Carbon Stocks, Species Diversity and Their Spatial Relationships in the Yucatán Peninsula, Mexico
Abstract
:1. Introduction
2. Materials and Methods
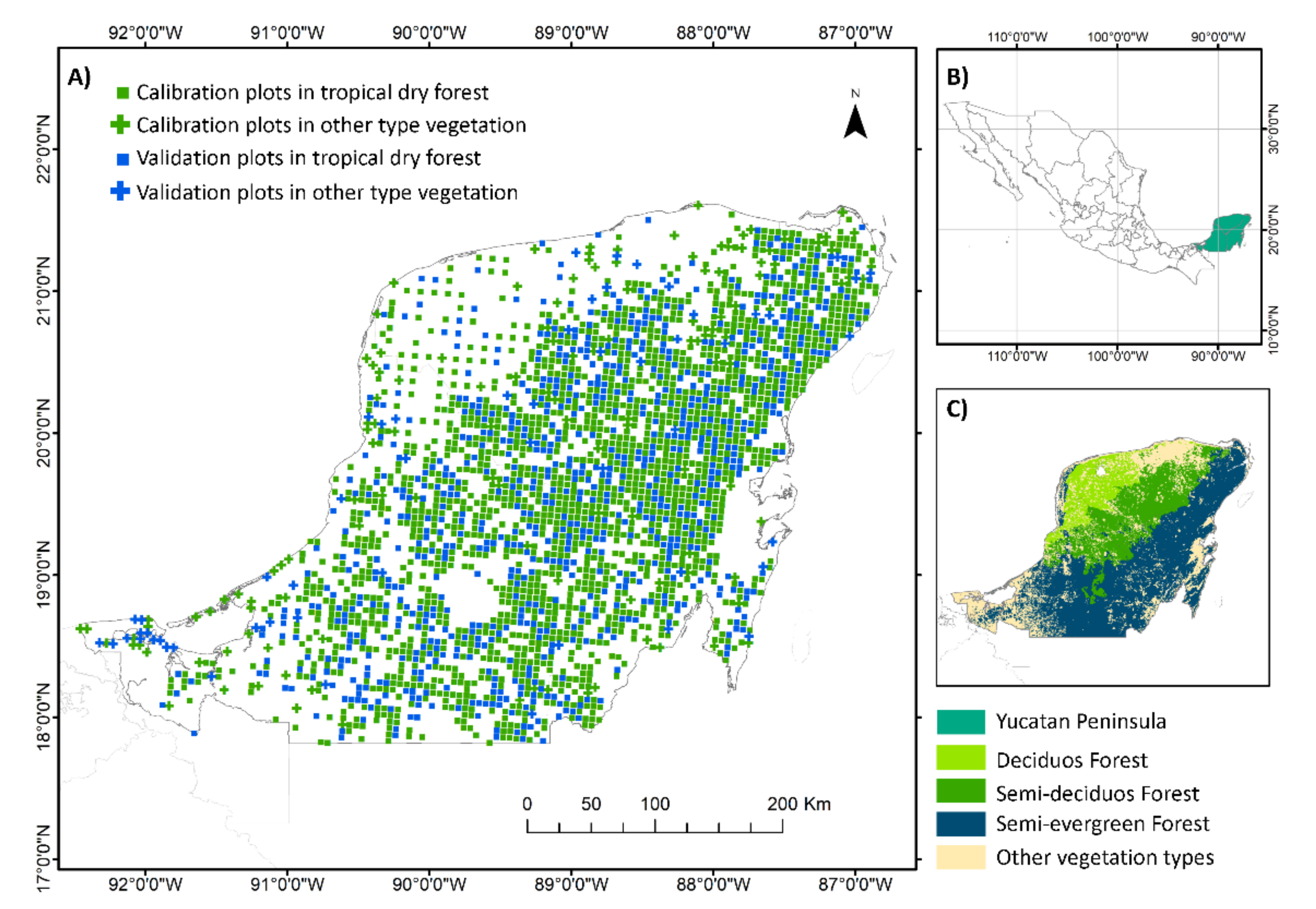
2.1. Study Area
2.2. Field Data and Calculations of Tree Species Richness and Aboveground Biomass
2.3. SAR Data Acquisition and Processing
2.4. Climate Data
2.5. Model Development and Validation
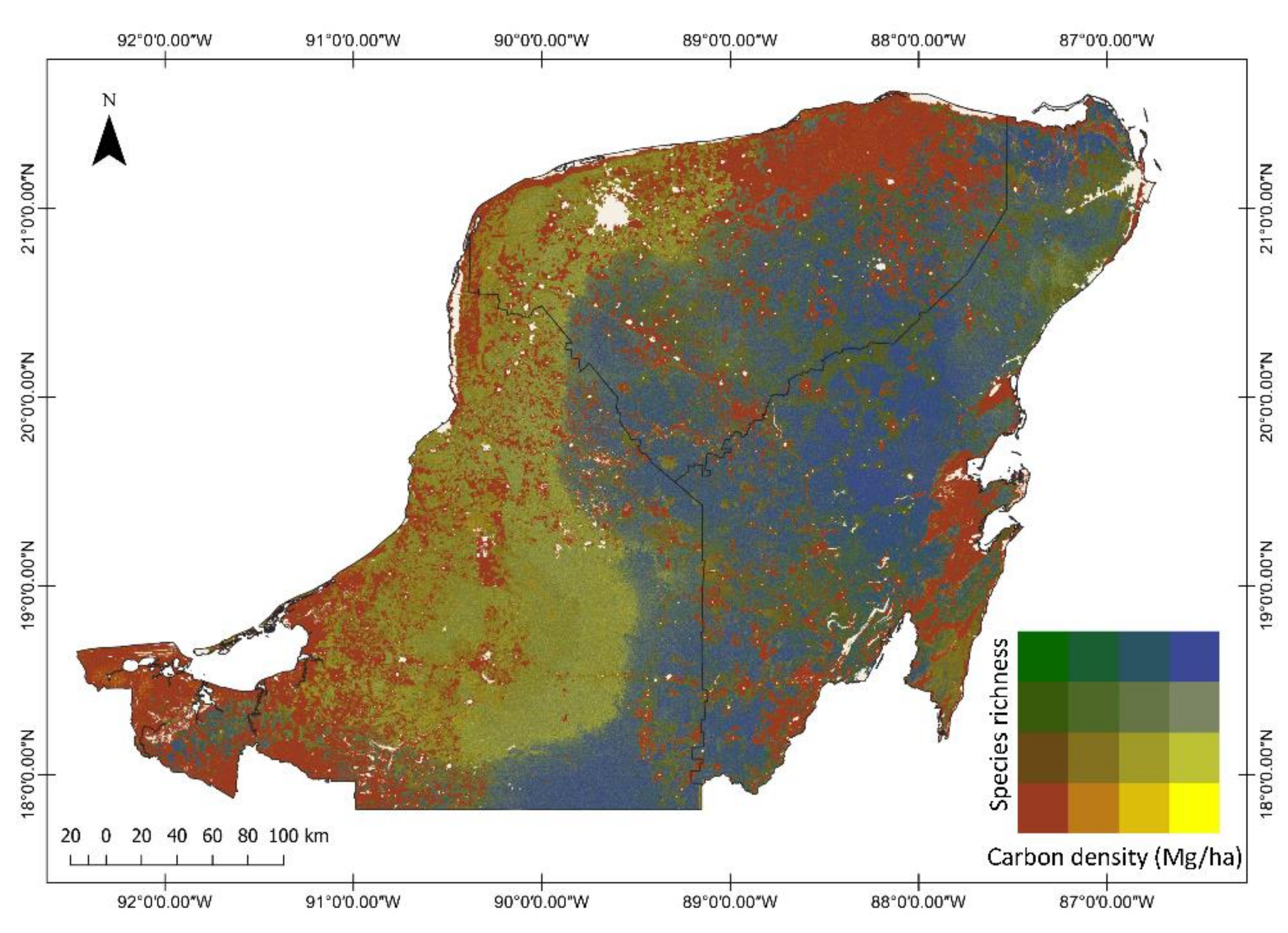
2.6. Generation of Carbon Density, Species Richness and Bivariate Maps
2.7. Statistical Analyses
3. Results
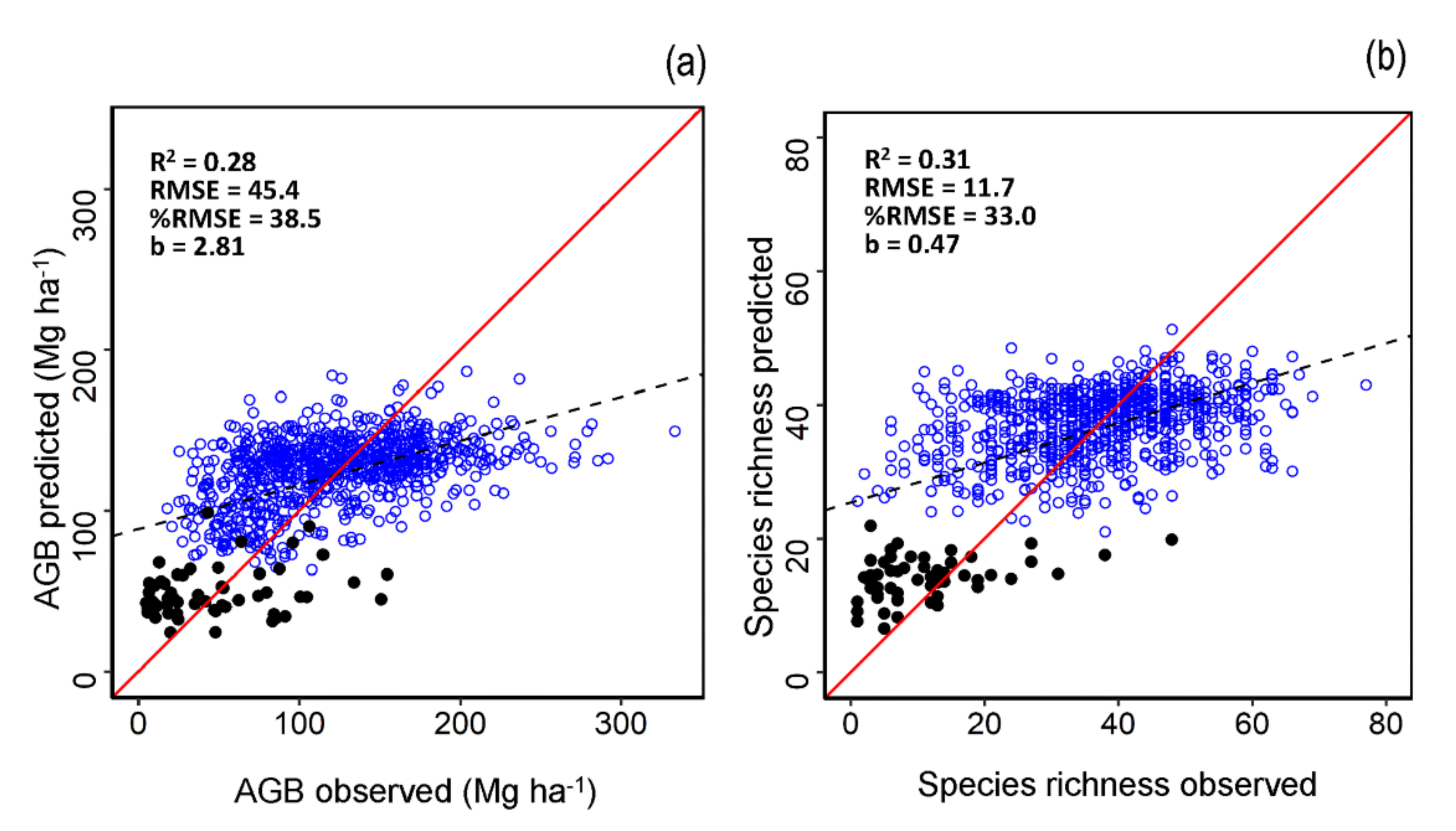
3.1. AGB and Species Richness Models Performance
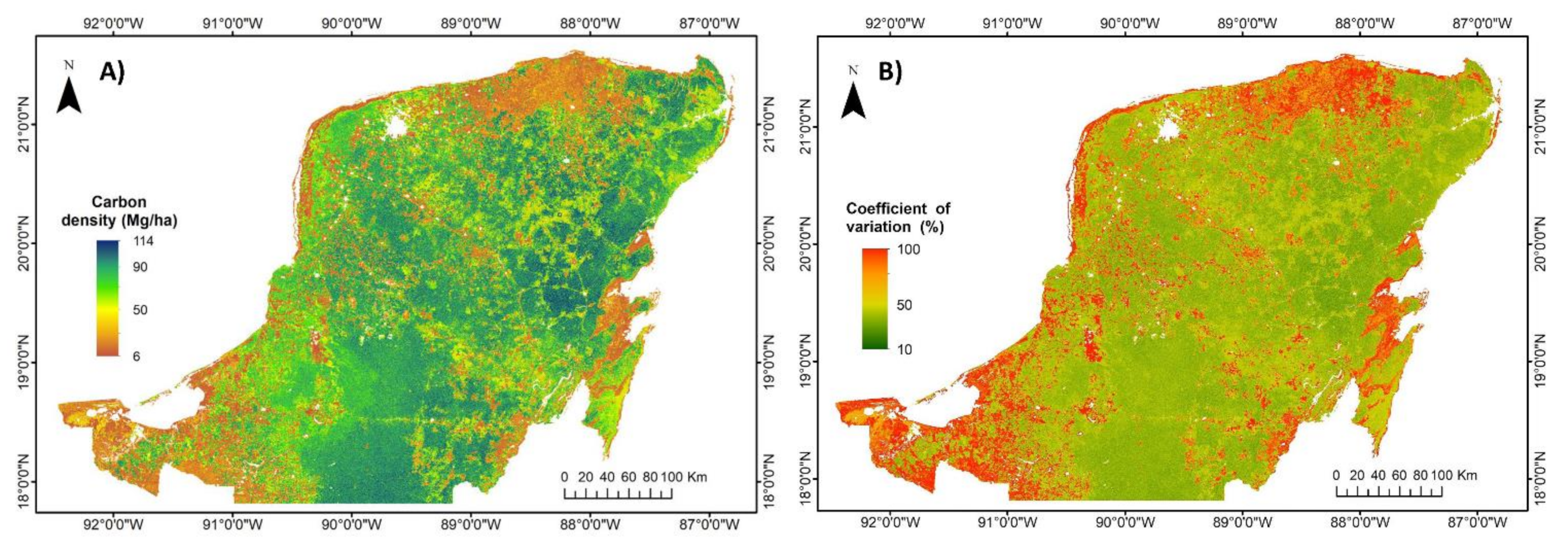
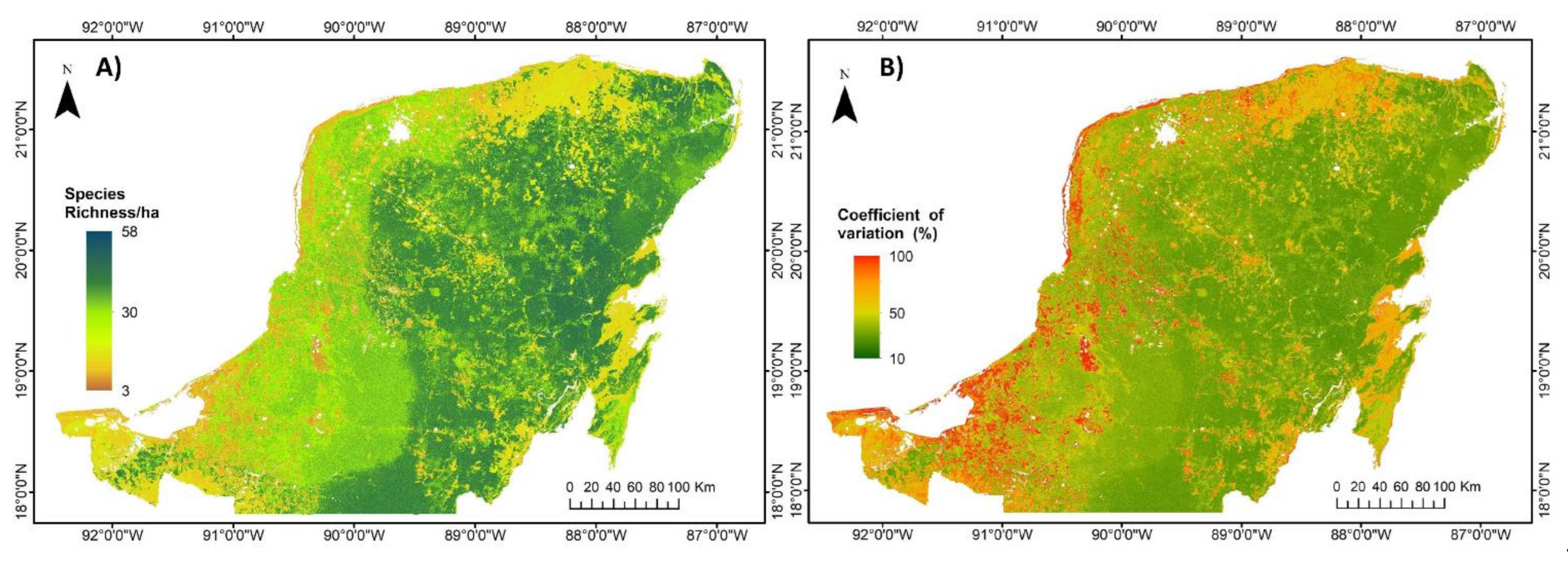
3.2. Regional Carbon Density, Species Richness and Bivariate Maps over the Yucatan Peninsula
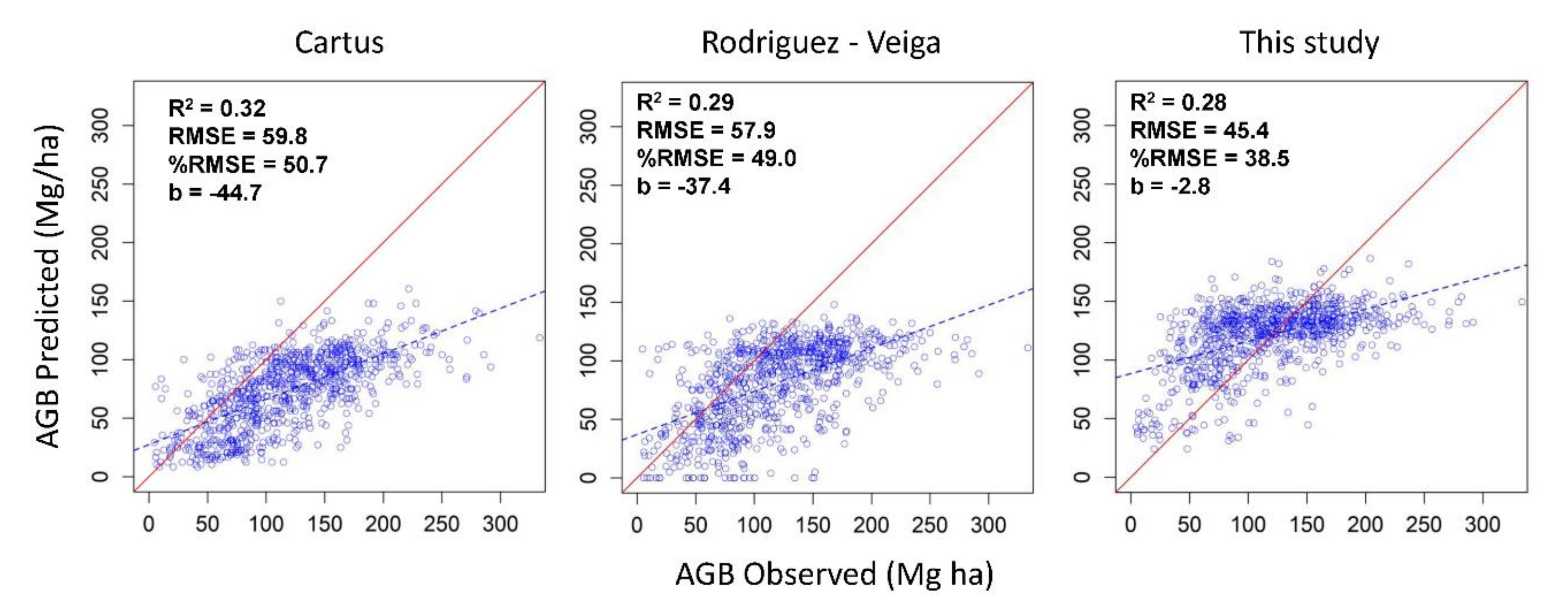
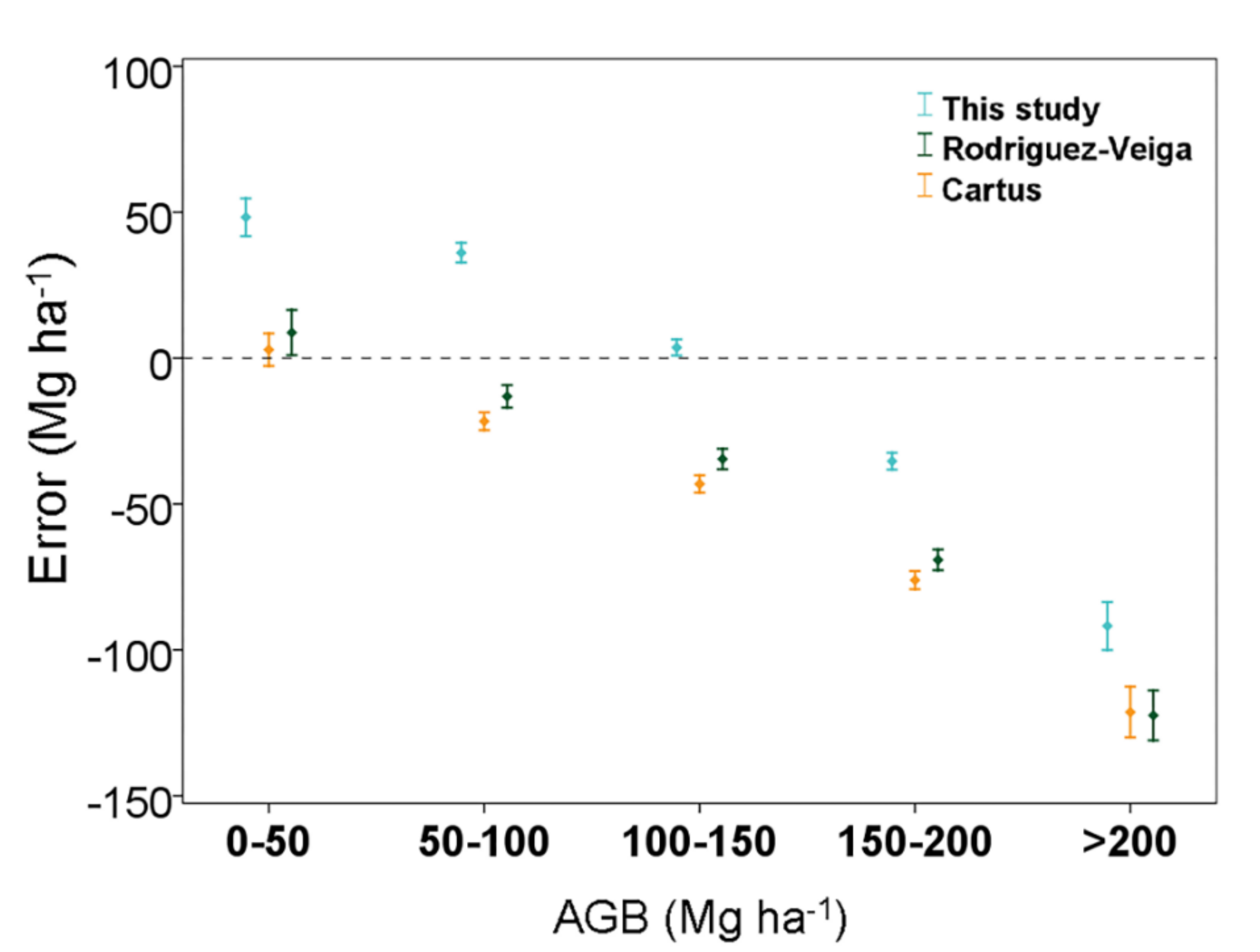
3.3. Comparison of the Aboveground Biomass Map with Previous Studies in the Yucatan Peninsula
3.4. Importance of Variables to Estimate Carbon Density and Species Richness
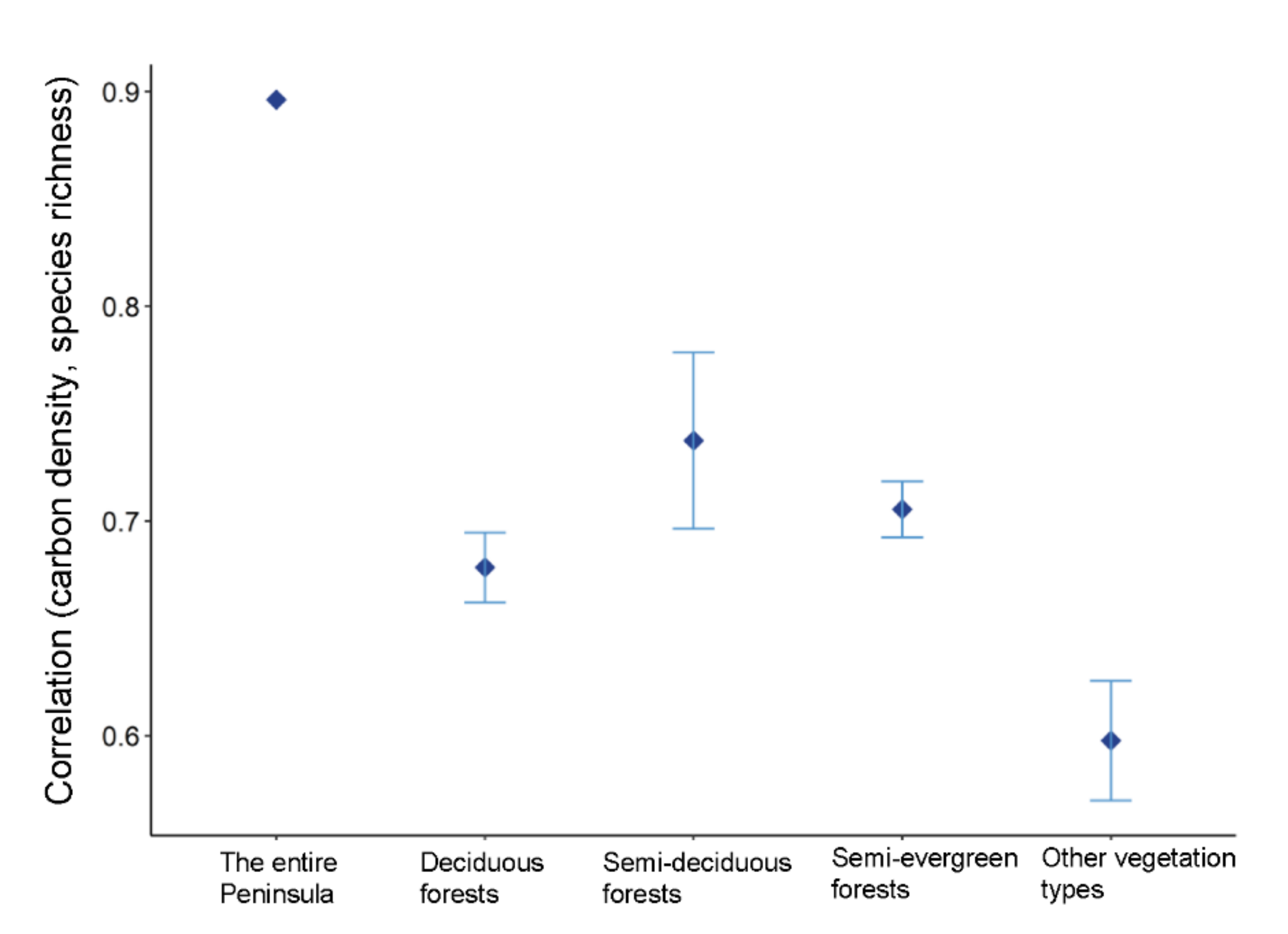
3.5. Relationships between Carbon Density and Species Richness
4. Discussion
4.1. Evaluation of Carbon Density and Species Richness Maps
4.2. Associations between Carbon Density and Species Richness in the Yucatan Peninsula
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poorter, L.; van der Sande, M.T.; Thompson, J.; Arets, E.J.; Alarcón, A.; Álvarez-Sánchez, J.; Ascarrunz, N.; Balvanera, P.; Barajas-Guzmán, G.; Boit, A.; et al. Diversity enhances carbon storage in tropical forests. Glob. Ecol. Biogeogr. 2015, 24, 1314–1328. [Google Scholar] [CrossRef]
- Keith, H.; Mackey, B.G.; Lindenmayer, D.B. Re-evaluation of forest biomass carbon stocks and lessons from the world’s most carbon-dense forests. Proc. Natl. Acad. Sci. USA 2009, 106, 11635–11640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portillo-Quintero, C.A.; Sánchez-Azofeifa, G.A. Extent and conservation of tropical dry forests in the Americas. Biol. Conserv. 2010, 143, 144–155. [Google Scholar] [CrossRef]
- Fine, P.V.A.; Ree, R.H.; Burnham, R.J. The disparity in tree species richness among tropical, temperate, and boreal biomes: The geographical area and age hypothesis. In Tropical Forest Community Ecology; Carson, R.P., Schnitzer, S.A., Eds.; Blackwell: Oxford, UK, 2008; pp. 31–45. [Google Scholar]
- Banda, K.; Delgado-Salinas, A.; Dexter, K.G.; Linares-Palomino, R.; Oliveira-Filho, A.; Prado, D.; Pullan, M.; Quintana, C.; Riina, R.; Rodríguez, G.M.; et al. Plant diversity patterns in neotropical dry forests and their conservation implications. Science 2016, 353, 1383–1387. [Google Scholar] [CrossRef] [Green Version]
- Friedlingstein, P.; Jones, M.W.; O’sullivan, M.; Andrew, R.M.; Hauck, J.; Peters, G.P.; Peters, W.; Pongratz, J.; Sitch, S.; Le Quéré, C.; et al. Global carbon budget 2019. Earth Syst. Sci. Data 2019, 11, 1783–1838. [Google Scholar] [CrossRef] [Green Version]
- Watson, J.E.; Dudley, N.; Segan, D.B.; Hockings, M. The performance and potential of protected areas. Nature 2014, 515, 67–73. [Google Scholar] [CrossRef]
- Saatchi, S.S.; Harris, N.L.; Brown, S.; Lefsky, M.; Mitchard, E.T.; Salas, W.; Zutta, B.R.; Buermann, W.; Lewis, S.L.; Hagen, S.; et al. Benchmark map of forest carbon stocks in tropical regions across three continents. Proc. Natl. Acad. Sci. USA 2011, 108, 9899–9904. [Google Scholar] [CrossRef] [Green Version]
- Baccini, A.G.S.J.; Goetz, S.J.; Walker, W.S.; Laporte, N.T.; Sun, M.; Sulla-Menashe, D.; Hackler, J.; Beck, P.S.A.; Dubayah, R.; Friedl, M.A.; et al. Estimated carbon dioxide emissions from tropical deforestation improved by carbon-density maps. Nat. Clim. Chang. 2012, 2, 182–185. [Google Scholar] [CrossRef]
- Kier, G.; Mutke, J.; Dinerstein, E.; Ricketts, T.H.; Küper, W.; Kreft, H.; Barthlott, W. Global patterns of plant diversity and floristic knowledge. J. Biogeogr. 2005, 32, 1107–1116. [Google Scholar] [CrossRef]
- Kreft, H.; Jetz, W. Global patterns and determinants of vascular plant diversity. Proc. Natl. Acad. Sci. USA 2007, 104, 5925–5930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houghton, R.A.; Hall, F.; Goetz, S.J. Importance of biomass in the global carbon cycle. J. Geophys. Res. Biogeosci. 2009, 114, 1–13. [Google Scholar] [CrossRef]
- Keil, P.; Chase, J.M. Global patterns and drivers of tree diversity integrated across a continuum of spatial grains. Nat. Ecol. Evol. 2019, 3, 390–399. [Google Scholar] [CrossRef]
- Cartus, O.; Kellndorfer, J.; Walker, W.; Franco, C.; Bishop, J.; Santos, L.; Fuentes, J. A national, detailed map of forest aboveground carbon stocks in Mexico. Remote. Sens. 2014, 6, 5559–5588. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Veiga, P.; Saatchi, S.; Tansey, K.; Balzter, H. Magnitude, spatial distribution and uncertainty of forest biomass stocks in Mexico. Remote. Sens. Environ. 2016, 183, 265–281. [Google Scholar] [CrossRef] [Green Version]
- Réjou-Méchain, M.; Barbier, N.; Couteron, P.; Ploton, P.; Vincent, G.; Herold, M.; Mermoz, S.; Saatchi, S.; Chave, J.; de Boissieu, F.; et al. Upscaling Forest biomass from field to satellite measurements: Sources of errors and ways to reduce them. Surv. Geophys. 2019, 40, 881–911. [Google Scholar] [CrossRef]
- Marselis, S.M.; Abernethy, K.; Alonso, A.; Armston, J.; Baker, T.R.; Bastin, J.F.; Bogaert, J.; Boyd, D.S.; Boeckx, P.; Burslem, D.F.R.P.; et al. Evaluating the potential of full-waveform lidar for mapping pan-tropical tree species richness. Glob. Ecol. Biogeogr. 2020, 29, 1799–1816. [Google Scholar] [CrossRef]
- Dupuy, J.M.; Durán García, R.; García Contreras, G.; Arellano Morin, J.; Acosta Lugo, E.; Méndez González, M.E.; Andrade Hernandez, M. Chapter 8: Conservation and Use. In Biodiversity and Conservation of the Yuca-tán Peninsula; Islebe, G.A., Schmook, B., Calmé, S., León-Cortés, J.L., Eds.; Springer: Cham, Switzerland, 2015; pp. 1–5. [Google Scholar]
- Read, L.; Lawrence, D. Recovery of biomass following shifting cultivation in dry tropical forests of the Yucatan. Ecol. Appl. 2003, 13, 85–97. [Google Scholar] [CrossRef] [Green Version]
- Fricker, G.A.; Wolf, J.A.; Saatchi, S.S.; Gillespie, T.W. Predicting spatial variations of tree species richness in trop-ical forests from high-resolution remote sensing. Ecol. Appl. 2015, 25, 1776–1789. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, J.M.; Hernández-Stefanoni, J.L.; Hernández-Juárez, R.A.; Tetetla-Rangel, E.; López-Martínez, J.O.; Leyequién-Abarca, E.; May-Pat, F. Patterns and correlates of tropical dry forest structure and composition in a highly replicated chronosequence in Yucatan, Mexico. Biotropica 2012, 44, 151–162. [Google Scholar] [CrossRef]
- CONAFOR. Inventario nacional forestal y de suelos. Inf. Result. 2018, 1, 2009–2014. [Google Scholar]
- Hernández-Stefanoni, J.L.; Castillo-Santiago, M.Á.; Mas, J.F.; Wheeler, C.E.; Andres-Mauricio, J.; Tun-Dzul, F.; George-Chacón, S.P.; Reyes-Palomeque, G.; Castellanos-Basto, B.; Vaca, R.; et al. Improving aboveground biomass maps of tropical dry forests by integrating LiDAR, ALOS PALSAR, climate and field data. Carbon Balance Manag. 2020, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Veiga, P.; Quegan, S.; Carreiras, J.; Persson, H.J.; Fransson, J.E.; Hoscilo, A.; Ziółkowski, D.; Stereńczak, K.; Lohberger, S.; Stängel, M.; et al. Forest biomass retrieval approaches from earth observation in different biomes. Int. J. Appl. Earth Obs. Geoinf. 2019, 77, 53–68. [Google Scholar] [CrossRef]
- Wang, R.; Gamon, J.A. Remote sensing of terrestrial plant biodiversity. Remote. Sens. Environ. 2019, 231, 111218. [Google Scholar] [CrossRef]
- Ploton, P.; Barbier, N.; Couteron, P.; Antin, C.M.; Ayyappan, N.; Balachandran, N.; Barathan, N.; Bastin, J.F.; Chuyong, G.; Dauby, G.; et al. Toward a general trop-ical forest biomass prediction model from very high resolution optical satellite images. Remote. Sens. Environ. 2017, 200, 140–153. [Google Scholar] [CrossRef]
- Thapa, R.B.; Watanabe, M.; Motohka, T.; Shimada, M. Potential of high-resolution ALOS–PALSAR mosaic texture for aboveground forest carbon tracking in tropical region. Remote. Sens. Environ. 2015, 160, 122–133. [Google Scholar] [CrossRef]
- Joshi, N.; Mitchard, E.T.; Brolly, M.; Schumacher, J.; Fernández-Landa, A.; Johannsen, V.K.; Marchamalo, M.; Fensholt, R. Understanding ‘saturation’of radar signals over forests. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Rocchini, D.; Balkenhol, N.; Carter, G.A.; Foody, G.M.; Gillespie, T.W.; He, K.S.; Kark, S.; Levin, N.; Lucas, K.; Luoto, M.; et al. Remotely sensed spectral heterogeneity as a proxy of species diversity: Recent advances and open challenges. Ecol. Inform. 2010, 5, 318–329. [Google Scholar] [CrossRef]
- Stein, A.; Gerstner, K.; Kreft, H. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 2014, 17, 866–880. [Google Scholar] [CrossRef] [PubMed]
- George-Chacon, S.P.; Dupuy, J.M.; Peduzzi, A.; Hernandez-Stefanoni, J.L. Combining high resolution satellite imagery and lidar data to model woody species diversity of tropical dry forests. Ecol. Indic. 2019, 101, 975–984. [Google Scholar] [CrossRef]
- Poorter, L.; Bongers, F.; Aide, T.M.; Zambrano, A.M.A.; Balvanera, P.; Becknell, J.M.; Boukili, V.; Brancalion, P.H.S.; Broadbent, E.N.; Chazdon, R.L.; et al. Biomass resili-ence of Neotropical secondary forests. Nature (London) 2016, 530, 211–214. [Google Scholar] [CrossRef]
- Rozendaal, D.M.A.; Bongers, F.; Aide, T.M.; Alvarez-Dávila, E.; Ascarrunz, N.; Balvanera, P.; Becknell, J.M.; Bentos, T.V.; Brancalion, P.H.S.; Cabral, G.A.L.; et al. Biodiversity recovery of Neotropical secondary forests. Sci. Adv. 2019, 5, eaau3114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittelbach, G.G.; Steiner, C.F.; Scheiner, S.M.; Gross, K.L.; Reynolds, H.L.; Waide, R.B.; Willig, W.R.; Dodson, S.I.; Gough, L. What is the observed relationship between species richness and productivity? Ecology 2001, 82, 2381–2396. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Talbot, J.; Lewis, S.L.; Phillips, O.L.; Qie, L.; Begne, S.K.; Chave, J.; Cuni-Sanchez, A.; Hubau, W.; Lopez-Gonzalez, G.; et al. Diversity and carbon storage across the tropical forest biome. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Murray, J.P.; Grenyer, R.; Wunder, S.; Raes, N.; Jones, J.P. Spatial patterns of carbon, biodiversity, deforestation threat, and REDD+ projects in Indonesia. Conserv. Biol. 2005, 29, 1434–1445. [Google Scholar] [CrossRef] [Green Version]
- Ramírez Ramírez, G.; Dupuy Rada, J.M.; Ramírez y Avilés, L.; Solorio Sánchez, F.J. Evaluación de ecuaciones alométricas de biomasa epigea en una selva mediana subcaducifolia de Yucatán. Madera bosques 2017, 23, 163–179. [Google Scholar] [CrossRef] [Green Version]
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.Q.; Eamus, D.; Fölster, H.; Fromard, F.; Higuchi, N.; Kira, T.; et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef]
- Guyot, J. Estimation du Stock de Carbone dans la Végétation des Zones Humides de la Péninsule du Yucatan. Memoire de fin d’etudes. Licentiate Thesis, AgroParis Tech-El Colegio de la Frontera Sur, Chiapas, Mexico, 2011; p. 110. [Google Scholar]
- Cairns, M.A.; Olmsted, I.; Granados, J.; Argaez, J. Composition and aboveground tree biomass of a dry semi-evergreen forest on Mexico’s Yucatan Peninsula. For. Ecol. Manag. 2003, 186, 125–132. [Google Scholar] [CrossRef]
- Urquiza-Haas, T.; Dolman, P.M.; Peres, C.A. Regional scale variation in forest structure and biomass in the Yucatan Peninsula, Mexico: Effects of forest disturbance. For. Ecol. Manag. 2007, 247, 80–90. [Google Scholar] [CrossRef]
- Chave, J.; Condit, R.; Lao, S.; Caspersen, J.P.; Foster, R.B.; Hubbell, S.P. Spatial and temporal variation of biomass in a tropical forest: Results from a large census plot in Panama. J. Ecol. 2003, 91, 240–252. [Google Scholar] [CrossRef]
- Frangi, J.L.; Lugo, A.E. Ecosystem dynamics of a subtropical floodplain forest. Ecol. Monogr. 1985, 55, 351–369. [Google Scholar] [CrossRef]
- Shimada, M.; Ohtaki, T. Generating Large-Scale High-Quality SAR Mosaic Datasets: Application to PALSAR Data for Global Monitoring. IEEE J. Sel. Top. Appl. Earth Obs. Remote. Sens. 2010, 3, 637–656. [Google Scholar] [CrossRef]
- Almeida-Filho, R.; Shimabukuro, Y.E.; Rosenqvist, A.; Sánchez, G.A. Using dual-polarized ALOS PALSAR data for detecting new fronts of deforestation in the Brazilian Amazônia. Int. J. Remote. Sens. 2009, 30, 3735–3743. [Google Scholar] [CrossRef]
- Mitchard, E.T.A.; Saatchi, S.S.; White, L.J.T.; Abernethy, K.A.; Jeffery, K.J.; Lewis, S.L.; Collins, M.; Lefsky, M.A.; Leal, M.E.; Woodhouse, I.H.; et al. Mapping tropical forest biomass with radar and spaceborne LiDAR in Lopé National Park, Gabon: Overcoming problems of high biomass and persistent cloud. Biogeosciences 2012, 9, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Haralick, R.M.; Dinstein, I.; Shanmugam, K. Textural Features for Image Classification. IEEE Trans. Syst. Man Cybern. 1973, SMC-3, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Fischer, R.; Knapp, N.; Bohn, F.; Shugart, H.H.; Huth, A. The relevance of forest structure for biomass and productivity in temperate forests: New perspectives for remote sensing. Surv. Geophys. 2019, 40, 709–734. [Google Scholar] [CrossRef]
- Zvoleff, A. Calculate Textures from Grey-Level Co-Occurrence Matrices (GLCMs); R Package v 1.6.4; R. Package: Arlington, VA, USA, 2019. [Google Scholar]
- Freeman, E.A.; Frescino, T.S.; Moisen, G.G. ModelMap: And R Package for Model Creation and Map Production. 2018, p. 18. Available online: https://cran.r-project.org/web/packages/ModelMap/vignettes/VModelMap (accessed on 10 August 2021).
- INEGI. Conjunto Nacional de Uso del Suelo y Vegetación a escala 1:250,000, Serie IV; INEGI: Aguascalientes, Mexico, 2010. [Google Scholar]
- Borcard, D.; Legendre, P.; Avois-Jacquet, C.; Tuomisto, H. Disecting the spatial structure of ecological data al multiple scales. Ecology 2004, 85, 1826–1832. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Saatchi, S.S.; Shapiro, A.; Meyer, V.; Ferraz, A.; Yang, Y.; Bastin, J.F.; Banks, N.; Boeckx, P.; Verbeeck, H.; et al. Spatial distribution of carbon stored in forests of the Democratic Republic of Congo. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Guo, Q.; Xue, B.; Hu, T.; Alvarez, O.; Tao, S.; Fang, J. Spatial distribution of forest aboveground biomass in China: Estimation through combination of spaceborne lidar, optical imagery, and forest inventory data. Remote. Sens. Environ. 2016, 173, 187–199. [Google Scholar] [CrossRef] [Green Version]
- Santoro, M.; Cartus, O.; Carvalhais, N.; Rozendaal, D.; Avitabilie, V.; Araza, A.; De Bruin, S.; Herold, M.; Quegan, S.; Veiga, P.R.; et al. The global forest above-ground biomass pool for 2010 estimated from high-resolution satellite observations. Earth Syst. Sci. Data Discuss. 2020, 148, 1–38. [Google Scholar] [CrossRef]
- Luther, J.E.; Fournier, R.A.; van Lier, O.R.; Bujold, M. Extending ALS-based mapping of forest attributes with medium resolution satellite and environmental data. Remote. Sens. 2019, 11, 1092. [Google Scholar] [CrossRef] [Green Version]
- Cook, B.; Nelson, R.; Middleton, E.; Morton, D.; McCorkel, J.; Masek, J.; Ranson, K.; Ly, V.; Montesano, P. NASA Goddard’s LiDAR, hyperspectral and thermal (G-LiHT) airborne imager. Remote. Sens. 2013, 5, 4045–4066. [Google Scholar] [CrossRef] [Green Version]
- Urbazaev, M.; Thiel, C.; Cremer, F.; Dubayah, R.; Migliavacca, M.; Reichstein, M.; Schmullius, C. Estimation of forest aboveground biomass and uncertainties by integration of field measurements, airborne LiDAR, and SAR and optical satellite data in Mexico. Carbon Balance Manag. 2018, 13, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mermoz, S.; Réjou-Méchain, M.; Villard, L.; Le Toan, T.; Rossi, V.; Gourlet-Fleury, S. Decrease of L-band SAR backscatter with biomass of dense forests. Remote. Sens. Environ. 2015, 159, 307–317. [Google Scholar] [CrossRef]
- Morel, A.C.; Saatchi, S.S.; Malhi, Y.; Berry, N.J.; Banin, L.; Burslem, D.; Nilus, R.; Ong, R.C. Estimating aboveground biomass in forest and oil palm plantation in Sabah, Malaysian Borneo using ALOS PALSAR data. For. Ecol. Manag. 2011, 262, 1786–1798. [Google Scholar] [CrossRef]
- Champion, I.; Germain, C.; Da Costa, J.P.; Alborini, A.; Dubois-Fernandez, P. Retrieval of forest stand age from SAR image texture for varying distance and orientation values of the gray level co-occurrence matrix. IEEE Geosci. Remote. Sens-Ing Lett. 2014, 11, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Sarker, M.L.R.; Nichol, J.; Iz, H.B.; Ahmad, B.B.; Rahman, A.A. Forest biomass estimation using texture measurements of high-resolution dual-polarization C-band SAR data. IEEE Trans. Geosci. Remote. Sens. 2013, 51, 3371–3384. [Google Scholar] [CrossRef]
- Andres-Mauricio, J.; Valdez-Lazalde, J.R.; George-Chacón, S.P.; Hernández-Stefanoni, J.L. Mapping structural attributes of tropical dry forests by combining Synthetic Aperture Radar and high-resolution satellite imagery data. Appl. Veg. Sci. 2021, 24, e12580. [Google Scholar] [CrossRef]
- Rocchini, D.; Boyd, D.S.; Féret, J.B.; Foody, G.M.; He, K.S.; Lausch, A.; Nagendra, H.; Wegmann, M.; Pettorelli, N. Satellite remote sensing to monitor species diversity: Potential and pitfalls. Remote. Sens. Ecol. Conserv. 2016, 2, 25–36. [Google Scholar] [CrossRef]
- Palmer, M.W.; Earls, P.G.; Hoagland, B.W.; White, P.S.; Wohlgemuth, T. Quantitative tools for perfecting species lists. Environmetrics 2002, 13, 121–137. [Google Scholar] [CrossRef]
- Soto-Navarro, C.; Ravilious, C.; Arnell, A.; De Lamo, X.; Harfoot, M.; Hill, S.L.L.; Wearn, O.R.; Santoro, M.; Bouvet, A.; Mermoz, S.; et al. Mapping co-benefits for carbon storage and biodiversity to inform conservation policy and action. Philos. Trans. R. Soc. B 2020, 375, 20190128. [Google Scholar] [CrossRef] [Green Version]
- Strassburg, B.B.; Kelly, A.; Balmford, A.; Davies, R.G.; Gibbs, H.K.; Lovett, A.; Miles, L.; Orme, C.D.L.; Price, J.; Turner, R.K.; et al. Global congruence of carbon storage and biodiversity in terrestrial ecosystems. Conserv. Lett. 2010, 3, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Cavanaugh, K.C.; Gosnell, J.S.; Davis, S.L.; Ahumada, J.; Boundja, P.; Clark, D.B.; Mugerwa, B.; Jansen, P.A.; O’Brien, T.G.; Rovero, F.; et al. Carbon storage in tropical forests correlates with taxonomic diversity and functional dominance on a global scale. Glob. Ecol. Biogeog-Raphy 2014, 23, 563–573. [Google Scholar] [CrossRef]
- Di Marco, M.; Watson, J.E.; Currie, D.J.; Possingham, H.P.; Venter, O. The extent and predictability of the biodiversity–carbon correlation. Ecol. Lett. 2018, 21, 365–375. [Google Scholar] [CrossRef] [Green Version]

| Author of Equation | Type of Vegetation | Growth Form/Class Size |
|---|---|---|
| Ramírez et al. [37] | Deciduos and semi-deciduous forest | Tree/DBH < 10 cm |
| Chave et al. [38] | Deciduos and semi-deciduous forest | Tree/DBH ≥ 10 cm |
| Guyot [39] | Semi-evergreen forest | Tree/DBH < 10 cm |
| Cairns modified [40] by Urquiza-Haas et al. [41] | Semi-evergreen forest | Tree/DBH ≥ 10 cm |
| Chave et al. [42] | Mangrove forest | Tree |
| Chave et al. [42] | Deciduos and semi-deciduos forest | Liana |
| Frangi and Lugo [43] | Deciduos, semi-deciduos and semi-evergreen forest | Palm/DBH ≥ 10 cm |
| Variable | Type of Data | Number of Plots | R2 | RMSE | %RMSE |
|---|---|---|---|---|---|
| AGB | Calibration | 1993 | 0.16 | 47.6 | 38.1 |
| Validation | 854 | 0.28 | 45.4 | 38.5 | |
| Species Richness | Calibration | 1993 | 0.12 | 12.0 | 31.9 |
| Validation | 854 | 0.31 | 11.72 | 33.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Stefanoni, J.L.; Castillo-Santiago, M.Á.; Andres-Mauricio, J.; Portillo-Quintero, C.A.; Tun-Dzul, F.; Dupuy, J.M. Carbon Stocks, Species Diversity and Their Spatial Relationships in the Yucatán Peninsula, Mexico. Remote Sens. 2021, 13, 3179. https://doi.org/10.3390/rs13163179
Hernández-Stefanoni JL, Castillo-Santiago MÁ, Andres-Mauricio J, Portillo-Quintero CA, Tun-Dzul F, Dupuy JM. Carbon Stocks, Species Diversity and Their Spatial Relationships in the Yucatán Peninsula, Mexico. Remote Sensing. 2021; 13(16):3179. https://doi.org/10.3390/rs13163179
Chicago/Turabian StyleHernández-Stefanoni, José Luis, Miguel Ángel Castillo-Santiago, Juan Andres-Mauricio, Carlos A. Portillo-Quintero, Fernando Tun-Dzul, and Juan Manuel Dupuy. 2021. "Carbon Stocks, Species Diversity and Their Spatial Relationships in the Yucatán Peninsula, Mexico" Remote Sensing 13, no. 16: 3179. https://doi.org/10.3390/rs13163179
APA StyleHernández-Stefanoni, J. L., Castillo-Santiago, M. Á., Andres-Mauricio, J., Portillo-Quintero, C. A., Tun-Dzul, F., & Dupuy, J. M. (2021). Carbon Stocks, Species Diversity and Their Spatial Relationships in the Yucatán Peninsula, Mexico. Remote Sensing, 13(16), 3179. https://doi.org/10.3390/rs13163179







