Res. Lett. 2003, 30, 1148. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Guo, J.; Ding, A.; Liao, H.; Liu, J.; Sun, Y.; Wang, T.; Xue, H.; Zhang, H.; Zhu, B. Aerosol and boundary-layer interactions and impact on air quality. Natl. Sci. Rev. 2017, 4, 810–833. [Google Scholar] [CrossRef]
- Veselovskii, I.; Goloub, P.; Podvin, T.; Tanre, D.; da Silva, A.; Colarco, P.; Castellanos, P.; Korenskiy, M.; Hu, Q.; Whiteman, D.N.; et al. Characterization of smoke/dust episode over West Africa: comparison of MERRA-2 modeling with multiwavelength Mie-Raman lidar observations. Atmos. Meas. Tech. Discuss. 2018, 11, 949–969. [Google Scholar] [CrossRef] [Green Version]
- Fernández, A.J.; Apituley, A.; Veselovskii, I.; Suvorina, A.; Henzing, J.; Pujadas, M.; Artíñano, B. Study of aerosol hygroscopic events over the Cabauw experimental site for atmospheric research (CESAR) using the multi-wavelength Raman lidar Caeli. Atmos. Environ. 2015, 120, 484–498. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.; Lv, M.; Wang, Y.; Wang, W.; Zhang, Y.; Wang, H.; Yan, X.; Sun, Y.; Cribb, M. Aerosol hygroscopic growth, contributing factors, and impact on haze events in a severely polluted region in northern China. Atmos. Chem. Phys. 2019, 19, 1327–1342. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Zhao, C.; Kuang, Y.; Tao, J.; Tan, W.; Bian, Y.; Li, J.; Li, C. Impact of aerosol hygroscopic growth on retrieving aerosol extinction coefficient profiles from elastic-backscatter lidar signals. Atmos. Chem. Phys. 2017, 17, 12133–12143. [Google Scholar] [CrossRef] [Green Version]
- Eichler, H.; Cheng, Y.F.; Birmili, W.; Nowak, A.; Wiedensohler, A.; Brüggemann, E.; Gnauk, T.; Herrmann, H.; Althausen, D.; Ansmann, A. Hygroscopic properties and extinction of aerosol particles at ambient relative humidity in South-Eastern China. Atmos. Environ. 2008, 42, 6321–6334. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Guo, J.; Zhao, C.; Cribb, M.C.; Dong, X.; Fan, J.; Gong, D.; Huang, J.; Jiang, M.; et al. East Asian Study of Tropospheric Aerosols and their Impact on Regional Clouds, Precipitation, and Climate (EAST-AIR CPC). J. Geophys. Res. Atmos. 2019. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Huang, J.; Bi, J.; Wang, G.; Wang, W.; Fu, Q.; Li, Z.; Tsay, S.-C.; Shi, J. Dust aerosol vertical structure measurements using three MPL lidars during 2008 China-U.S. joint dust field experiment. J. Geophys. Res. 2010, 115, D00K15. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Du, W.; Wang, Y.; Wang, Q.; Wang, H.; Zheng, H.; Zhang, F.; Shi, H.; Bian, Y.; Han, Y.; et al. Aerosol chemistry and particle growth events at an urban downwind site in North China Plain. Atmos. Chem. Phys. 2018, 18, 14637–14651. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Wang, F.; Sun, Y. Analysis of Chemical Composition, Source and Processing Characteristics of Submicron Aerosol during the Summer in Beijing, China. Aerosol Air Qual. Res. 2019, 19, 1450–1462. [Google Scholar] [CrossRef] [Green Version]
- Ng, N.L.; Herndon, S.C.; Trimborn, A.; Canagaratna, M.R.; Croteau, P.L.; Onasch, T.B.; Sueper, D.; Worsnop, D.R.; Zhang, Q.; Sun, Y.L.; et al. An Aerosol Chemical Speciation Monitor (ACSM) for Routine Monitoring of the Composition and Mass Concentrations of Ambient Aerosol. Aerosol Sci. Technol. 2011, 45, 780–794. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, W.; Zhang, Q.; Jiang, Q.; Canonaco, F.; Prévôt, A.S.H.; Fu, P.; Li, J.; Jayne, J.; Worsnop, D.R.; et al. Source apportionment of organic aerosol from 2-year highly time-resolved measurements by an aerosol chemical speciation monitor in Beijing, China. Atmos. Chem. Phys. 2018, 18, 8469–8489. [Google Scholar] [CrossRef] [Green Version]
- Hitchins, J.; Morawska, L.; Wolff, R.; Gilbert, D. Concentrations of submicrometre particles from vehicle emissions near a major road. Atmos. Environ. 2000, 34, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Covert, D.S.; Wiedensohler, A.; Aalto, P.; Heintzenberg, J.; Mcmurry, P.H.; Leck, C. Aerosol number size distributions from 3 to 500 nm diameter in the arctic marine boundary layer during summer and autumn. Tellus B Chem. Phys. Meteorol. 1996, 48, 197–212. [Google Scholar] [CrossRef] [Green Version]
- Bian, J.; Chen, H.; Vömel, H.; Duan, Y.; Xuan, Y.; Lü, D. Intercomparison of humidity and temperature sensors: GTS1, Vaisala RS80, and CFH. Adv. Atmos. Sci. 2011, 28, 139–146. [Google Scholar] [CrossRef]
- Guo, J.-P.; Zhang, X.-Y.; Che, H.-Z.; Gong, S.-L.; An, X.; Cao, C.-X.; Guang, J.; Zhang, H.; Wang, Y.-Q.; Zhang, X.-C.; et al. Correlation between PM concentrations and aerosol optical depth in eastern China. Atmos. Environ. 2009, 43, 5876–5886. [Google Scholar] [CrossRef]
- Wu, T.; Fan, M.; Tao, J.; Su, L.; Wang, P.; Liu, D.; Li, M.; Han, X.; Chen, L. Aerosol Optical Properties over China from RAMS-CMAQ Model Compared with CALIOP Observations. Atmosphere 2017, 8, 201. [Google Scholar] [CrossRef] [Green Version]
- Fernald, F.G. Analysis of atmospheric lidar observations: Some comments. Appl. Opt. 1984, 23, 652. [Google Scholar] [CrossRef]
- Li, C.; Pan, Z.; Mao, F.; Gong, W.; Chen, S.; Min, Q. De-noising and retrieving algorithm of Mie lidar data based on the particle filter and the Fernald method. Opt. Express 2015, 23, 26509. [Google Scholar] [CrossRef]
- Whiteman, D.N.; Melfi, S.H.; Ferrare, R.A. Raman lidar system for the measurement of water vapor and aerosols in the Earth’s atmosphere. Appl. Opt. 1992, 31, 3068. [Google Scholar] [CrossRef]
- Miloshevich, L.M.; Vömel, H.; Whiteman, D.N.; Lesht, B.M.; Schmidlin, F.J.; Russo, F. Absolute accuracy of water vapor measurements from six operational radiosonde types launched during AWEX-G and implications for AIRS validation. J. Geophys. Res. 2006, 111, D09S10. [Google Scholar] [CrossRef] [Green Version]
- Melfi, S.H. Remote Measurements of the Atmosphere Using Raman Scattering. Appl. Opt. 1972, 11, 1605. [Google Scholar] [CrossRef]
- Granados-Muñoz, M.J.; Navas-Guzmán, F.; Bravo-Aranda, J.A.; Guerrero-Rascado, J.L.; Lyamani, H.; Valenzuela, A.; Titos, G.; Fernández-Gálvez, J.; Alados-Arboledas, L. Hygroscopic growth of atmospheric aerosol particles based on active remote sensing and radiosounding measurements: Selected cases in southeastern Spain. Atmos. Meas. Tech. 2015, 8, 705–718. [Google Scholar] [CrossRef] [Green Version]
- Lv, M.; Liu, D.; Li, Z.; Mao, J.; Sun, Y.; Wang, Z.; Wang, Y.; Xie, C. Hygroscopic growth of atmospheric aerosol particles based on lidar, radiosonde, and in situ measurements: Case studies from the Xinzhou field campaign. J. Quant. Spectrosc. Radiat. Transf. 2017, 188, 60–70. [Google Scholar] [CrossRef]
- Jeong, M.-J.; Li, Z.; Andrews, E.; Tsay, S.-C. Effect of aerosol humidification on the column aerosol optical thickness over the Atmospheric Radiation Measurement Southern Great Plains site: EFFECT of AEROSOL HUMIDIFICATION on AOT. J. Geophys. Res. Atmos. 2007, 112. [Google Scholar] [CrossRef]
- Adam, M.; Putaud, J.P.; Martins dos Santos, S.; Dell’Acqua, A.; Gruening, C. Aerosol hygroscopicity at a regional background site (Ispra) in Northern Italy. Atmos. Chem. Phys. 2012, 12, 5703–5717. [Google Scholar] [CrossRef]
- Reilly, P.J.; Wood, R.H. Prediction of the properties of mixed electrolytes from measurements on common ion mixtures. J. Phys. Chem. 1969, 73, 4292–4297. [Google Scholar] [CrossRef]
- Engelhart, G.J.; Hennigan, C.J.; Miracolo, M.A.; Robinson, A.L.; Pandis, S.N. Cloud condensation nuclei activity of fresh primary and aged biomass burning aerosol. Atmos. Chem. Phys. 2012, 12, 7285–7293. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.K.V.; Zhang, Q.; Jimenez, J.L.; Pike, M.; Carlton, A.G. Liquid Water: Ubiquitous Contributor to Aerosol Mass. Environ. Sci. Technol. Lett. 2016, 3, 257–263. [Google Scholar] [CrossRef]
- Tesche, M.; Ansmann, A.; Müller, D.; Althausen, D.; Engelmann, R.; Freudenthaler, V.; Groß, S. Vertically resolved separation of dust and smoke over Cape Verde using multiwavelength Raman and polarization lidars during Saharan Mineral Dust Experiment 2008. J. Geophys. Res. 2009, 114, D13202. [Google Scholar] [CrossRef]
- Mamouri, R.E.; Ansmann, A. Fine and coarse dust separation with polarization lidar. Atmos. Meas. Tech. 2014, 7, 3717–3735. [Google Scholar] [CrossRef] [Green Version]
- Tesche, M.; Müller, D.; Gross, S.; Ansmann, A.; Althausen, D.; Freudenthaler, V.; Weinzierl, B.; Veira, A.; Petzold, A. Optical and microphysical properties of smoke over Cape Verde inferred from multiwavelength lidar measurements. Tellus B Chem. Phys. Meteorol. 2011, 63, 677–694. [Google Scholar] [CrossRef] [Green Version]
- Sicard, M.; Guerrero-Rascado, J.L.; Navas-Guzmán, F.; Preißler, J.; Molero, F.; Tomás, S.; Bravo-Aranda, J.A.; Comerón, A.; Rocadenbosch, F.; Wagner, F.; et al. Monitoring of the Eyjafjallajökull volcanic aerosol plume over the Iberian Peninsula by means of four EARLINET lidar stations. Atmos. Chem. Phys. 2012, 12, 3115–3130. [Google Scholar] [CrossRef] [Green Version]
- Feingold, G. Aerosol hygroscopic properties as measured by lidar and comparison with in situ measurements. J. Geophys. Res. 2003, 108, 4327. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Li, Z.; Fan, X.; Peng, L.; Yu, Y.; Cribb, M. Analysis of cloud layer structure in Shouxian, China using RS92 radiosonde aided by 95 GHz cloud radar. J. Geophys. Res. 2010, 115, D00K30. [Google Scholar] [CrossRef]
- Veselovskii, I.; Whiteman, D.N.; Kolgotin, A.; Andrews, E.; Korenskii, M. Demonstration of Aerosol Property Profiling by Multiwavelength Lidar under Varying Relative Humidity Conditions. J. Atmos. Ocean. Technol. 2009, 26, 1543–1557. [Google Scholar] [CrossRef]
- Bedoya-Velásquez, A.E.; Navas-Guzmán, F.; Granados-Muñoz, M.J.; Titos, G.; Román, R.; Casquero-Vera, J.A.; Ortiz-Amezcua, P.; Benavent-Oltra, J.A.; de Arruda Moreira, G.; Montilla-Rosero, E.; et al. Hygroscopic growth study in the framework of EARLINET during the SLOPE I campaign: synergy of remote sensing and in situ instrumentation. Atmos. Chem. Phys. 2018, 18, 7001–7017. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.G.; Li, J.; Qu, Y.; Han, T.; Hou, L.; Gu, J.; Chen, C.; Yang, Y.; Liu, X.; Yang, T.; et al. Formation and evolution mechanism of regional haze: a case study in the megacity Beijing, China. Atmos. Chem. Phys. 2013, 13, 4501–4514. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Al-Yaari, A.; Schwank, M.; Fan, L.; Frappart, F.; Swenson, J.; Wigneron, J.-P. Compared performances of SMOS-IC soil moisture and vegetation optical depth retrievals based on Tau-Omega and Two-Stream microwave emission models. Remote Sens. Environ. 2020, 236, 111502. [Google Scholar] [CrossRef]
- Pan, X.; Ge, B.; Wang, Z.; Tian, Y.; Liu, H.; Wei, L.; Yue, S.; Uno, I.; Kobayashi, H.; Nishizawa, T.; et al. Synergistic effect of water-soluble species and relative humidity on morphological changes in aerosol particles in the Beijing megacity during severe pollution episodes. Atmos. Chem. Phys. 2019, 19, 219–232. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Uno, I.; Wang, Z.; Nishizawa, T.; Sugimoto, N.; Yamamoto, S.; Kobayashi, H.; Sun, Y.; Fu, P.; Tang, X.; et al. Real-time observational evidence of changing Asian dust morphology with the mixing of heavy anthropogenic pollution. Sci. Rep. 2017, 7, 335. [Google Scholar] [CrossRef] [Green Version]
- Tian, P.; Zhang, L.; Ma, J.; Tang, K.; Xu, L.; Wang, Y.; Cao, X.; Liang, J.; Ji, Y.; Jiang, J.H.; et al. Radiative absorption enhancement of dust mixed with anthropogenic pollution over East Asia. Atmos. Chem. Phys. 2018, 18, 7815–7825. [Google Scholar] [CrossRef] [Green Version]
- Di Girolamo, P.; Summa, D.; Bhawar, R.; Di Iorio, T.; Cacciani, M.; Veselovskii, I.; Dubovik, O.; Kolgotin, A. Raman lidar observations of a Saharan dust outbreak event: Characterization of the dust optical properties and determination of particle size and microphysical parameters. Atmos. Environ. 2012, 50, 66–78. [Google Scholar] [CrossRef]
- Zieger, P.; Fierz-Schmidhauser, R.; Weingartner, E.; Baltensperger, U. Effects of relative humidity on aerosol light scattering: Results from different European sites. Atmos. Chem. Phys. 2013, 13, 10609–10631. [Google Scholar] [CrossRef] [Green Version]







| Instruments | Parameters | Type | Time Resolution | Vertical Spatial Resolution |
|---|---|---|---|---|
| MPL | Extinction coefficient profile, backscatter coefficient profile, volume depolarization ratio profile | SigmaSpace micropulse lidar system 4202 | 30 s | 30 m |
| Raman Lidar | Water vapor mixing ratio profile | Vibrational–Rotation polarization Raman lidar | 15 min | 7.5 m |
| ACSM | Aerosol chemical composition | Aerodyne Q-ACSM | 15 min | |
| SMPS | Particle number size distribution (0.01~0.05 μm) | TSI 3938 | 5 min | |
| APS | Particle number size distribution (0.5~20 μm) | TSI 3321 | 5 min | |
| Nano-SMPS | Particle number size distribution (5 nm~0.05 μm) | TSI 3756 | 5 min | |
| Radiosondes | Temperature profile, relative humidity profile, water vapor mixing ratio profile | L-band GTS1, GRAW | Twice a day (11:00 and 23:00 UTC) | one data per second during ascent |
| Species | Organics | ||||
|---|---|---|---|---|---|
| 1.725 | 1.78 | 1.76 | 1.83 | 1.4 | |
| 0.68 | 0.56 | 0.52 | 0.91 | 0.1 |
| Case I | Case II | |||||
|---|---|---|---|---|---|---|
| Range | Gradient | Range | Gradient | |||
| Altitude (m) | 410 | 540 | 130 | 580 | 1000 | 420 |
| () | 1.788 | 2.285 | 0.497 | 1.402 | 1.488 | 0.086 |
| (°C) | −2.309 | −1.638 | 0.671 | −5.204 | −4.352 | 0.852 |
| () | 0.023 | 0.034 | 0.011 | 0.008 | 0.026 | 0.018 |
| Depolarization ratio | 0.125 | 0.071 | −0.054 | 0.077 | 0.035 | −0.042 |
| Color ratio | 0.351 | 0.544 | 0.193 | 0.388 | 1.60 | 1.212 |
| Case I | Case II | |||||
|---|---|---|---|---|---|---|
| a | b | R2 | a | b | R2 | |
| Kasten | 0.68 0.083 | 0.33 0.103 | 0.93 | 0.2 0.017 | 1.34 0.091 | 0.97 |
| R2 | R2 | |||||
| Hänel | 0.307 0.100 | 0.87 | 1.138 0.179 | 0.90 | ||
| Case I | Case II | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Organics | Organics | |||||||||
| VF | 0.227 0.019 | 0.210 0.093 | 0.068 0.090 | 0 | 0.493 0.040 | 0.252 0.047 | 0.089 0.084 | 0.136 0.102 | 0 | 0.523 0.046 |
| 0.357 0.024 | 0.344 0.026 | |||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Li, Z.; Chen, J.; Wang, Y.; Wu, H.; Jin, X.; Liang, C.; Li, S.; Wang, W.; Cribb, M. Hygroscopicity of Different Types of Aerosol Particles: Case Studies Using Multi-Instrument Data in Megacity Beijing, China. Remote Sens. 2020, 12, 785. https://doi.org/10.3390/rs12050785
Wu T, Li Z, Chen J, Wang Y, Wu H, Jin X, Liang C, Li S, Wang W, Cribb M. Hygroscopicity of Different Types of Aerosol Particles: Case Studies Using Multi-Instrument Data in Megacity Beijing, China. Remote Sensing. 2020; 12(5):785. https://doi.org/10.3390/rs12050785
Chicago/Turabian StyleWu, Tong, Zhanqing Li, Jun Chen, Yuying Wang, Hao Wu, Xiao’ai Jin, Chen Liang, Shangze Li, Wei Wang, and Maureen Cribb. 2020. "Hygroscopicity of Different Types of Aerosol Particles: Case Studies Using Multi-Instrument Data in Megacity Beijing, China" Remote Sensing 12, no. 5: 785. https://doi.org/10.3390/rs12050785
APA StyleWu, T., Li, Z., Chen, J., Wang, Y., Wu, H., Jin, X., Liang, C., Li, S., Wang, W., & Cribb, M. (2020). Hygroscopicity of Different Types of Aerosol Particles: Case Studies Using Multi-Instrument Data in Megacity Beijing, China. Remote Sensing, 12(5), 785. https://doi.org/10.3390/rs12050785






