Drone-Based Tracking of the Fine-Scale Movement of a Coastal Stingray (Bathytoshia brevicaudata)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Focal Species
2.2. Study Site
2.3. Sampling Technique
2.3.1. Tracking Accuracy
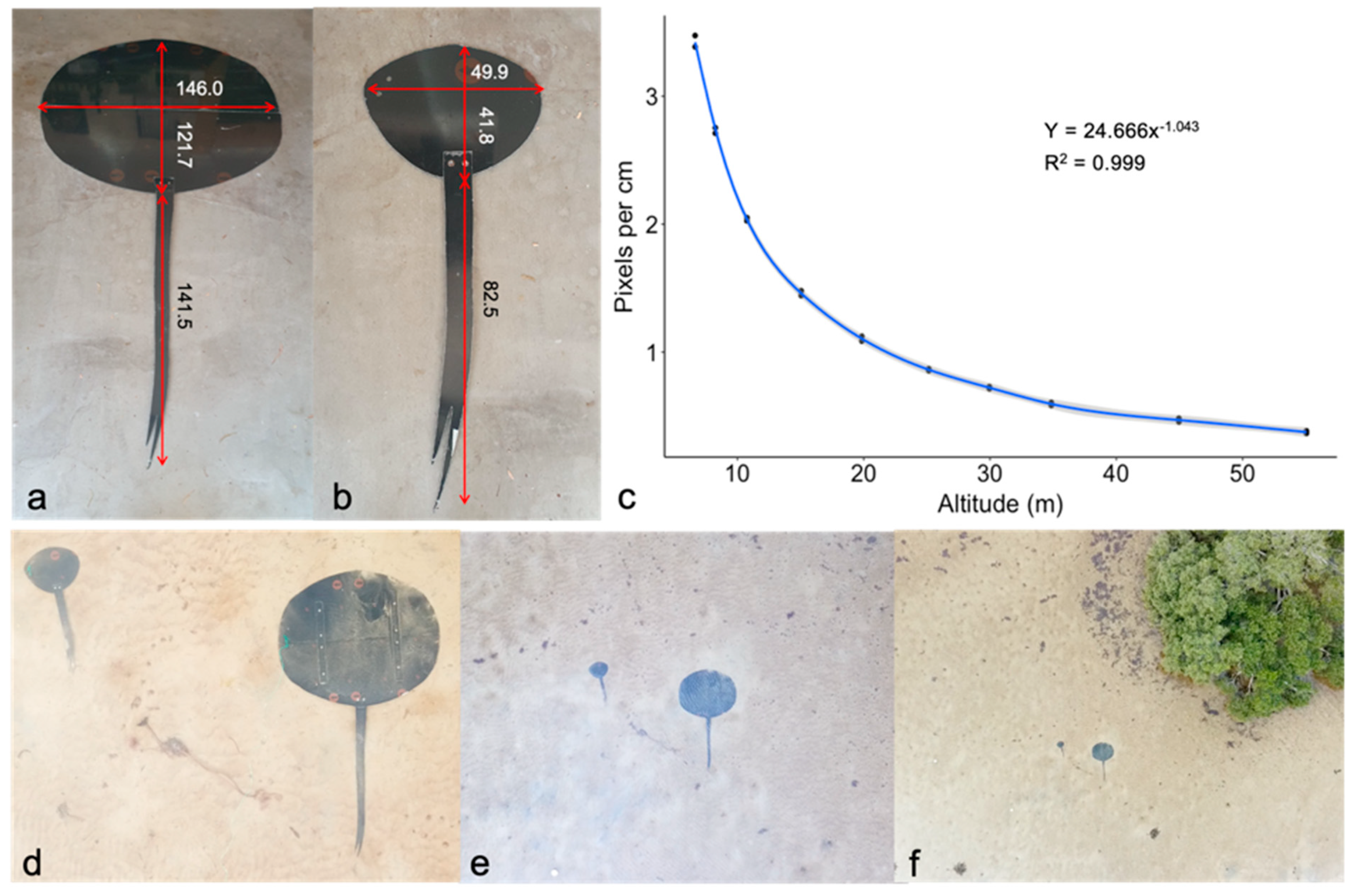
2.3.2. Photogrammetric Accuracy
2.4. Data Preparation
2.5. Data Analysis
2.5.1. Search Effort
2.5.2. Selection of Movement Characteristics for Modeling
2.5.3. Effect of Body Size on Ray Movement
2.5.4. Effect of Tide and Time of Day on Ray Movement
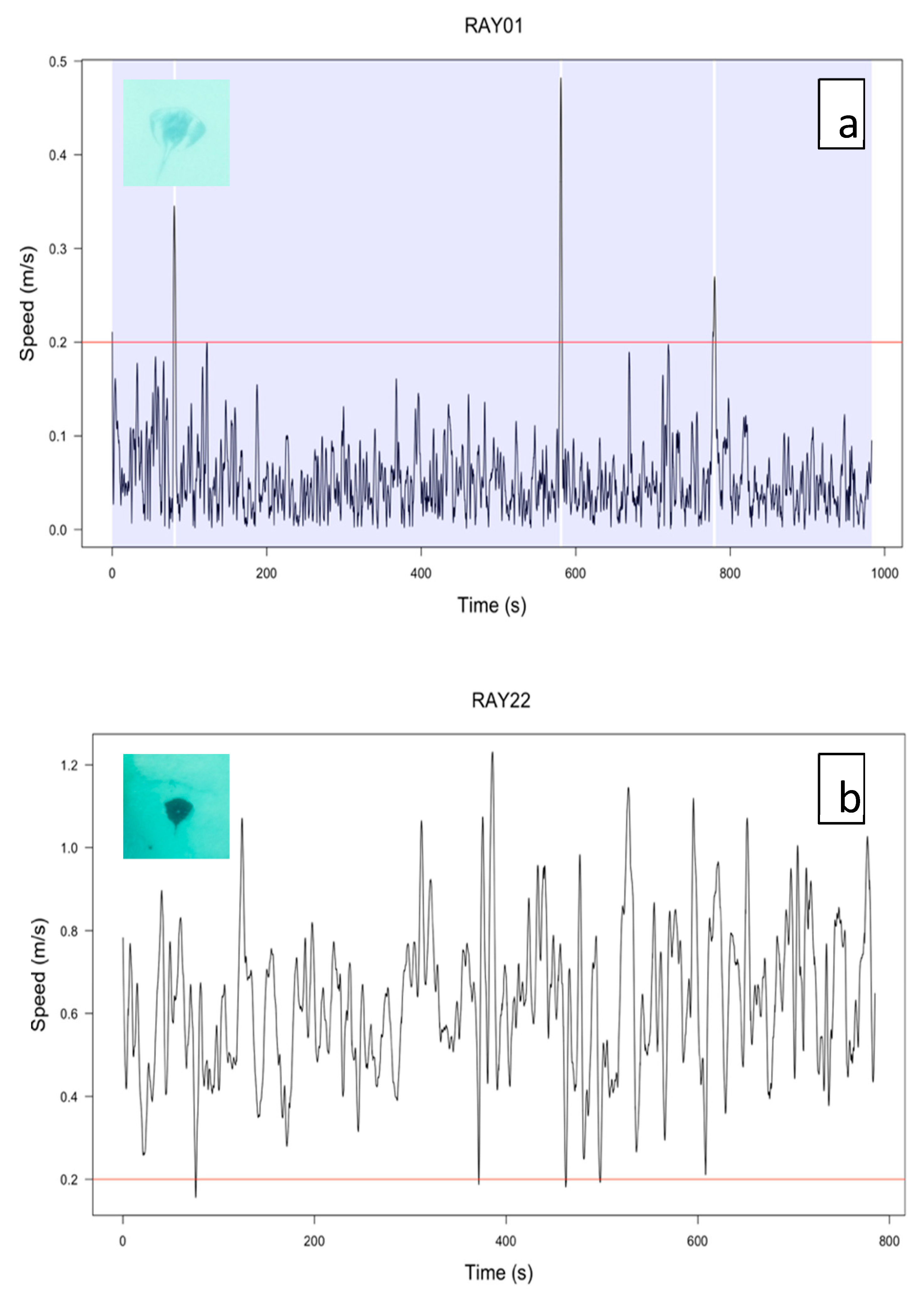
2.5.5. Categorizing Behavior
3. Results
3.1. Search Effort
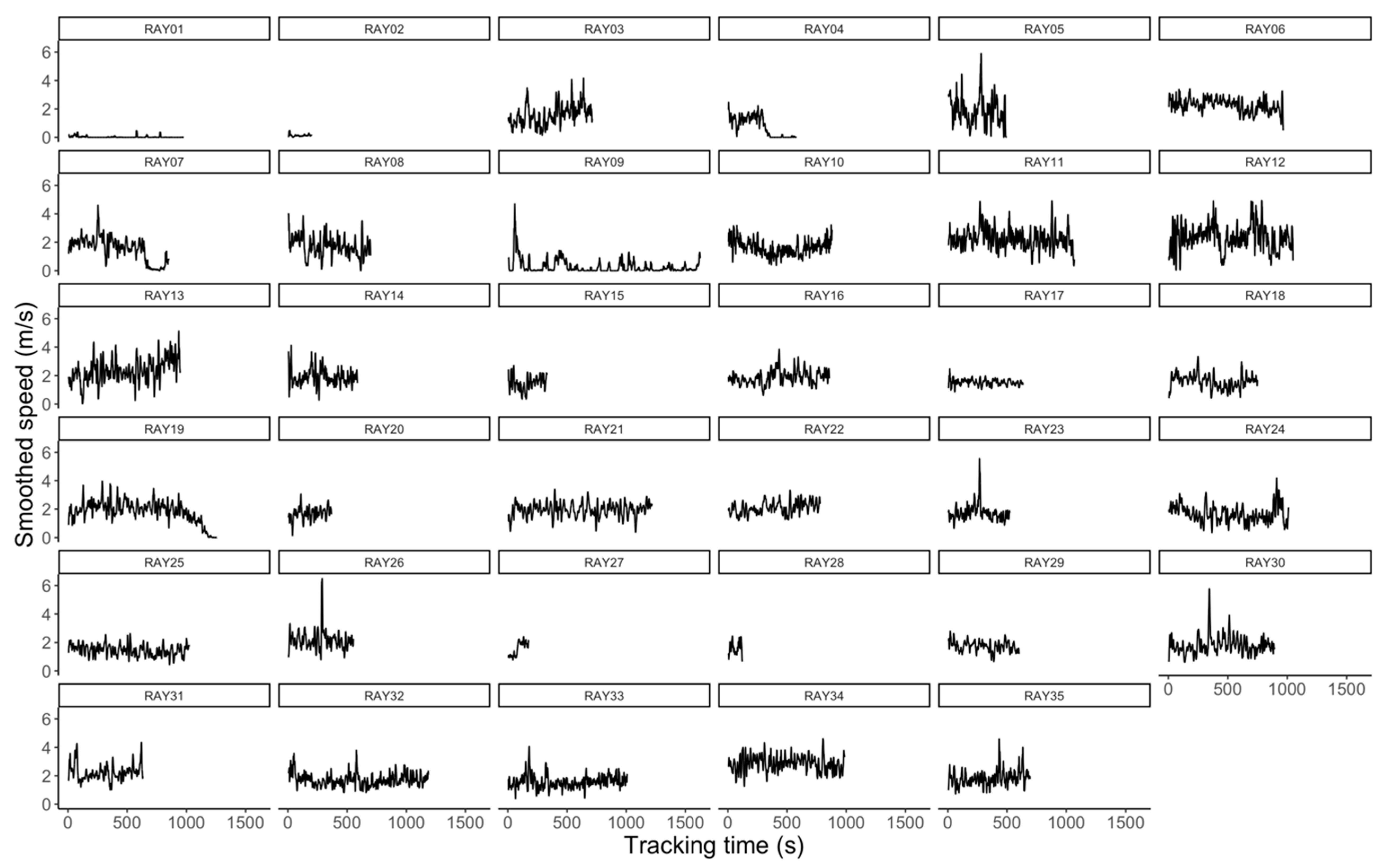
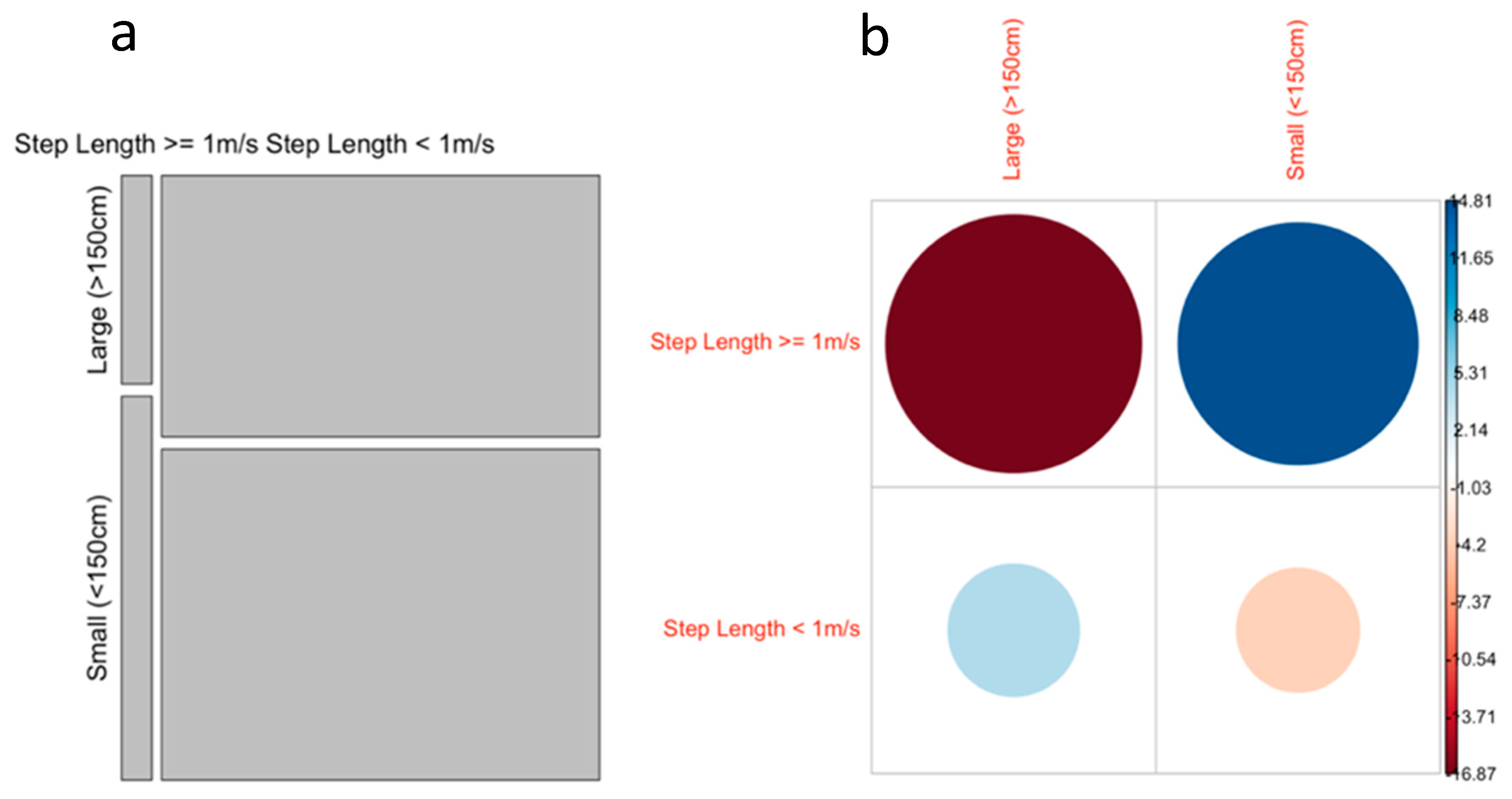
3.2. Effect of Body Size on Ray Movement
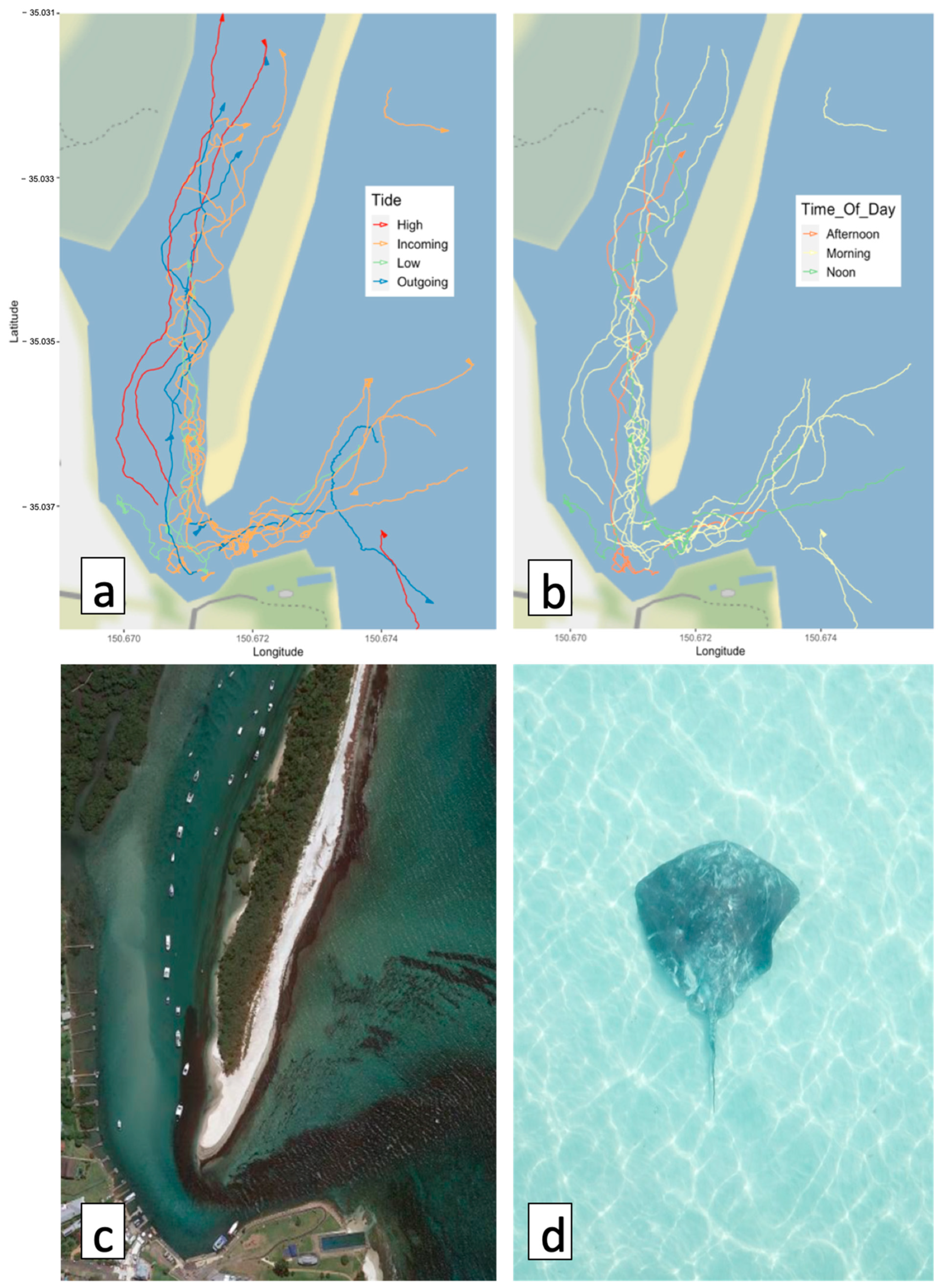
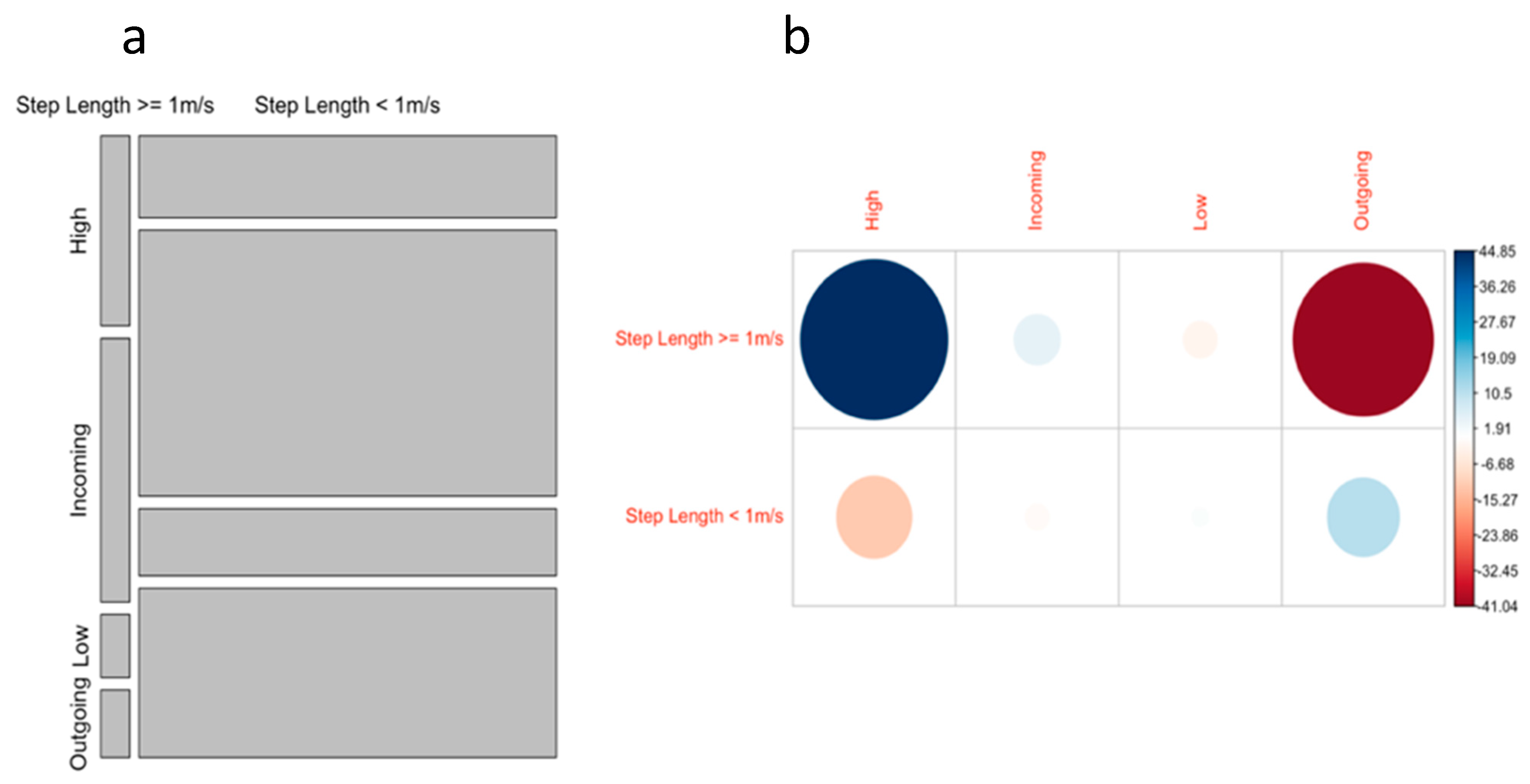
3.3. Effect of Tide on Ray Movement
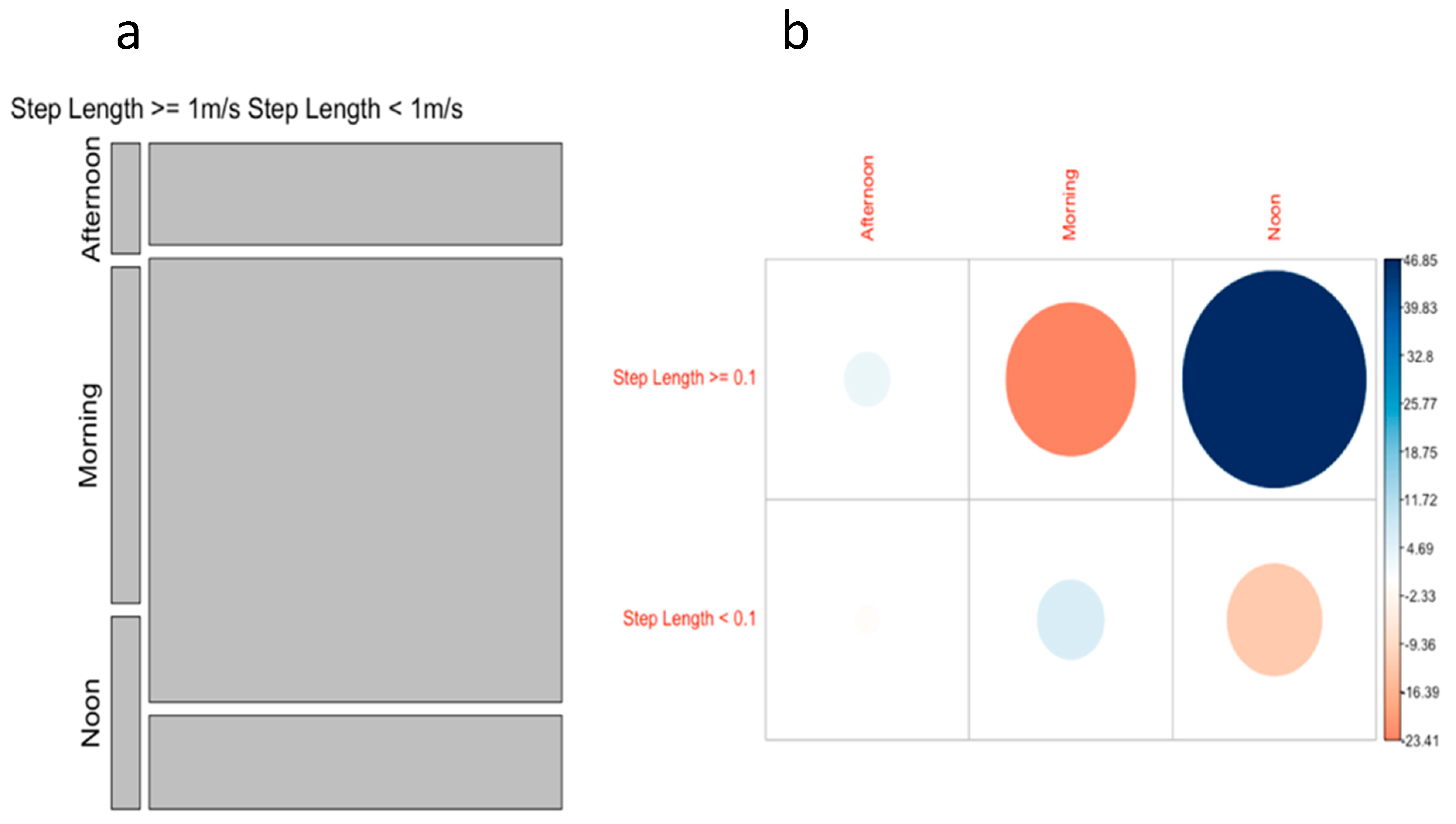
3.4. Effect of Time of Day on Ray Movement
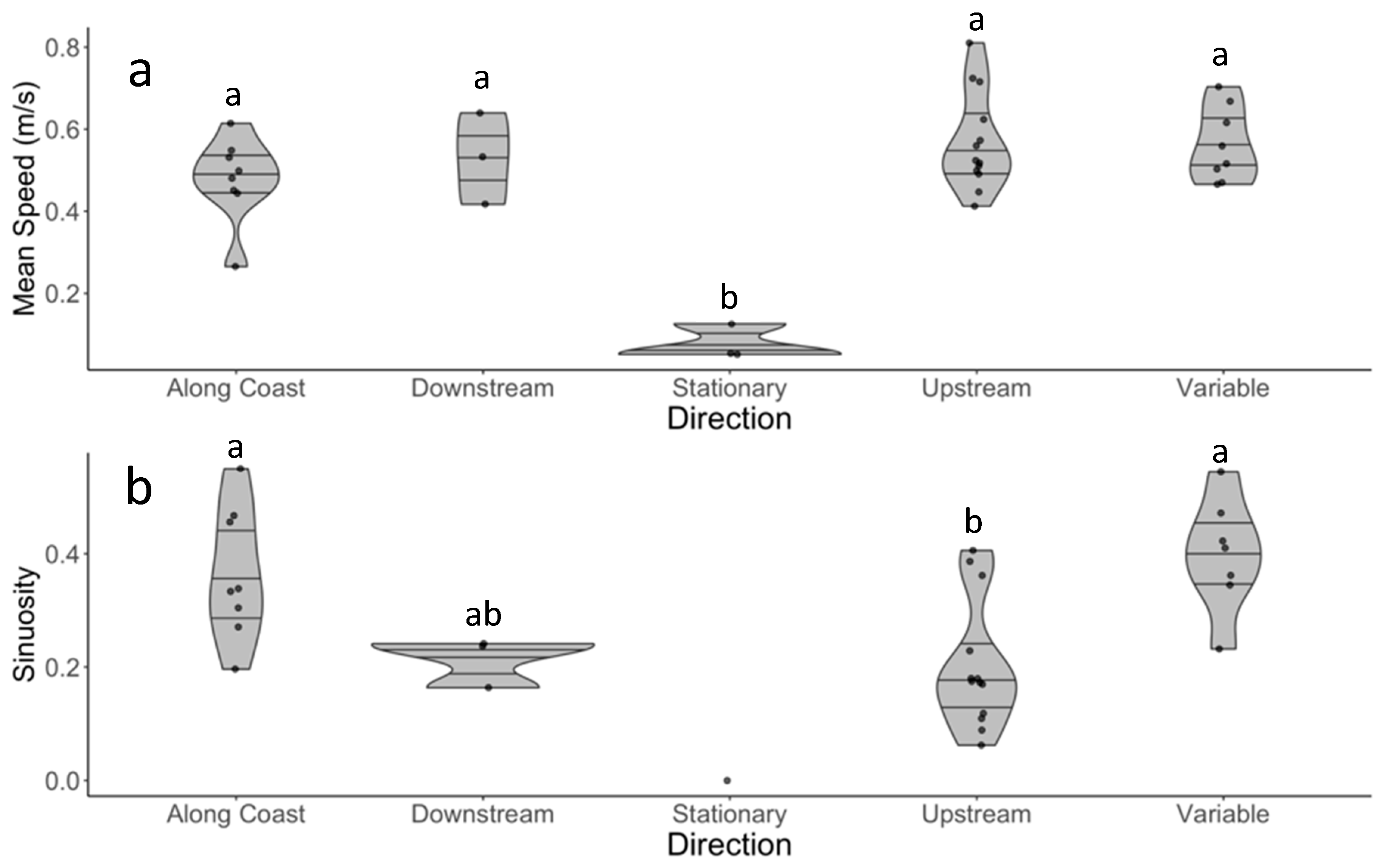
3.5. Categorising Behavior
4. Discussion
4.1. Effect of Body Size on Ray Movement
4.2. Effect of Tide on Ray Movement
4.3. Effect of Time of Day on Ray Movement
4.4. Site and Species Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fahrig, L. Non-optimal animal movement in human-altered landscapes. Funct. Ecol. 2007, 21, 1003–1015. [Google Scholar] [CrossRef]
- Doherty, T.S.; Driscoll, D.A. Coupling movement and landscape ecology for animal conservation in production landscapes. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172272. [Google Scholar] [CrossRef]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; Agrosa, C.; Watson, R. A Global Map of Human Impact on Marine Ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef] [Green Version]
- Halpern, B.S.; Frazier, M.; Potapenko, J.; Casey, K.S.; Koenig, K.; Longo, C.; Walbridge, S. Spatial and temporal changes in cumulative human impacts on the world’s ocean. Nat. Commun. 2015, 6, 7615. [Google Scholar] [CrossRef] [Green Version]
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Warner, R.R. Historical Overfishing and the Recent Collapse of Coastal Ecosystems. Science 2001, 293, 629–637. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Silliman, B.R. Climate Change, Human Impacts, and Coastal Ecosystems in the Anthropocene. Curr. Biol. 2019, 29, R1021–R1035. [Google Scholar] [CrossRef]
- Beck, M.W.; Heck, K.L.; Able, K.W.; Childers, D.L.; Eggleston, D.B.; Gillanders, B.M.; Minello, T.J. The Role of Nearshore Ecosystems as Fish and Shellfish Nurseries. 2003. Available online: https://www.epa.gov/watershedacademy/role-nearshore-ecosystems-fish-and-shellfish-nurseries (accessed on 3 December 2020).
- Sheaves, M.; Baker, R.; Nagelkerken, I.; Connolly, R.M. True value of estuarine and coastal nurseries for fish: Incorporating complexity and dynamics. Estuaries Coasts 2015, 38, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Fauchald, P.; Tveraa, T. Hierarchical patch dynamics and animal movement pattern. Oecologia 2006, 149, 383–395. [Google Scholar] [CrossRef]
- Schofield, G.; Katselidis, K.A.; Lilley, M.K.S.; Reina, R.D.; Hays, G.C. Detecting elusive aspects of wildlife ecology using drones: New insights on the mating dynamics and operational sex ratios of sea turtles. Funct. Ecol. 2017, 31, 2310–2319. [Google Scholar] [CrossRef]
- Feinsinger, P.; Colwell, R.K.; Terborgh, J.; Chaplin, S.B. Elevation and the Morphology, Flight Energetics, and Foraging Ecology of Tropical Hummingbirds. Am. Nat. 1979, 113, 481–497. [Google Scholar] [CrossRef]
- Braña, F. Morphological correlates of burst speed and field movement patterns: The behavioural adjustment of locomotion in wall lizards (Podarcis muralis). Biol. J. Linn. Soc. 2003, 80, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Braccini, M.; Aires-Da-Silva, A.; Taylor, I. Incorporating movement in the modelling of shark and ray population dynamics: Approaches and management implications. Rev. Fish Biol. Fish. 2016, 26, 13–24. [Google Scholar] [CrossRef]
- Porreca, A.P.; Hintz, W.D.; Whitledge, G.W.; Rude, N.P.; Heist, E.J.; Garvey, J.E. Establishing ecologically relevant management boundaries: Linking movement ecology with the conservation of Scaphirhynchus sturgeon. Can. J. Fish. Aquat. Sci. 2016, 73, 877–884. [Google Scholar] [CrossRef]
- Ogburn, M.B.; Harrison, A.-L.; Whoriskey, F.G.; Cooke, S.J.; Mills Flemming, J.E.; Torres, L.G. Addressing Challenges in the Application of Animal Movement Ecology to Aquatic Conservation and Management. Front. Mar. Sci. 2017, 4, 70. Available online: https://www.frontiersin.org/article/10.3389/fmars.2017.00070 (accessed on 3 December 2020). [CrossRef] [Green Version]
- Allen, A.M.; Singh, N.J. Linking Movement Ecology with Wildlife Management and Conservation. Front. Ecol. Evol. 2016, 3, 155. Available online: https://www.frontiersin.org/article/10.3389/fevo.2015.00155 (accessed on 3 December 2020). [CrossRef] [Green Version]
- Katzner, T.; Arlettaz, R. Evaluating Contributions of Recent Tracking-Based Animal Movement Ecology to Conservation Management. Front. Ecol. Evol. 2019, 7, 519. [Google Scholar] [CrossRef] [Green Version]
- Stein, R.W.; Mull, C.G.; Kuhn, T.S.; Aschliman, N.C.; Davidson, L.N.K.; Joy, J.B.; Mooers, A.O. Global priorities for conserving the evolutionary history of sharks, rays and chimaeras. Nat. Ecol. Evol. 2018, 2, 288–298. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, O.R.; Thums, M.; van Keulen, M.; Meekan, M. Bioturbation by stingrays at Ningaloo Reef, Western Australia. Mar. Freshw. Res. 2012, 63, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Bornatowski, H.; Navia, A.F.; Braga, R.R.; Abilhoa, V.; Corrêa, M.F.M. Ecological importance of sharks and rays in a structural foodweb analysis in southern Brazil. ICES J. Mar. Sci. 2014, 71, 1586–1592. [Google Scholar] [CrossRef] [Green Version]
- Flowers, K.I.; Heithaus, M.R.; Papastamatiou, Y.P. Buried in the sand: Uncovering the ecological roles and importance of rays. Fish Fish. 2020. [Google Scholar] [CrossRef]
- Young, H.J.; Raoult, V.; Platell, M.E.; Williamson, J.E.; Gaston, T.F. Within-genus differences in catchability of elasmobranchs during trawling. Fish. Res. 2019, 211, 141–147. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Baum, J.K.; Clarke, S.; Compagno, L.J.V.; Cortés, E.; Domingo, A.; Gibson, C. You can swim but you can’t hide: The global status and conservation of oceanic pelagic sharks and rays. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 459–482. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; White, W.T. Extinction risk and conservation of the world’s sharks and rays. Elife 2014, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, I.C.; Meekan, M.G.; Buckworth, R.C.; Bradshaw, C.J.A. Susceptibility of sharks, rays and chimaeras to global extinction. Adv. Mar. Biol. 2009, 56, 275–363. [Google Scholar] [PubMed]
- Rogers, C.; Roden, C.; Lohoefener, R.; Mullin, K.; Hoggard, W. Behavior, distribution, and relative abundance of cownose ray schools Rhinoptera bonasus in the northern Gulf of Mexico. Gulf Mex. Sci. 1990, 11, 8. [Google Scholar] [CrossRef]
- James, P. Observations on shoals of the Javanese Cownose Ray Rhinoptera javanica Muller & Henle from the Gulf of Mannar, with additional notes on the species. J. Mar. Biol. Assoc. India 1962, 4, 217–223. [Google Scholar]
- Schwartz, F.J. Mass Migratory Congregations and Movements of Several Species of Cownose Rays, Genus Rhinoptera: A World-Wide Review. J. Elisha Mitchell Sci. Soc. 1990, 106, 10–13. Available online: www.jstor.org/stable/24333475 (accessed on 3 December 2020).
- Merriner, J.V.; Smith, J.W. A report to the oyster industry of Virginia on the biology and management of the cownose ray (Rhinoptera bonasus, Mitchill) in lower Chesapeake Bay. Spec. Rep. Appl. Mar. Sci. Ocean Eng. 1979, 216, 957. [Google Scholar]
- Smith, J.W.; Merriner, J. Observations on The Reproductive-Biology of The Cownose Ray, Rhinoptera-Bonasus, In Chesapeake Bay. Fish. Bull. 1986, 84, 871. [Google Scholar]
- Smith, J.W.; Merriner, J.V. Age and Growth, Movements and Distribution of the Cownose Ray, Rhinoptera bonasus, in Chesapeake Bay. Estuaries 1987, 10, 153. [Google Scholar] [CrossRef]
- Gray, A.E.; Mulligan, T.J.; Hannah, R.W. Food habits, occurrence, and population structure of the bat ray, Myliobatis californica, in Humboldt Bay, California. Environ. Biol. Fishes 1997, 49, 227–238. [Google Scholar] [CrossRef]
- Hopkins, T.E.; Cech, J.J. Effect of temperature on oxygen consumption of the bat ray, Myliobatis californica (Chondrichthyes, Myliobatididae). Copeia 1994, 529–532. [Google Scholar] [CrossRef]
- Le Port, A.; Sippel, T.; Montgomery, J.C. Observations of mesoscale movements in the short-tailed stingray, Dasyatis brevicaudata from New Zealand using a novel PSAT tag attachment method. J. Exp. Mar. Biol. Ecol. 2008, 359, 110–117. [Google Scholar] [CrossRef]
- Le Port, A.; Lavery, S.; Montgomery, J.C. Conservation of coastal stingrays: Seasonal abundance and population structure of the short-tailed stingray Dasyatis brevicaudata at a Marine Protected Area. ICES J. Mar. Sci. 2012, 69, 1427–1435. [Google Scholar] [CrossRef] [Green Version]
- Collins, A.B.; Heupel, M.R.; Simpfendorfer, C.A. Spatial Distribution and Long-term Movement Patterns of Cownose Rays Rhinoptera bonasus Within an Estuarine River. Estuaries Coasts 2008, 31, 1174–1183. [Google Scholar] [CrossRef]
- Drymon, J.M. Distributions of Coastal Sharks in the Northern Gulf of Mexico: Consequences for Trophic Transfer and Foodweb Dynamics; University of South Alabama: Mobile, Alabama, 2010. [Google Scholar]
- Ajemian, M.J.; Powers, S.P. Seasonality and Ontogenetic Habitat Partitioning of Cownose Rays in the Northern Gulf of Mexico. Estuaries Coasts 2016, 39, 1234–1248. [Google Scholar] [CrossRef]
- Smith, J.W.; Merriner, J.V. Food Habits and Feeding Behavior of the Cownose Ray, Rhinoptera bonasus, in Lower Chesapeake Bay. Estuaries 1985, 8, 305. [Google Scholar] [CrossRef]
- Coles, R.J. Observations on the Habits and Distribution of Certain Fishes Taken on the Coast of North Carolina; Order of the Trustees; American Museum of Natural History: New York, NY, USA, 1910. [Google Scholar]
- Davy, L.E.; Simpfendorfer, C.A.; Heupel, M.R. Movement patterns and habitat use of juvenile mangrove whiprays (Himantura granulata). Mar. Freshw. Res. 2015, 66, 481. [Google Scholar] [CrossRef]
- Kanno, S.; Schlaff, A.; Heupel, M.; Simpfendorfer, C. Stationary video monitoring reveals habitat use of stingrays in mangroves. Mar. Ecol. Prog. Ser. 2019, 621, 155–168. [Google Scholar] [CrossRef]
- Martins, A.P.B.; Heupel, M.R.; Bierwagen, S.L.; Chin, A.; Simpfendorfer, C. Diurnal activity patterns and habitat use of juvenile Pastinachus ater in a coral reef flat environment. PLoS ONE 2020, 15, e0228280. [Google Scholar] [CrossRef] [Green Version]
- Hoisington, G.; Lowe, C.G. Abundance and distribution of the round stingray, Urobatis halleri, near a heated effluent outfall. Mar. Environ. Res. 2005, 60, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Vaudo, J.J.; Lowe, C.G. Movement patterns of the round stingray Urobatis halleri (Cooper) near a thermal outfall. J. Fish Biol. 2006, 68, 1756–1766. [Google Scholar] [CrossRef] [Green Version]
- Matern, S.A.; Cech, J.J.; Hopkins, T.E. Diel movements of bat rays, Myliobatis californica, in Tomales Bay, California: Evidence for behavioral thermoregulation? Environ. Biol. Fishes 2000, 58, 173–182. [Google Scholar] [CrossRef]
- Tagliafico, A.; Butcher, P.A.; Colefax, A.P.; Clark, G.F.; Kelaher, B.P. Variation in cownose ray Rhinoptera neglecta abundance and group size on the central east coast of Australia. J. Fish Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Crossin, G.T.; Hinch, S.G.; Farrell, A.P.; Higgs, D.A.; Lotto, A.G.; Oakes, J.D.; Healey, M.C. Energetics and morphology of sockeye salmon: Effects of upriver migratory distance and elevation. J. Fish Biol. 2004, 65, 788–810. [Google Scholar] [CrossRef]
- Pettersson, L.B.; Hedenström, A. Energetics, cost reduction and functional consequences of fish morphology. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2000, 267, 759–764. [Google Scholar] [CrossRef] [Green Version]
- Schlaff, A.M.; Heupel, M.R.; Simpfendorfer, C.A. Influence of environmental factors on shark and ray movement, behaviour and habitat use: A review. Rev. Fish Biol. Fish. 2014, 24, 1089–1103. [Google Scholar] [CrossRef]
- Strong, W.R.; Snelson, F.F.; Gruber, S.H. Hammerhead Shark Predation on Stingrays: An Observation of Prey Handling by Sphyrna mokarran. Copeia 1990, 836–840. [Google Scholar] [CrossRef]
- Myers, R.A.; Baum, J.K.; Shepherd, T.D.; Powers, S.P.; Peterson, C.H. Cascading Effects of the Loss of Apex Predatory Sharks from a Coastal Ocean. Science 2007, 315, 1846–1850. [Google Scholar] [CrossRef]
- Heithaus, M.R.; Frid, A.; Wirsing, A.J.; Worm, B. Predicting ecological consequences of marine top predator declines. Trends Ecol. Evol. 2008, 23, 202–210. [Google Scholar] [CrossRef]
- Bond, M.E.; Valentin-Albanese, J.; Babcock, E.A.; Heithaus, M.R.; Grubbs, R.D.; Cerrato, R.; Chapman, D.D. Top predators induce habitat shifts in prey within marine protected areas. Oecologia 2019, 190, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Semeniuk, C.A.D.; Rothley, K.D. Costs of group-living for a normally solitary forager: Effects of provisioning tourism on southern stingrays Dasyatis americana. Mar. Ecol. Prog. Ser. 2008, 357, 271–282. [Google Scholar] [CrossRef]
- Lobel, P.S. Underwater acoustic ecology: Boat noises and fish behavior. In Proceedings of the AAUS Diving for Science 2009 Symposium, Atlanta, GA, USA, 13–14 March 2009; pp. 31–42. [Google Scholar]
- Caldwell, I.R.; Gergel, S.E. Thresholds in seascape connectivity: Influence of mobility, habitat distribution, and current strength on fish movement. Landsc. Ecol. 2013, 28, 1937–1948. [Google Scholar] [CrossRef]
- Berthe, C.; Lecchini, D. Influence of boat noises on escape behaviour of white-spotted eagle ray Aetobatus ocellatus at Moorea Island (French Polynesia). Compt. Rendus Biol. 2016, 339, 99–103. [Google Scholar] [CrossRef]
- Simpson, S.D.; Radford, A.N.; Nedelec, S.L.; Ferrari, M.C.O.; Chivers, D.P.; McCormick, M.I.; Meekan, M.G. Anthropogenic noise increases fish mortality by predation. Nat. Commun. 2016, 7, 10544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butcher, P.C.; Kajiura, S.M.; Lopez, N.A.; Mourier, J.; Purcell, C.R.; Skomal, G.B.; Tucker, J.P.; Walsh, A.J.; Williamson, J.E.; Raoult, V.; et al. The Drone Revolution of Shark Science: A Review. Drones. (under review).
- Lower, N.; Moore, A.; Scott, A.P.; Ellis, T.; James, J.D.; Russell, I.C. A non-invasive method to assess the impact of electronic tag insertion on stress levels in fishes. J. Fish Biol. 2005, 67, 1202–1212. [Google Scholar] [CrossRef]
- Klefoth, T.; Kobler, A.; Arlinghaus, R. The impact of catch-and-release angling on short-term behaviour and habitat choice of northern pike (Esox lucius L.). Hydrobiologia 2008, 601, 99–110. [Google Scholar] [CrossRef]
- Jepsen, N.; Thorstad, E.B.; Havn, T.; Lucas, M.C. The use of external electronic tags on fish: An evaluation of tag retention and tagging effects. Anim. Biotelemetry 2015, 3, 49. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-García, D.; Adams, K.; Brown, H.; Davis, A.R. Determining Stingray Movement Patterns in a Wave-Swept Coastal Zone Using a Blimp for Continuous Aerial Video Surveillance. Fishes 2020, 5, 31. [Google Scholar] [CrossRef]
- Oleksyn, S.; Tosetto, L.; Raoult, V.; Joyce, K.; Williamson, J.E. Going Batty: The Challenges and Opportunities for Drone Researchers In Monitoring Behaviour And Habitat Use Of Rays. Drones. (under review).
- Christiansen, F.; Rojano-Doñate, L.; Madsen, P.T.; Bejder, L. Noise Levels of Multi-Rotor Unmanned Aerial Vehicles with Implications for Potential Underwater Impacts on Marine Mammals. Front. Mar. Sci. 2016, 3, 277. Available online: https://www.frontiersin.org/article/10.3389/fmars.2016.00277 (accessed on 3 December 2020). [CrossRef] [Green Version]
- Mulero-Pázmány, M.; Jenni-Eiermann, S.; Strebel, N.; Sattler, T.; Negro, J.J.; Tablado, Z. Unmanned aircraft systems as a new source of disturbance for wildlife: A systematic review. PLoS ONE 2017, 12, e0178448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, C.A.J.; Paul, L.J.; Chin, A. Bathytoshia brevicaudata. The IUCN Red List of Threatened Species 2016. 2016. Available online: https://dx.doi.org/10.2305/IUCN.UK.2016-1.RLTS.T41796A68618154.en (accessed on 3 December 2020).
- Pini-Fitzsimmons, J.; Knott, N.; Brown, C. Effects of food provisioning on site use in the short-tail stingray Bathytoshia brevicaudata. Mar. Ecol. Prog. Ser. 2018, 600, 99–110. [Google Scholar] [CrossRef]
- Last, P.R.; Manjaji-Matsumoto, B.M.; Naylor, G.J.P.; White, W.T. 25. Stingrays. Family Dasyatidae. Rays World. Csiro Publ. Comstock Publ. Assoc. Ithaca Lond. 2016, 1, 522–618. [Google Scholar]
- Raoult, V.; Tosetto, L.; Williamson, E.J. Drone-Based High-Resolution Tracking of Aquatic Vertebrates. Drones 2018, 2, 37. [Google Scholar] [CrossRef] [Green Version]
- Hugenholtz, C.; Brown, O.; Walker, J.; Barchyn, T.; Nesbit, P.; Kucharczyk, M.; Myshak, S. Spatial accuracy of UAV-derived orthoimagery and topography: Comparing photogrammetric models processed with direct geo-referencing and ground control points. Geomatica 2016, 70, 21–30. [Google Scholar] [CrossRef]
- Colefax, A.P.; Kelaher, B.P.; Pagendam, D.E.; Butcher, P.A. Assessing White Shark (Carcharodon carcharias) Behavior Along Coastal Beaches for Conservation-Focused Shark Mitigation. Front. Mar. Sci. 2020, 7, 268. [Google Scholar] [CrossRef]
- Microsoft Corporation. Microsoft Excel. 2020. Available online: https://office.microsoft.com/excel (accessed on 3 December 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 3 December 2020).
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2019; Available online: http://www.rstudio.com/ (accessed on 3 December 2020).
- NSW Department of Planning, Industry, and Environment. Currambene Creek. 2018. Available online: https://www.environment.nsw.gov.au/topics/water/estuaries/estuaries-of-nsw/currambene-creek (accessed on 3 December 2020).
- Scheider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Kahle, D.; Wickham, H. ggmap: Spatial Visualization with ggplot2. R J. 2013, 5, 144–161. [Google Scholar] [CrossRef] [Green Version]
- Bivand, R.; Keitt, T.; Rowlingson, B.; Pebesma, E.; Sumner, M.; Hijmans, R.; Bivand, M.R. Package ‘rgdal’. Bind. Geospat. Data Abstr. Libr. 2015. Available online: https://cran.r-project.org/web/packages/rgdal/index.html (accessed on 3 December 2020).
- McLean, D.J.; Skowron Volponi, M.A. trajr: An R package for characterisation of animal trajectories. Ethology 2018, 124, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Edelhoff, H.; Signer, J.; Balkenhol, N. Path segmentation for beginners: An overview of current methods for detecting changes in animal movement patterns. Mov. Ecol. 2016, 4, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bainbridge, R. The Speed of Swimming of Fish as Related to Size and to the Frequency and Amplitude of the Tail Beat. J. Exp. Biol. 1958, 35, 109. Available online: http://jeb.biologists.org/content/35/1/109.abstract (accessed on 3 December 2020).
- Ware, D.M. Bioenergetics of Pelagic Fish: Theoretical Change in Swimming Speed and Ration with Body Size. J. Fish. Res. Board Can. 1978, 35, 220–228. [Google Scholar] [CrossRef]
- Martins, A.P.B.; Heupel, M.R.; Oakley-Cogan, A.; Chin, A.; Simpfendorfer, C.A. Towed-float GPS telemetry: A tool to assess movement patterns and habitat use of juvenile stingrays. Mar. Freshw. Res. 2019, 71, 89–98. [Google Scholar] [CrossRef]
- Dickinson, M.H. How Animals Move: An Integrative View. Science 2000, 288, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Daley, M.A.; Bertram, J. Non-steady locomotion. In Understanding Mammalian Locomotion: Concepts and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 277–306. [Google Scholar]
- Di Santo, V.; Kenaley, C.P.; Lauder, G.V. High postural costs and anaerobic metabolism during swimming support the hypothesis of a U-shaped metabolism–speed curve in fishes. Proc. Natl. Acad. Sci. USA 2017, 114, 13048–13053. [Google Scholar] [CrossRef] [Green Version]
- Trump, C.L.; Leggett, W.C. Optimum swimming speeds in fish: The problem of currents. Can. J. Fish. Aquat. Sci. 1980, 37, 1086–1092. [Google Scholar] [CrossRef]
- Bovet, P.; Benhamou, S. Optimal sinuosity in central place foraging movements. Anim. Behav. 1991, 42, 57–62. [Google Scholar] [CrossRef]
- Guttridge, T.L.; Myrberg, A.A.; Porcher, I.F.; Sims, D.W.; Krause, J. The role of learning in shark behaviour. Fish Fish. 2009, 10, 450–469. [Google Scholar] [CrossRef]
- Henningsen, A.D.; Leaf, R.T. Observations on the Captive Biology of the Southern Stingray. Trans. Am. Fish. Soc. 2010, 139, 783–791. [Google Scholar] [CrossRef]
- Webb, P.W. Speed, Acceleration and Manoeuvrability of Two Teleost Fishes. J. Exp. Biol. 1983, 102, 115. Available online: http://jeb.biologists.org/content/102/1/115.abstract (accessed on 3 December 2020).
- Rosenberger, L.J.; Westneat, M.W. Functional morphology of undulatory pectoral fin locomotion in the stingray taeniura lymma (Chondrichthyes: Dasyatidae). J. Exp. Biol. 1999, 202, 3523. Available online: http://jeb.biologists.org/content/202/24/3523.abstract (accessed on 3 December 2020). [PubMed]
- Seamone, S.G.; McCaffrey, T.M.; Syme, D.A. Disc starts: The pectoral disc of stingrays promotes omnidirectional fast starts across the substrate. Can. J. Zool. 2019, 97, 597–605. [Google Scholar] [CrossRef]
- Casella, E.; Collin, A.; Harris, D.; Ferse, S.; Bejarano, S.; Parravicini, V.; Rovere, A. Mapping coral reefs using consumer-grade drones and structure from motion photogrammetry techniques. Coral Reefs 2017, 36, 269–275. [Google Scholar] [CrossRef]
- Snelson, F.F. Notes on the occurrence, distribution, and biology of elasmobranch fishes in the Indian River lagoon system, Florida. Estuaries 1981, 4, 110–120. [Google Scholar] [CrossRef]
- Walker, P.; Howlett, G.; Millner, R. Distribution, movement and stock structure of three ray species in the North Sea and eastern English Channel. ICES J. Mar. Sci. 1997, 54, 797–808. [Google Scholar] [CrossRef]
- Montgomery, J.; Skipworth, E. Detection of Weak Water Jets by the Short-Tailed Stingray Dasyatis brevicaudata (Pisces: Dasyatidae). Copeia 1997, 4, 881–883. [Google Scholar] [CrossRef]
- Semeniuk, C.A.D.; Dill, L.M. Anti-Predator Benefits of Mixed-Species Groups of Cowtail Stingrays (Pastinachus sephen) and Whiprays (Himantura uarnak) at Rest. Ethology 2006, 112, 33–43. [Google Scholar] [CrossRef]
- Seamone, S.; Blaine, T.; Higham, T.E. Sharks modulate their escape behavior in response to predator size, speed and approach orientation. Zoology 2014, 117, 377–382. [Google Scholar] [CrossRef]
- Brinton, C.P.; Curran, M.C. Tidal and diel movement patterns of the Atlantic stingray (Dasyatis sabina) along a stream-order gradient. Mar. Freshw. Res. 2017, 68, 1716. [Google Scholar] [CrossRef]
- Braekevelt, C.R. Retinal photoreceptor fine structure in the short-tailed stingray Dasyatis brevicaudata. Histol. Histopathol. 1994, 9, 507–514. [Google Scholar] [PubMed]
- Braekevelt, C.R. Fine structure of the tapetum lucidum in the short-tailed stingray Dasyatis brevicaudata. Histol. Histopathol. 1994, 9, 495–500. [Google Scholar] [PubMed]
- Cartamil, D.P.; Vaudo, J.J.; Lowe, C.G.; Wetherbee, B.M.; Holland, K.N. Diel movement patterns of the Hawaiian stingray, Dasyatis lata: Implications for ecological interactions between sympatric elasmobranch species. Mar. Biol. 2003, 142, 841–847. [Google Scholar] [CrossRef]
- Rizzari, J.R.; Semmens, J.M.; Fox, A.; Huveneers, C. Observations of marine wildlife tourism effects on a non-focal species. J. Fish Biol. 2017, 91, 981–988. [Google Scholar] [CrossRef]
- Last, P.R.; Stevens, J.D. Sharks and Rays of Australia; CSIRO Australia: East Melbourne, Australia, 1994.





| Step | Process |
|---|---|
| 1 | Assess the weather conditions and launch drone, systematically sweeping the area of interest while searching for a ray. |
| 2 | Once a ray is located, center it in the viewing screen and descend to 5–25 m, selecting a height within this range that can be maintained for the duration of the track while avoiding any potential hazards including trees and boats. |
| 3 | Start recording video to capture the behavior of the ray once the drone is positioned directly above it. |
| 4 | Use gentle pilot inputs to accurately maintain the position of the drone above the ray and a flight path consistent with the nature of the ray’s movement. |
| 5 | Continue tracking until the ray is lost from sight or the battery of the drone, controller or phone go below 30%. |
| 6 | Stop recording, ensure that the flight path is clear, and return the drone to the home point. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oleksyn, S.; Tosetto, L.; Raoult, V.; Williamson, J.E. Drone-Based Tracking of the Fine-Scale Movement of a Coastal Stingray (Bathytoshia brevicaudata). Remote Sens. 2021, 13, 40. https://doi.org/10.3390/rs13010040
Oleksyn S, Tosetto L, Raoult V, Williamson JE. Drone-Based Tracking of the Fine-Scale Movement of a Coastal Stingray (Bathytoshia brevicaudata). Remote Sensing. 2021; 13(1):40. https://doi.org/10.3390/rs13010040
Chicago/Turabian StyleOleksyn, Semonn, Louise Tosetto, Vincent Raoult, and Jane E. Williamson. 2021. "Drone-Based Tracking of the Fine-Scale Movement of a Coastal Stingray (Bathytoshia brevicaudata)" Remote Sensing 13, no. 1: 40. https://doi.org/10.3390/rs13010040
APA StyleOleksyn, S., Tosetto, L., Raoult, V., & Williamson, J. E. (2021). Drone-Based Tracking of the Fine-Scale Movement of a Coastal Stingray (Bathytoshia brevicaudata). Remote Sensing, 13(1), 40. https://doi.org/10.3390/rs13010040







