Characterizing Spatial Patterns of Pine Wood Nematode Outbreaks in Subtropical Zone in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Methods
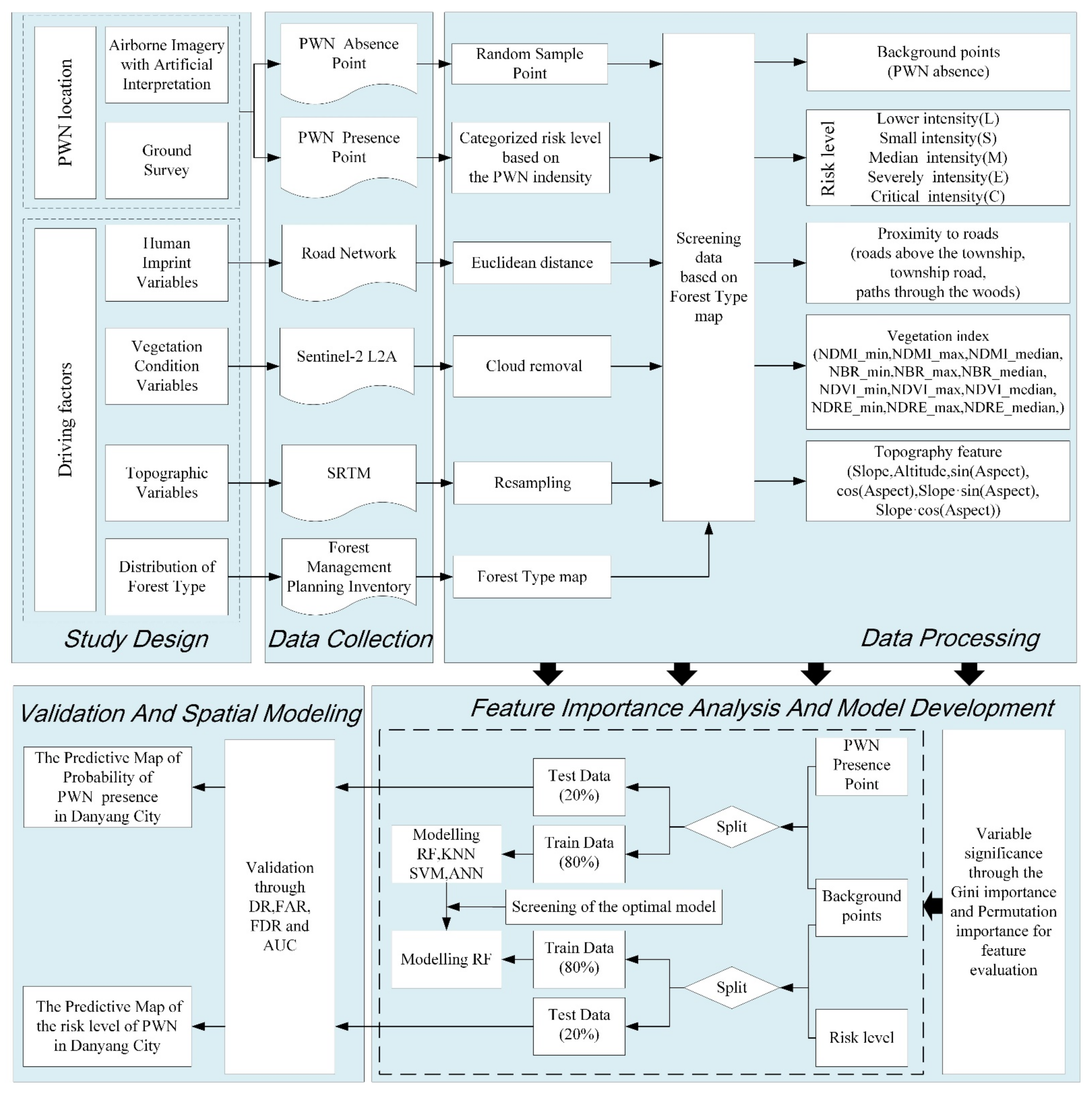
2.2.1. Study Design
2.2.2. Data Collection
2.2.3. Data Processing
2.2.4. Feature Importance Analysis and Model Development
2.2.5. Validation and Spatial Modeling
3. Results
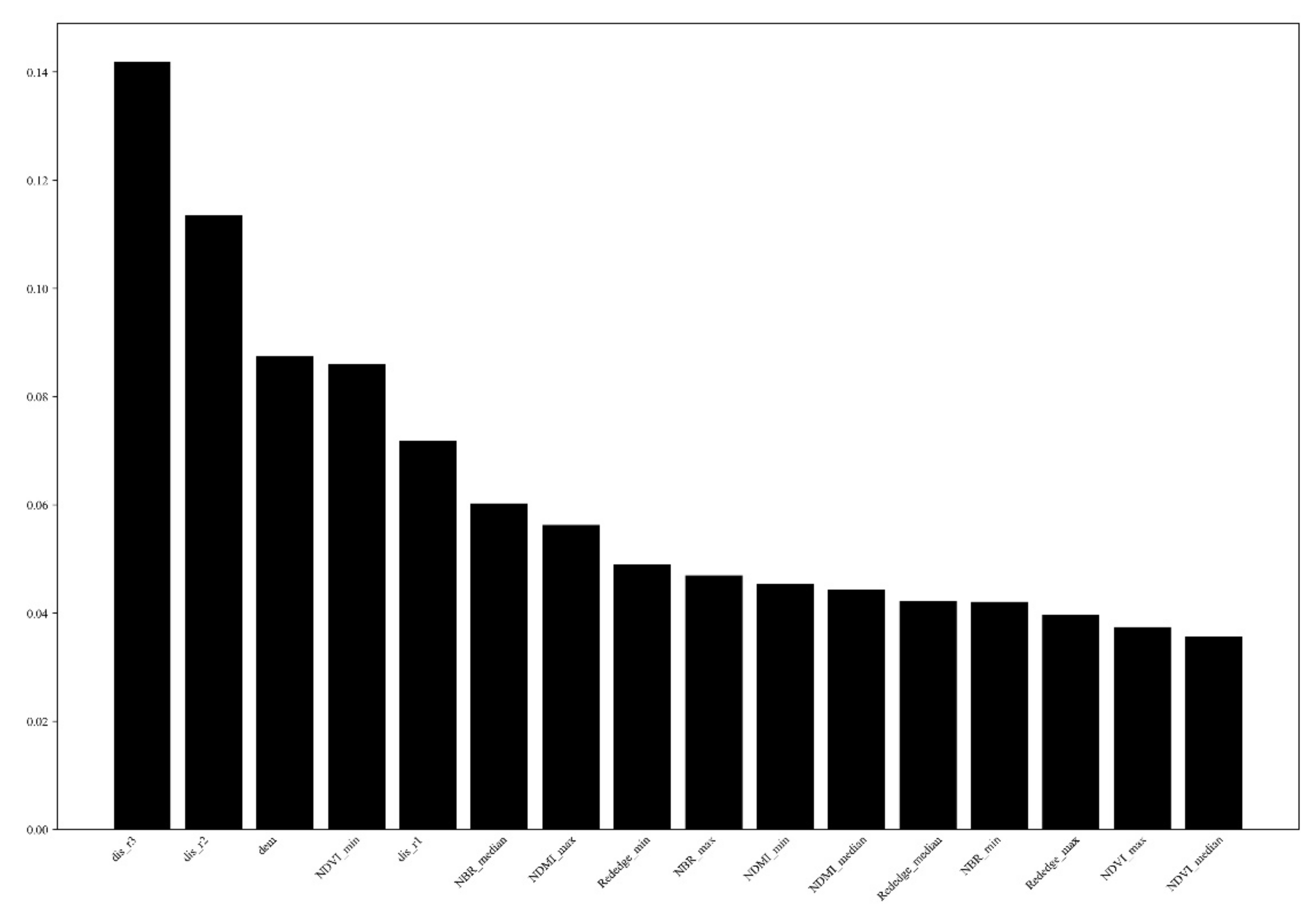
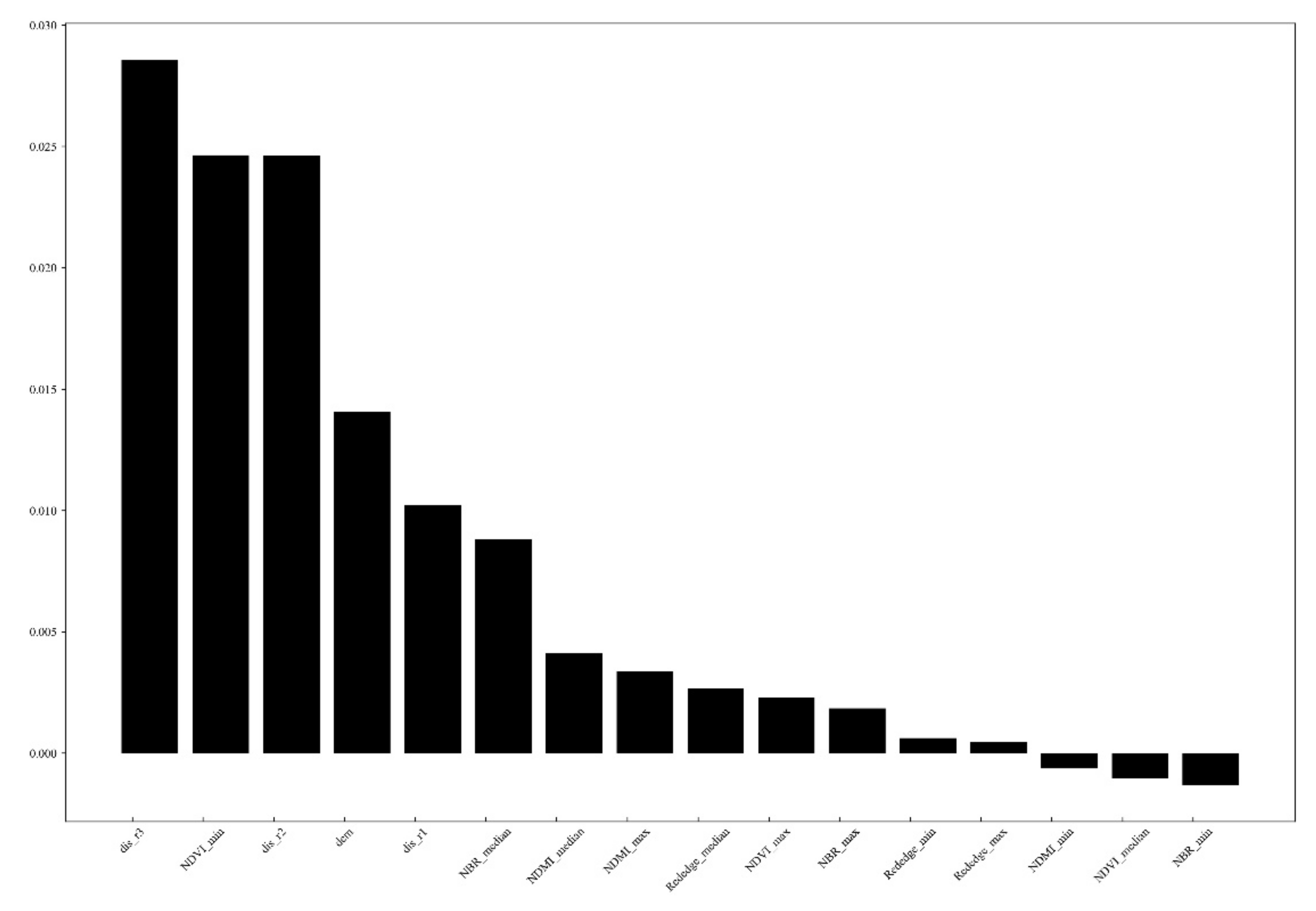
3.1. Variable Importance Analysis
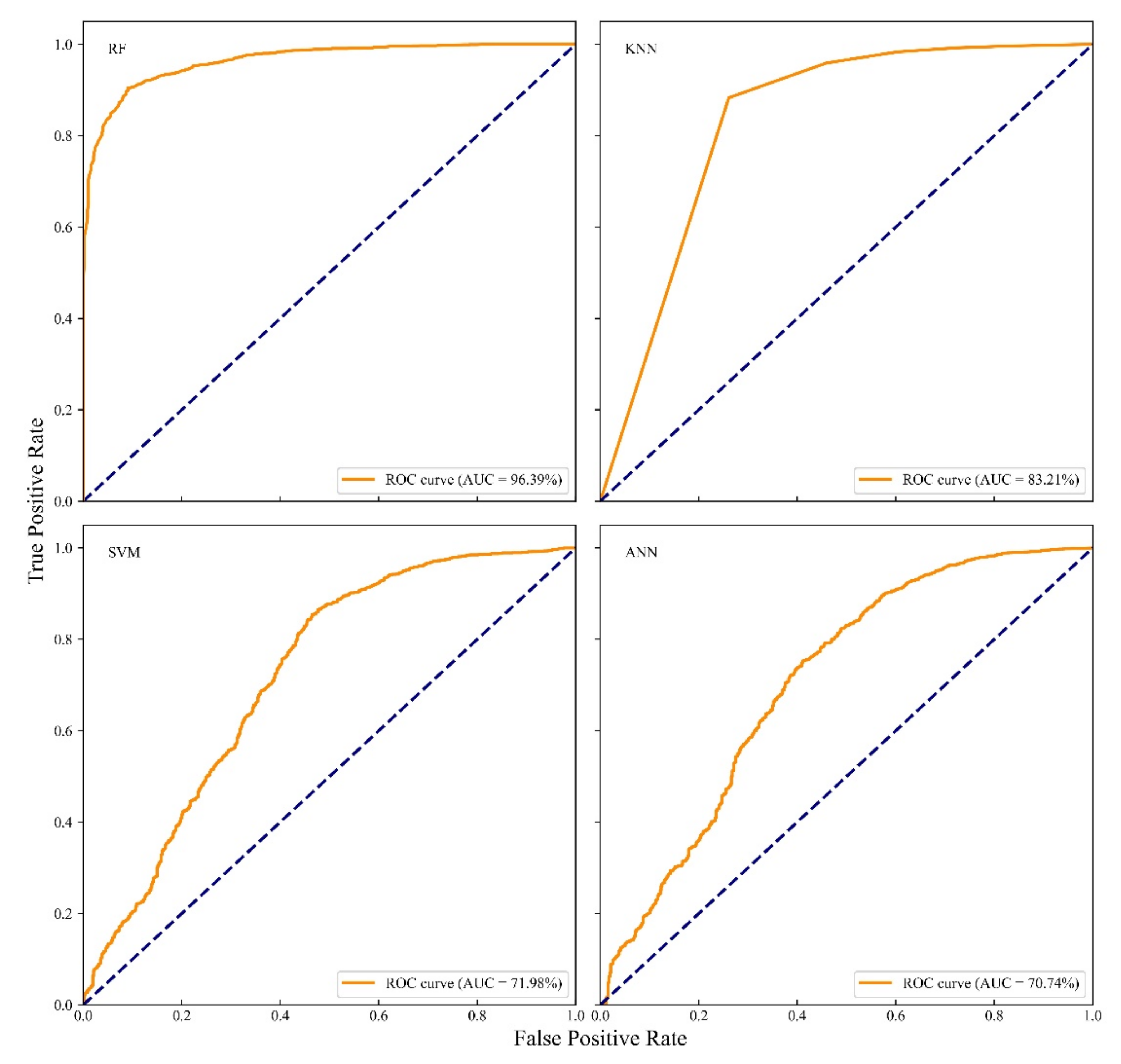
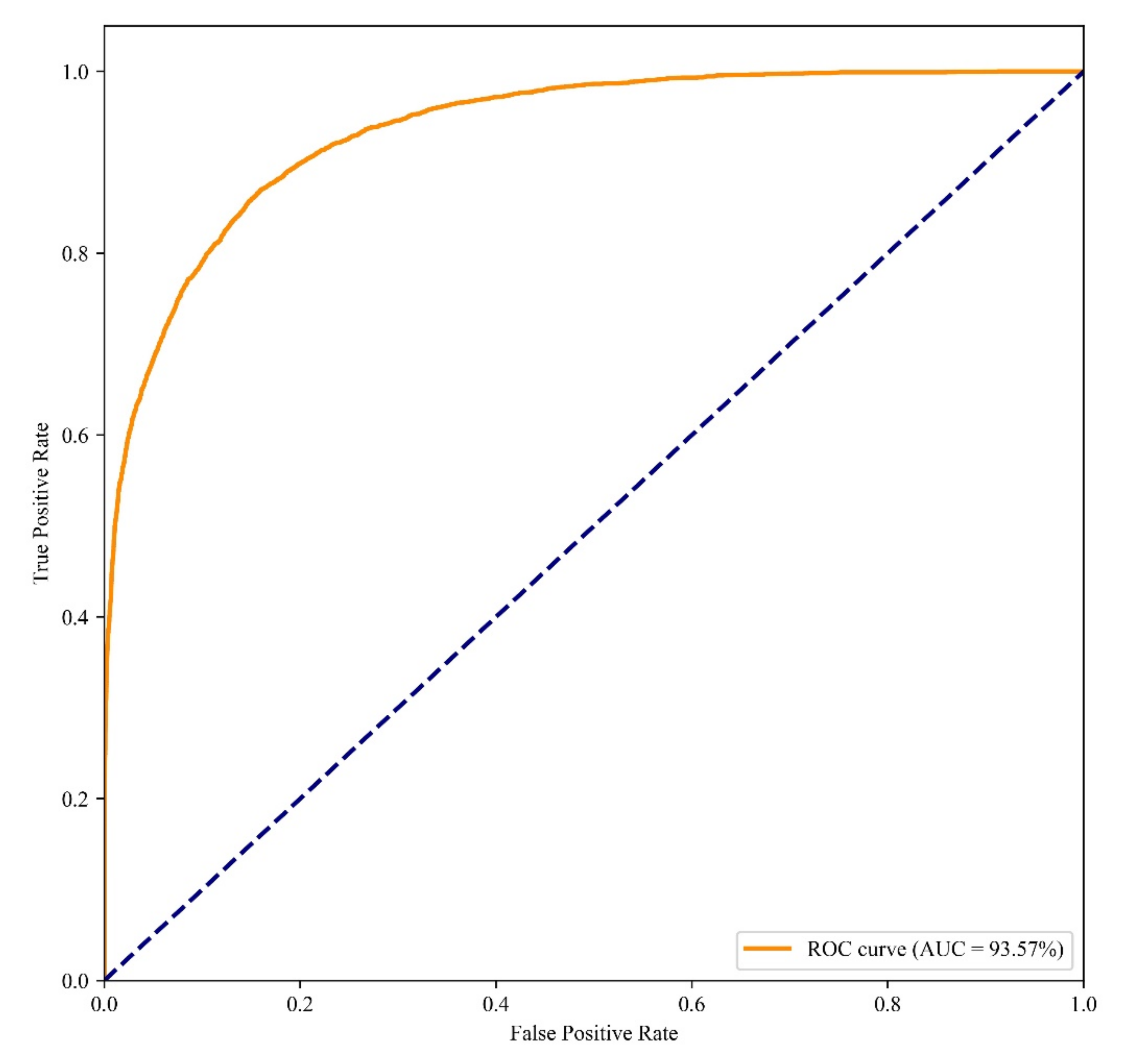
3.2. Comparison of Different Models
3.3. Analysis of Risk Levels
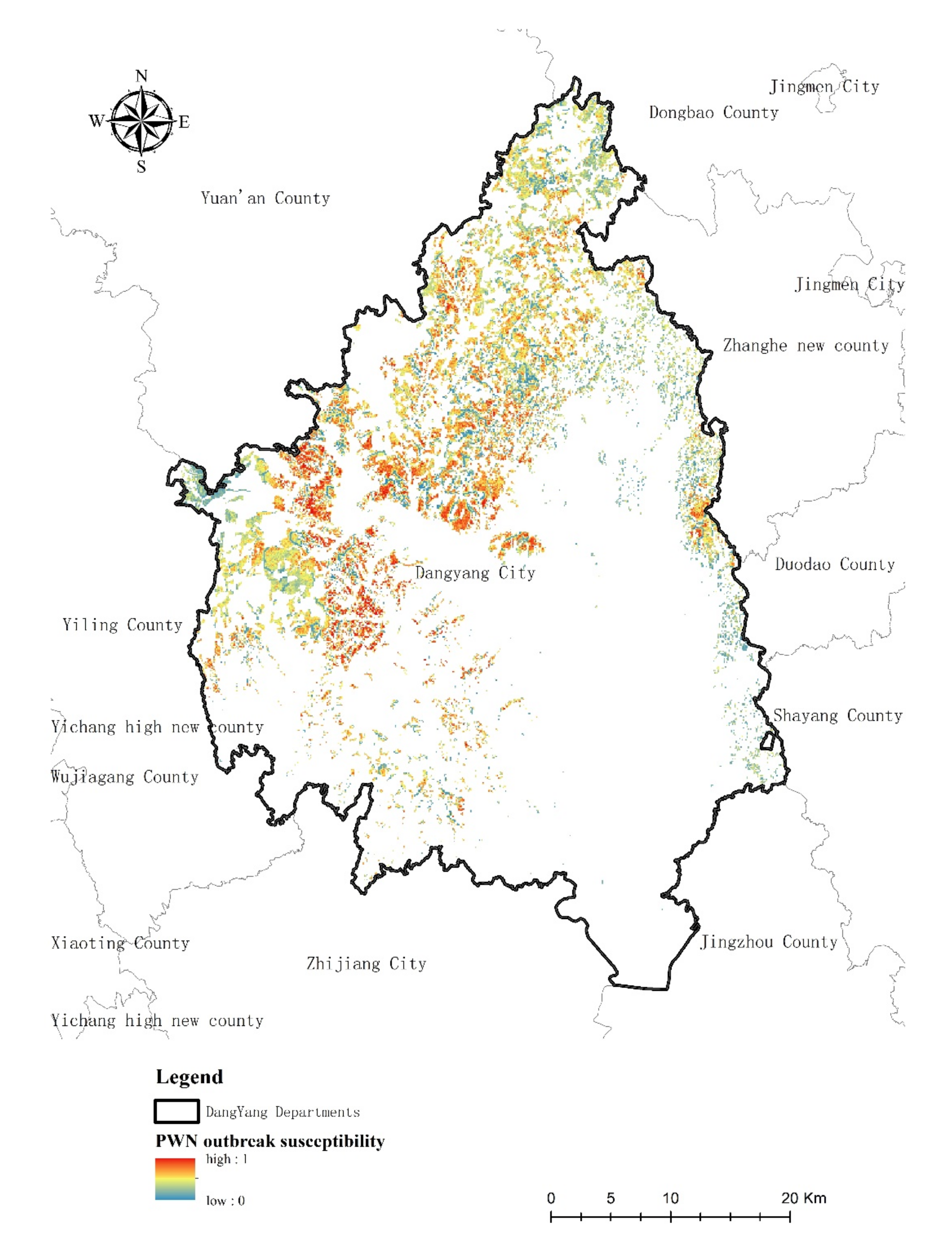
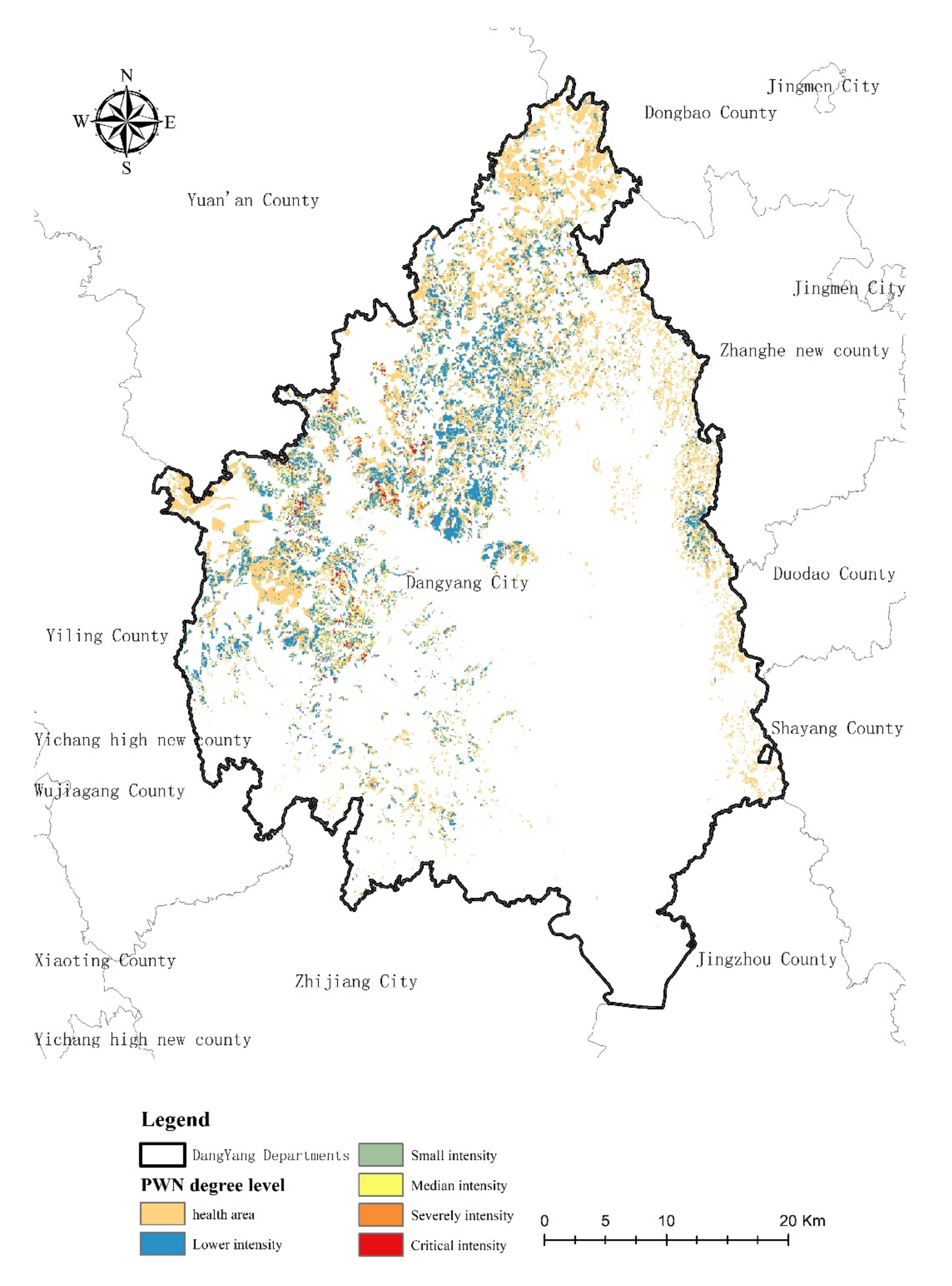
3.4. PWN Risk Levels Distribution in Research Area
4. Discussion
4.1. Analysis and Optimization of the Predictor Variables
4.2. Models Performance and Evaluation
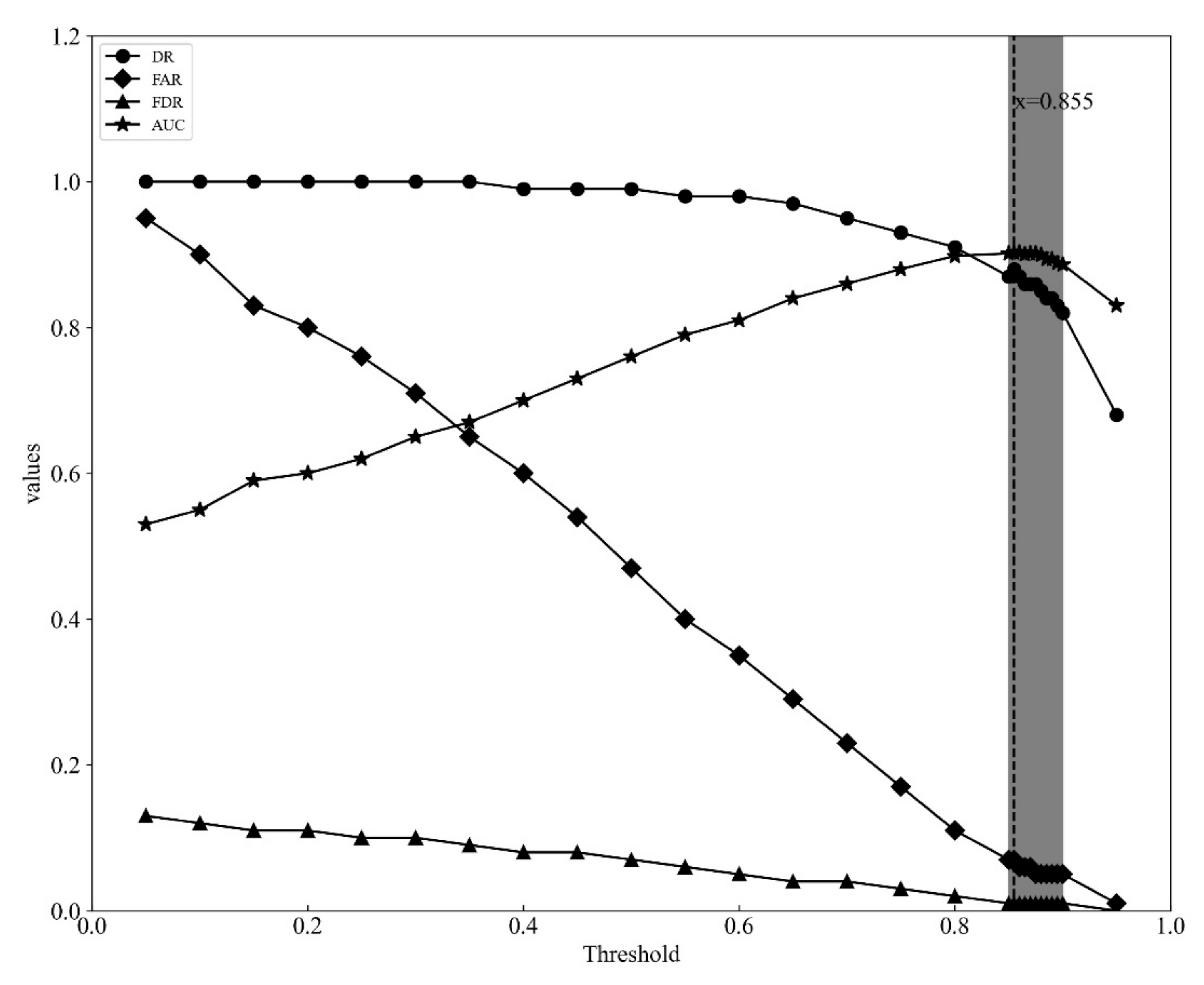
4.3. Threshold for Model
4.4. The Risk Level of PWN Classification
5. Conclusions
- (1)
- It is possible to achieve the prediction probability of the presence of PWN in a large extended area with remote sensing data combined with topography, anthropogenic activities, and other variables. The overall DR can be up to 96%, FAR lower than 28%, FDR lower than 5%. Moreover, different risk levels of PWN have a certain predictive effect, especially for areas with a high risk level. Different predictor variables have different effects on PWN susceptibility, and in the Dangyang region, PWN outbreaks are highly correlated with anthropogenic activity factors.
- (2)
- Different models have different performances on the prediction of PWN. The performance of the different models is sensitive to many factors as shown in our evaluation, such as the selection of hyper-parameter, the use of training and testing datasets. In this study, we found that the RF method consistently outperforms other models that we used. Therefore, we recommend using RF first in similar applications, and only tires other models if the FR cannot provide the modeling result with sufficient accuracy.
- (3)
- The threshold value plays an important role in model performance, which balances the trade-off between true and false detection rates. However, the selection of optimal threshold value will depend on the context and can be difficult, similar to selecting optimal hyperparameters for a machine learning algorithm.
- (4)
- The predictor variables showed different importance in predicting PWN. The distance to path through the wood and distance to township roads and the elevation and minimal value of NDVI were was the most relevant explanatory variables, followed by the distance to above township roads, and other vegetation indices, the topographic variables such as slope, aspect showed the least importance. Based on the results of the study, it is inferred that the source of the initial PWN disease in the region may have been brought in by anthropogenic activities.
Author Contributions
Funding
Conflicts of Interest
References
- Suzuki, K. PATHOLOGY|Pine Wilt and the Pine Wood Nematode. Encyclopedia of Forest Sciences; Elsevier: Amsterdam, The Netherlands, 2004; pp. 773–777. [Google Scholar]
- Mota, M.M.; Vieira, P. (Eds.) Pine Wilt Disease: A Worldwide Threat to Forest Ecosystems; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Hulme, P.E. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Perrings, C.; Dehnen-Schmutz, K.; Touza, J.; Williamson, M. How to manage biological invasions under globalization. Trends Ecol. Evol. 2005, 20, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Juan, S.; Youqing, L.; Haiwei, W.; Heliovaara, K.; Lizhuang, L. Impact of the invasion by Bursaphelenchus xylophilus on forest growth and related growth models of Pinus massoniana population. Acta Ecol. Sin. 2008, 28, 3193–3204. [Google Scholar] [CrossRef]
- Ferrenberg, S. Landscape Features and Processes Influencing Forest Pest Dynamics. Curr. Landsc. Ecol. Rep. 2016, 1, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, L.; Cardoso, J.; Lopes, A.; Pestana, M.; Abreu, F.; Nunes, N.; Mota, M.; Abrantes, I. The pinewood nematode, Bursaphelenchus xylophilus, in Madeira Island. Helminthologia 2012, 49, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.G.; Chung, M.S.; DeMarsilis, A.J.; Holland, C.K.; Jaswaney, R.V.; Jiang, C.; Kroboth, J.H.; Kulshrestha, K.; Marcelo, R.Z.; Meyyappa, V.M.; et al. Structural and biochemical analysis of phosphoethanolamine methyltransferase from the pine wilt nematode Bursaphelenchus xylophilus. Mol. Biochem. Parasitol. 2020, 238, 111291. [Google Scholar] [CrossRef]
- Kim, A.Y.; Osabutey, A.F.; Yoon, K.A.; Choi, B.-H.; Lee, S.-H.; Han, H.R.; Koh, Y.H. Identification of Aldose Reductase 1 as a pine wood nematode secretory enzyme and generation and characterization of its monoclonal antibodies. J. Asia-Pac. Èntomol. 2018, 22, 233–238. [Google Scholar] [CrossRef]
- Chiluwal, K.; Roh, G.H.; Kim, J.; Park, C.G. Acaricidal activity of the aggregation pheromone of Japanese pine sawyer against two-spotted spider mite. J. Asia-Pac. Èntomol. 2019, 23, 86–90. [Google Scholar] [CrossRef]
- Gastón, A.; Viñas, J.I.G. Modelling species distributions with penalised logistic regressions: A comparison with maximum entropy models. Ecol. Model. 2011, 222, 2037–2041. [Google Scholar] [CrossRef]
- Louis, M.; Toffin, E.; Gregoire, J.-C.; Deneubourg, J.-L. Modelling collective foraging in endemic bark beetle populations. Ecol. Model. 2016, 337, 188–199. [Google Scholar] [CrossRef]
- Bonneau, M.; Johnson, F.A.; Romagosa, C.M. Spatially explicit control of invasive species using a reaction–diffusion model. Ecol. Model. 2016, 337, 15–24. [Google Scholar] [CrossRef]
- Mulder, O.; Sleith, R.; Mulder, K.; Coe, N.R. A Bayesian analysis of topographic influences on the presence and severity of beech bark disease. For. Ecol. Manag. 2020, 472, 118198. [Google Scholar] [CrossRef]
- Kamińska, A.; Lisiewicz, M.; Kraszewski, B.; Stereńczak, K. Habitat and stand factors related to spatial dynamics of Norway spruce dieback driven by Ips typographus (L.) in the Białowieża Forest District. For. Ecol. Manag. 2020, 476, 118432. [Google Scholar] [CrossRef]
- Calvão, T.; Duarte, C.M.; Pimentel, C.S. Climate and landscape patterns of pine forest decline after invasion by the pinewood nematode. For. Ecol. Manag. 2019, 433, 43–51. [Google Scholar] [CrossRef]
- Ikegami, M.; Jenkins, T.A. Estimate global risks of a forest disease under current and future climates using species distribution model and simple thermal model—Pine Wilt disease as a model case. For. Ecol. Manag. 2018, 409, 343–352. [Google Scholar] [CrossRef]
- Togashi, K.; Jikumaru, S. Evolutionary change in a pine wilt system following the invasion of Japan by the pinewood nematode, Bursaphelenchus xylophilus. Ecol. Res. 2007, 22, 862–868. [Google Scholar] [CrossRef]
- Syifa, M.; Park, S.-J.; Lee, C.-W. Detection of the Pine Wilt Disease Tree Candidates for Drone Remote Sensing Using Artificial Intelligence Techniques. Engineering 2020, 6, 919–926. [Google Scholar] [CrossRef]
- Wu, B.; Liang, A.; Zhang, H.; Zhu, T.; Zou, Z.; Yang, D.; Tang, W.; Li, J.; Su, J. Application of conventional UAV-based high-throughput object detection to the early diagnosis of pine wilt disease by deep learning. For. Ecol. Manag. 2021, 486, 118986. [Google Scholar] [CrossRef]
- Vasquez, M.C.V.; Chen, C.-F.; Lin, Y.-J.; Kuo, Y.-C.; Chen, Y.-Y.; Medina, D.; Diaz, K. Characterizing spatial patterns of pine bark beetle outbreaks during the dry and rainy season’s in Honduras with the aid of geographic information systems and remote sensing data. For. Ecol. Manag. 2020, 467, 118162. [Google Scholar] [CrossRef]
- Assal, T.; Sibold, J.; Reich, R. Modeling a Historical Mountain Pine Beetle Outbreak Using Landsat MSS and Multiple Lines of Evidence. Remote Sens. Environ. 2014, 155, 275–288. [Google Scholar] [CrossRef]
- Hu, G.; Yin, C.; Wan, M.; Zhang, Y.; Fang, Y. Recognition of diseased Pinus trees in UAV images using deep learning and AdaBoost classifier. Biosyst. Eng. 2020, 194, 138–151. [Google Scholar] [CrossRef]
- Sylvain, J.-D.; Drolet, G.; Brown, N. Mapping dead forest cover using a deep convolutional neural network and digital aerial photography. ISPRS J. Photogramm. Remote Sens. 2019, 156, 14–26. [Google Scholar] [CrossRef]
- Senf, C.; Seidl, R.; Hostert, P. Remote sensing of forest insect disturbances: Current state and future directions. Int. J. Appl. Earth Obs. Geoinf. 2017, 60, 49–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, R.; Castilla, G.; White, J.; Cooke, B.; Skakun, R. Remote sensing of forest pest damage: A review and lessons learned from a Canadian perspective. Can. Èntomol. 2016, 148, S296–S356. [Google Scholar] [CrossRef]
- Eitel, J.U.H.; Vierling, L.A.; Litvak, M.E.; Long, D.S.; Schulthess, U.; Ager, A.A.; Krofcheck, D.J.; Stoscheck, L. Broadband, red-edge information from satellites improves early stress detection in a New Mexico conifer woodland. Remote Sens. Environ. 2011, 115, 3640–3646. [Google Scholar] [CrossRef]
- Shin, S.-C. Pine Wilt Disease in Korea. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, R.J., Takeuchi, Y., Eds.; Springer: Tokyo, Japan, 2008; pp. 26–32. [Google Scholar]
- Yang, Z.-Q.; Wang, X.; Zhang, Y.-N. Recent advances in biological control of important native and invasive forest pests in China. Biol. Control 2014, 68, 117–128. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-resolution global maps of 21st-century forest cover change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef] [Green Version]
- Kenji, F.; Taizo, H.; Kazuo, S. Photosynthesis and Water Status of Pine-Wood Nematode-Infected Pine Seedlings. J. Jpn. For. Soc. 1992, 74, 1–8. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.; Hornero, A.; Beck, P.; Kattenborn, T.; Kempeneers, P.; Hernández-Clemente, R. Chlorophyll content estimation in an open-canopy conifer forest with Sentinel-2A and hyperspectral imagery in the context of forest decline. Remote Sens. Environ. 2019, 223, 320–335. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Sader, S.A. Comparison of time series tasseled cap wetness and the normalized difference moisture index in detecting forest disturbances. Remote Sens. Environ. 2005, 94, 364–372. [Google Scholar] [CrossRef]
- Datt, B. Remote Sensing of Chlorophyll a, Chlorophyll b, Chlorophyll a+b, and Total Carotenoid Content in Eucalyptus Leaves. Remote Sens. Environ. 1998, 66, 111–121. [Google Scholar] [CrossRef]
- Gao, B.-C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- White, J.; Wulder, M.; Hermosilla, T.; Coops, N.C.; Hobart, G.W. A nationwide annual characterization of 25 years of forest disturbance and recovery for Canada using Landsat time series. Remote Sens. Environ. 2017, 194, 303–321. [Google Scholar] [CrossRef]
- Verbesselt, J.; Hyndman, R.; Newnham, G.; Culvenor, D. Detecting trend and seasonal changes in satellite image time series. Remote Sens. Environ. 2010, 114, 106–115. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Fassnacht, F.E.; Latifi, H.; Ghosh, A.; Joshi, P.K.; Koch, B. Assessing the potential of hyperspectral imagery to map bark beetle-induced tree mortality. Remote Sens. Environ. 2014, 140, 533–548. [Google Scholar] [CrossRef]
- Dash, J.P.; Watt, M.; Pearse, G.D.; Heaphy, M.; Dungey, H. Assessing very high resolution UAV imagery for monitoring forest health during a simulated disease outbreak. ISPRS J. Photogramm. Remote Sens. 2017, 131, 1–14. [Google Scholar] [CrossRef]
- Gordon, A.D.; Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees. Biometrics 1984, 40, 874. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-C.; Lin, C.-J. LIBSVM. ACM Trans. Intell. Syst. Technol. 2011, 2, 1–27. [Google Scholar] [CrossRef]
- Mountrakis, G.; Im, J.; Ogole, C. Support vector machines in remote sensing: A review. ISPRS J. Photogramm. Remote Sens. 2011, 66, 247–259. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, X.; Lin, L.; Wang, Q.; Liu, J.; Yu, K.; Chen, C. BP neural networks and random forest models to detect damage by Dendrolimus punctatus Walker. J. For. Res. 2018, 31, 107–121. [Google Scholar] [CrossRef]
- Zhang, G.; Patuwo, B.E.; Hu, M.Y. Forecasting with artificial neural networks:: The state of the art. Int. J. Forecast. 1998, 14, 35–62. [Google Scholar] [CrossRef]
- Carrington, A.M.; Fieguth, P.W.; Qazi, H.; Holzinger, A.; Chen, H.H.; Mayr, F.; Manuel, D.G. A new concordant partial AUC and partial c statistic for imbalanced data in the evaluation of machine learning algorithms. BMC Med. Inform. Decis. Mak. 2020, 20, 4. [Google Scholar] [CrossRef]
- Spruce, J.P.; Sader, S.; Ryan, R.E.; Smoot, J.; Kuper, P.; Ross, K.; Prados, D.; Russell, J.; Gasser, G.; McKellip, R. Assessment of MODIS NDVI time series data products for detecting forest defoliation by gypsy moth outbreaks. Remote Sens. Environ. 2011, 115, 427–437. [Google Scholar] [CrossRef]
- Pasquarella, V.J.; Bradley, B.A.; Woodcock, C.E. Near-Real-Time Monitoring of Insect Defoliation Using Landsat Time Series. Forests 2017, 8, 275. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Tian, C.; Liang, Y. Mixed effects of landscape structure, tree diversity and stand’s relative position on insect and pathogen damage in riparian poplar forests. For. Ecol. Manag. 2020, 479, 118555. [Google Scholar] [CrossRef]

| Dataset | Variable | Describe |
|---|---|---|
| Sentinel-2 | NDMI_MIN | Normalized difference moisture index [35] |
| NDMI-MIDIAN | ||
| NDMI-MAX | ||
| NBR_MIN | Normalized burn ratio [36] | |
| NBR_MIDIAN | ||
| NBR_MAX | ||
| NDVI_MIN | Normalized difference vegetation index [37] | |
| NDVI_MIDIAN | ||
| NDVI_MAX | ||
| NDRE_MIN | Normalized difference red-edge index [27] | |
| NDRE_MIDIAN | ||
| NDRE_MAX | ||
| SRTM | Slope | The degree of steepness of the surface unit [degrees] |
| altitude | Vertical distance above sea level(dem) | |
| SIN(Aspect) | Sine of the aspect(sinasp) | |
| COS(Aspect) | Cosine of the aspect(cosasp) | |
| Slope*SIN(Aspect)() | Product of slope and sine of the aspect(slop_sinasp) | |
| Slope*COS(Aspect) | Product of slope and cosine of the aspect(slop_cosasp) | |
| roads above the township | Euclidean distance [m] (dis_r1) | |
| Road network | township roads | Euclidean distance [m] (dis_r2) |
| paths through the woods | Euclidean distance [m] (dis_r3) |
| Risk Level | Number of Dead Trees Caused by PWN in 50 m Radius | Number of Points |
|---|---|---|
| Lower intensity (L) | 1 ≤ n ≤ 3 | 4308 |
| Small intensity (S) | 3 < n ≤ 6 | 3780 |
| Median intensity (M) | 6 < n ≤ 10 | 3684 |
| Severely intensity (E) | 10 < n ≤ 16 | 3394 |
| Critical intensity (C) | 16 < n ≤ 95 | 3880 |
| Models | DR | FAR | FDR | AUC |
|---|---|---|---|---|
| RF | 98.84% | 46.61% | 6.66% | 96.39% |
| KNN | 98.37% | 60.87% | 8.56% | 83.21% |
| SVM | 99.24% | 92.17% | 12.32% | 71.98% |
| ANN | 93.27% | 70.08% | 10.21% | 70.74% |
| Risk Level | BK | L | S | M | E | C | DR | FAR | FDR |
|---|---|---|---|---|---|---|---|---|---|
| BK | 401 | 128 | 19 | 9 | 8 | 10 | 76.11% | 3.80% | 25.22% |
| L | 87 | 581 | 103 | 58 | 23 | 23 | 64.56% | 8.95% | 35.89% |
| S | 24 | 140 | 422 | 115 | 27 | 16 | 62.99% | 8.41% | 41.67% |
| M | 9 | 58 | 97 | 471 | 65 | 28 | 63.90% | 6.54% | 32.42% |
| E | 5 | 20 | 11 | 98 | 458 | 64 | 72.45% | 6.43% | 30.64% |
| C | 1 | 9 | 6 | 12 | 50 | 721 | 84.35% | 2.18% | 9.64% |
| PWN Risk Level | Area (ha) | Proportion |
|---|---|---|
| PWN absence area | 22,975 | 61.12% |
| Lower intensity | 10,347 | 27.53% |
| Small intensity | 1858 | 4.94% |
| Median intensity | 1150 | 3.06% |
| Severely intensity | 607 | 1.61% |
| Critical intensity | 650 | 1.73% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Dian, Y.; Zhou, J.; Peng, S.; Hu, Y.; Hu, L.; Han, Z.; Fang, X.; Cui, H. Characterizing Spatial Patterns of Pine Wood Nematode Outbreaks in Subtropical Zone in China. Remote Sens. 2021, 13, 4682. https://doi.org/10.3390/rs13224682
Zhang Y, Dian Y, Zhou J, Peng S, Hu Y, Hu L, Han Z, Fang X, Cui H. Characterizing Spatial Patterns of Pine Wood Nematode Outbreaks in Subtropical Zone in China. Remote Sensing. 2021; 13(22):4682. https://doi.org/10.3390/rs13224682
Chicago/Turabian StyleZhang, Yahao, Yuanyong Dian, Jingjing Zhou, Shoulian Peng, Yue Hu, Lei Hu, Zemin Han, Xinwei Fang, and Hongxia Cui. 2021. "Characterizing Spatial Patterns of Pine Wood Nematode Outbreaks in Subtropical Zone in China" Remote Sensing 13, no. 22: 4682. https://doi.org/10.3390/rs13224682







