Phenology Effects on Physically Based Estimation of Paddy Rice Canopy Traits from UAV Hyperspectral Imagery
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Setup
2.2. Field Data Collection
2.3. Inversion Method
2.3.1. Look-Up Table Generation
2.3.2. Model Inversion and Evaluation
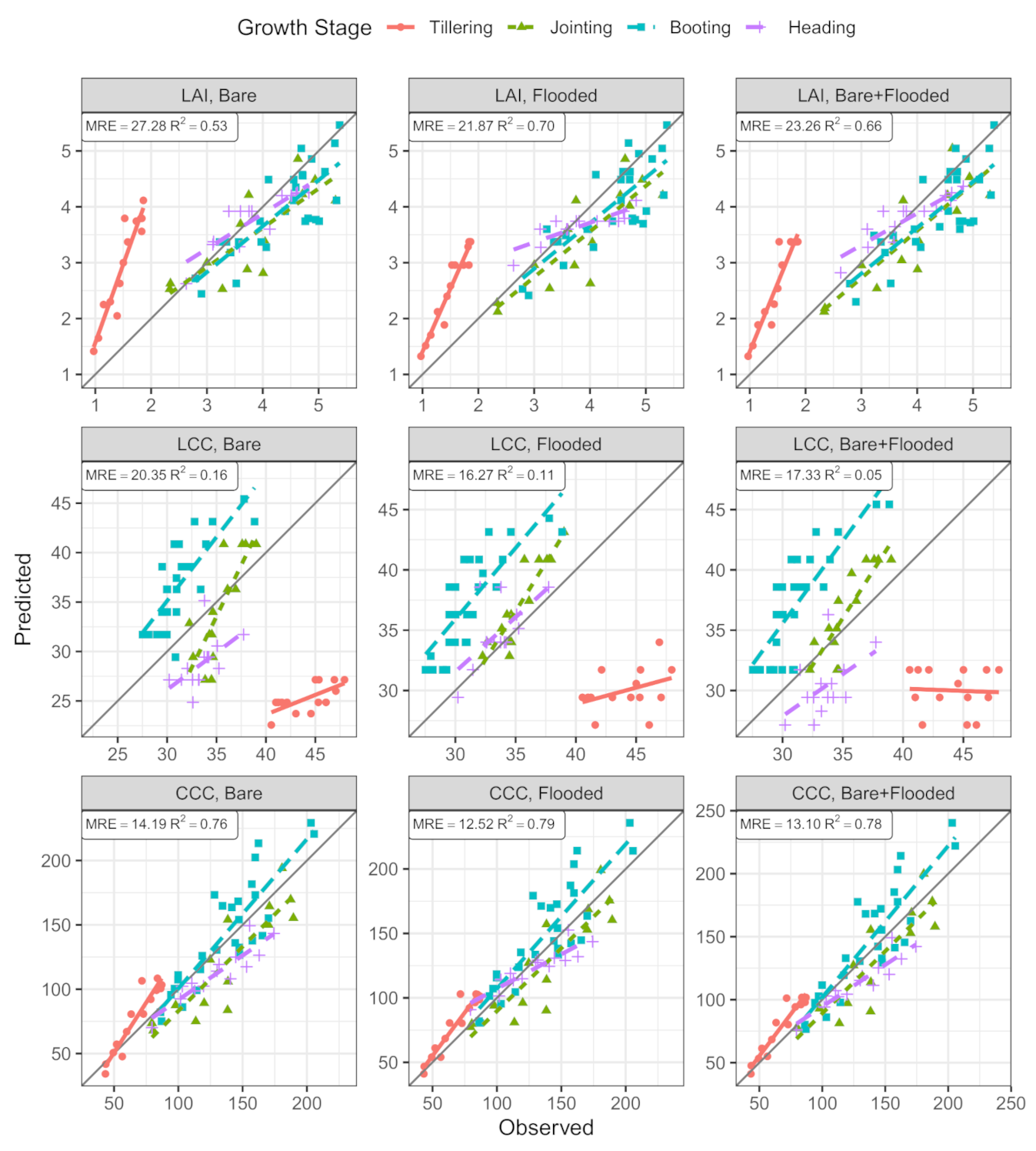
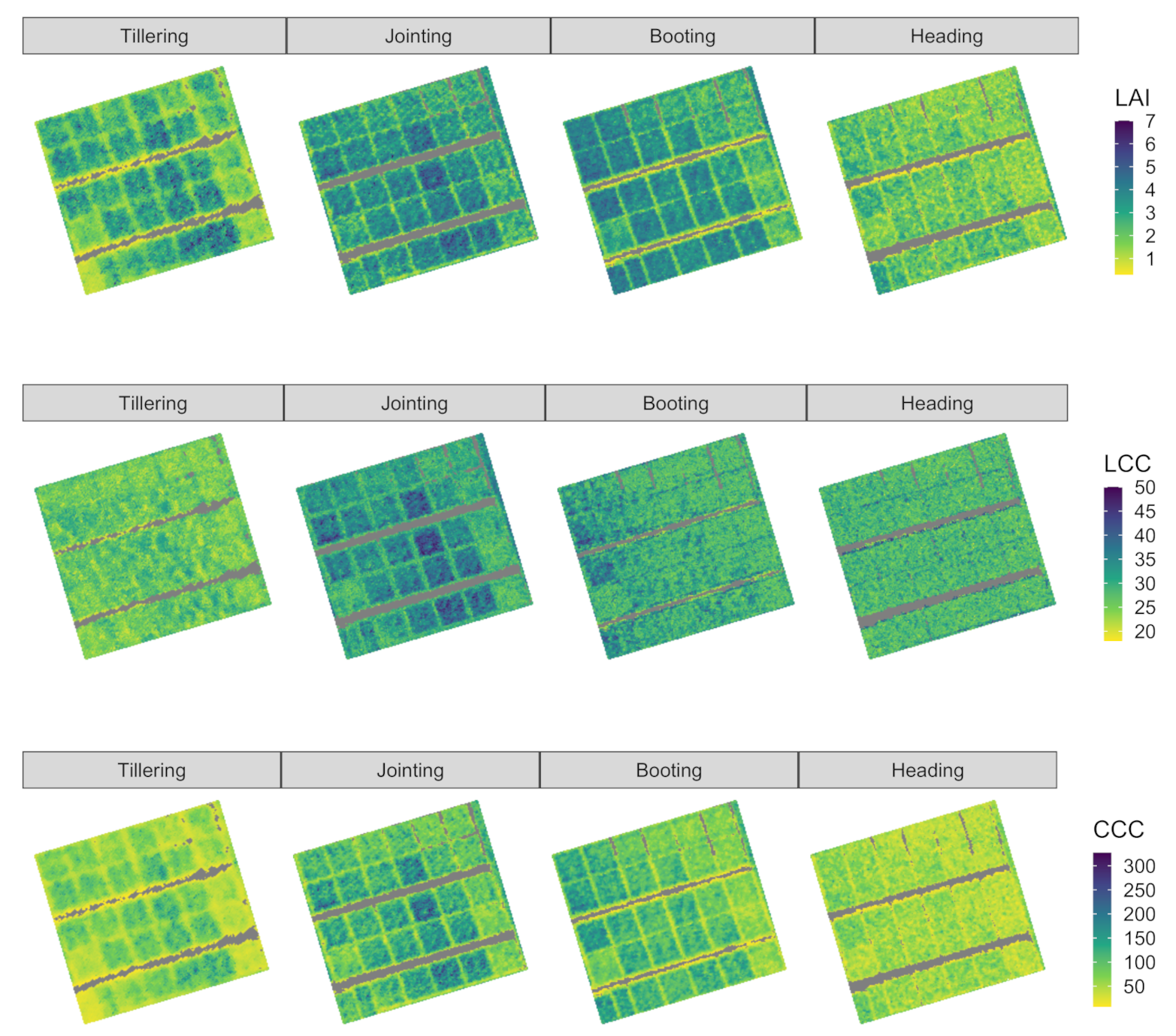
3. Results
3.1. Characteristics of Measured LAI, LCC, and CCC
3.2. PROSAIL-LUT Inversion Result
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LAI | Leaf Area Index |
| LCC | Leaf Chlorophyll Content |
| CCC | Canopy Chlorophyll Content |
| VI | Vegetation Index |
| PLSR | Partial Least-squares Regression |
| SVM | Support Vector Machine |
| RF | Random Forest |
| GPR | Gaussian Process Regression |
| ANN | Artificial Neural Network |
| RTM | Radiation Transform Model |
| LUT | Look-up Table |
| DAT | Day After Transplantation |
| N | Leaf Structure Parameter |
| Car | Leaf Carotenoid Content |
| Cbrown | Leaf Brown-Pigment Content |
| Cw | Leaf Equivalent Water Thickness |
| Cm | Leaf Dry Matter Content |
| ALA | Average Leaf Angle |
| hspot | Hotspot Parameter |
| tts | Solar Zenith Angle |
| tto | Observer Zenith Angle |
| psi | Relative Azimuth Angle |
| rsoil | Background Soil Reflectance |
| Look-up Table Generated from Bare Soil Reflectance Signature | |
| Look-up Table Generated from Flooded Soil Reflectance Signature | |
| Look-up Table Generated from both Bare Soil and Flooded Soil reflectance signature | |
| Root-Mean-Squared Error | |
| Coefficient of Determination | |
| Mean Relative Error |
References
- Weiss, M.; Jacob, F.; Duveiller, G. Remote sensing for agricultural applications: A meta-review. Remote Sens. Environ. 2020, 236, 111402. [Google Scholar] [CrossRef]
- Onojeghuo, A.O.; Blackburn, G.A.; Huang, J.; Kindred, D.; Huang, W. Applications of satellite ‘hyper-sensing’ in Chinese agriculture: Challenges and opportunities. Int. J. Appl. Earth Obs. Geoinf. 2018, 64, 62–86. [Google Scholar] [CrossRef]
- Pathak, H.S.; Brown, P.; Best, T. A systematic literature review of the factors affecting the precision agriculture adoption process. Precis. Agric. 2019, 20, 1292–1316. [Google Scholar] [CrossRef]
- Goetz, A.F. Three decades of hyperspectral remote sensing of the Earth: A personal view. Remote Sens. Environ. 2009, 113, S5–S16. [Google Scholar] [CrossRef]
- Kokaly, R.F.; Asner, G.P.; Ollinger, S.V.; Martin, M.E.; Wessman, C.A. Characterizing canopy biochemistry from imaging spectroscopy and its application to ecosystem studies. Remote Sens. Environ. 2009, 113, S78–S91. [Google Scholar] [CrossRef]
- Jonckheere, I.; Fleck, S.; Nackaerts, K.; Muys, B.; Coppin, P.; Weiss, M.; Baret, F. Review of methods for in situ leaf area index determination. Agric. For. Meteorol. 2004, 121, 19–35. [Google Scholar] [CrossRef]
- Wang, L.; Chang, Q.; Li, F.; Yan, L.; Huang, Y.; Wang, Q.; Luo, L. Effects of Growth Stage Development on Paddy Rice Leaf Area Index Prediction Models. Remote Sens. 2019, 11, 361. [Google Scholar] [CrossRef]
- Ustin, S.L.; Gitelson, A.A.; Jacquemoud, S.; Schaepman, M.; Asner, G.P.; Gamon, J.A.; Zarco-Tejada, P. Retrieval of foliar information about plant pigment systems from high resolution spectroscopy. Remote Sens. Environ. 2009, 113, S67–S77. [Google Scholar] [CrossRef]
- Verrelst, J.; Camps-Valls, G.; Muñoz-Marí, J.; Rivera, J.P.; Veroustraete, F.; Clevers, J.G.; Moreno, J. Optical remote sensing and the retrieval of terrestrial vegetation bio-geophysical properties—A review. ISPRS J. Photogramm. Remote Sens. 2015, 108, 273–290. [Google Scholar] [CrossRef]
- Wang, L.; Chang, Q.; Yang, J.; Zhang, X.; Li, F. Estimation of paddy rice leaf area index using machine learning methods based on hyperspectral data from multi-year experiments. PLoS ONE 2018, 13, e0207624. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Chen, D.; Huang, Y.; Kong, W.; Yuan, L.; Ye, H.; Huang, W. Assessment of Leaf Chlorophyll Content Models for Winter Wheat Using Landsat-8 Multispectral Remote Sensing Data. Remote Sens. 2020, 12, 2574. [Google Scholar] [CrossRef]
- Jacquemoud, S. Inversion of the PROSPECT + SAIL canopy reflectance model from AVIRIS equivalent spectra: Theoretical study. Remote Sens. Environ. 1993, 44, 281–292. [Google Scholar] [CrossRef]
- Chaabouni, S.; Kallel, A.; Houborg, R. Improving retrieval of crop biophysical properties in dryland areas using a multi-scale variational RTM inversion approach. Int. J. Appl. Earth Obs. Geoinf. 2021, 94, 102220. [Google Scholar] [CrossRef]
- César de Sá, N.; Baratchi, M.; Hauser, L.T.; van Bodegom, P. Exploring the Impact of Noise on Hybrid Inversion of PROSAIL RTM on Sentinel-2 Data. Remote Sens. 2021, 13, 648. [Google Scholar] [CrossRef]
- Danner, M.; Berger, K.; Wocher, M.; Mauser, W.; Hank, T. Efficient RTM-based training of machine learning regression algorithms to quantify biophysical & biochemical traits of agricultural crops. ISPRS J. Photogramm. Remote Sens. 2021, 173, 278–296. [Google Scholar] [CrossRef]
- Bacour, C.; Baret, F.; Béal, D.; Weiss, M.; Pavageau, K. Neural network estimation of LAI, fAPAR, fCover and LAI×Cab, from top of canopy MERIS reflectance data: Principles and validation. Remote Sens. Environ. 2006, 105, 313–325. [Google Scholar] [CrossRef]
- Baret, F.; Hagolle, O.; Geiger, B.; Bicheron, P.; Miras, B.; Huc, M.; Berthelot, B.; Niño, F.; Weiss, M.; Samain, O.; et al. LAI, fAPAR and fCover CYCLOPES global products derived from VEGETATION. Part 1: Principles of the algorithm. Remote Sens. Environ. 2007, 110, 275–286. [Google Scholar] [CrossRef]
- Durbha, S.S.; King, R.L.; Younan, N.H. Support vector machines regression for retrieval of leaf area index from multiangle imaging spectroradiometer. Remote Sens. Environ. 2007, 107, 348–361. [Google Scholar] [CrossRef]
- Campos-Taberner, M.; García-Haro, F.J.; Camps-Valls, G.; Grau-Muedra, G.; Nutini, F.; Crema, A.; Boschetti, M. Multitemporal and multiresolution leaf area index retrieval for operational local rice crop monitoring. Remote Sens. Environ. 2016, 187, 102–118. [Google Scholar] [CrossRef]
- Svendsen, D.H.; Martino, L.; Campos-Taberner, M.; Garcia-Haro, F.J.; Camps-Valls, G. Joint Gaussian Processes for Biophysical Parameter Retrieval. IEEE Trans. Geosci. Remote Sens. 2018, 56, 1718–1727. [Google Scholar] [CrossRef]
- Nguy-Robertson, A.L.; Peng, Y.; Gitelson, A.A.; Arkebauer, T.J.; Pimstein, A.; Herrmann, I.; Karnieli, A.; Rundquist, D.C.; Bonfil, D.J. Estimating green LAI in four crops: Potential of determining optimal spectral bands for a universal algorithm. Agric. Forest Meteorol. 2014, 192, 140–148. [Google Scholar] [CrossRef]
- Corti, M.; Cavalli, D.; Cabassi, G.; Marino Gallina, P.; Bechini, L. Does remote and proximal optical sensing successfully estimate maize variables? A review. Eur. J. Agron. 2018, 99, 37–50. [Google Scholar] [CrossRef]
- Berger, K.; Atzberger, C.; Danner, M.; D’Urso, G.; Mauser, W.; Vuolo, F.; Hank, T. Evaluation of the PROSAIL Model Capabilities for Future Hyperspectral Model Environments: A Review Study. Remote Sens. 2018, 10, 85. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Verhoef, W.; Baret, F.; Bacour, C.; Zarco-Tejada, P.J.; Asner, G.P.; François, C.; Ustin, S.L. PROSPECT + SAIL models: A review of use for vegetation characterization. Remote Sens. Environ. 2009, 113, S56–S66. [Google Scholar] [CrossRef]
- Plaza, A.; Benediktsson, J.A.; Boardman, J.W.; Brazile, J.; Bruzzone, L.; Camps-Valls, G.; Chanussot, J.; Fauvel, M.; Gamba, P.; Gualtieri, A.; et al. Recent advances in techniques for hyperspectral image processing. Remote Sens. Environ. 2009, 113, S110–S122. [Google Scholar] [CrossRef]
- Abdelbaki, A.; Schlerf, M.; Verhoef, W.; Udelhoven, T. Introduction of Variable Correlation for the Improved Retrieval of Crop Traits Using Canopy Reflectance Model Inversion. Remote Sens. 2019, 11, 2681. [Google Scholar] [CrossRef]
- Weiss, M.; Baret, F.; Myneni, R.B.; Pragnère, A.; Knyazikhin, Y. Investigation of a model inversion technique to estimate canopy biophysical variables from spectral and directional reflectance data. Agronomie 2000, 20, 3–22. [Google Scholar] [CrossRef]
- Darvishzadeh, R.; Matkan, A.A.; Dashti Ahangar, A. Inversion of a radiative transfer model for estimation of rice canopy chlorophyll content using a lookup-table approach. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2012, 5, 1222–1230. [Google Scholar] [CrossRef]
- Boren, E.J.; Boschetti, L. Landsat-8 and Sentinel-2 Canopy Water Content Estimation in Croplands through Radiative Transfer Model Inversion. Remote Sens. 2020, 12, 2803. [Google Scholar] [CrossRef]
- Feret, J.B.; François, C.; Asner, G.P.; Gitelson, A.A.; Martin, R.E.; Bidel, L.P.; Ustin, S.L.; le Maire, G.; Jacquemoud, S. PROSPECT-4 and 5: Advances in the leaf optical properties model separating photosynthetic pigments. Remote Sens. Environ. 2008, 112, 3030–3043. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Baret, F. PROSPECT: A model of leaf optical properties spectra. Remote Sens. Environ. 1990, 34, 75–91. [Google Scholar] [CrossRef]
- Verhoef, W. Light scattering by leaf layers with application to canopy reflectance modeling: The SAIL model. Remote Sens. Environ. 1984, 16, 125–141. [Google Scholar] [CrossRef]
- Verhoef, W.; Jia, L.; Xiao, Q.; Su, Z. Unified optical-thermal four-stream radiative transfer theory for homogeneous vegetation canopies. IEEE Trans. Geosci. Remote Sens. 2007, 45, 1808–1822. [Google Scholar] [CrossRef]
- Xu, X.Q.; Lu, J.S.; Zhang, N.; Yang, T.C.; He, J.Y.; Yao, X.; Cheng, T.; Zhu, Y.; Cao, W.X.; Tian, Y.C. Inversion of rice canopy chlorophyll content and leaf area index based on coupling of radiative transfer and Bayesian network models. ISPRS J. Photogramm. Remote Sens. 2019, 150, 185–196. [Google Scholar] [CrossRef]
- Campos-Taberner, M.; García-Haro, F.J.; Busetto, L.; Ranghetti, L.; Martínez, B.; Gilabert, M.A.; Camps-Valls, G.; Camacho, F.; Boschetti, M. A critical comparison of remote sensing Leaf Area Index estimates over rice-cultivated areas: From Sentinel-2 and Landsat-7/8 to MODIS, GEOV1 and EUMETSAT polar system. Remote Sens. 2018, 10, 763. [Google Scholar] [CrossRef]
- Richter, K.; Atzberger, C.; Vuolo, F.; D’Urso, G. Evaluation of Sentinel-2 Spectral Sampling for Radiative Transfer Model Based LAI Estimation of Wheat, Sugar Beet, and Maize. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2011, 4, 458–464. [Google Scholar] [CrossRef]
- Dorigo, W.A. Improving the robustness of cotton status characterisation by radiative transfer model inversion of multi-angular CHRIS/PROBA data. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2012, 5, 18–29. [Google Scholar] [CrossRef]
- Duan, S.B.; Li, Z.L.; Wu, H.; Tang, B.H.; Ma, L.; Zhao, E.; Li, C. Inversion of the PROSAIL model to estimate leaf area index of maize, potato, and sunflower fields from unmanned aerial vehicle hyperspectral data. Int. J. Appl. Earth Obs. Geoinf. 2014, 26, 12–20. [Google Scholar] [CrossRef]
- Moldenhauer, K.; Slaton, N. (Eds.) Rice growth and development. In Rice Production Handbook; University of Arkansas: Fayetteville, AR, USA, 2001; Volume 192, pp. 7–14. [Google Scholar]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef]
- Tian, M.; Ban, S.; Chang, Q.; Ma, W.; Yin, Z.; Wang, L. Estimation of SPAD value of cotton leaf using hyperspectral images from UAV-based imaging spectroradiometer. Trans. Chin. Soc. Agric. Eng. 2016, 47, 285–293. [Google Scholar]
- Botha, E.J.; Leblon, B.; Zebarth, B.; Watmough, J. Non-destructive estimation of potato leaf chlorophyll from canopy hyperspectral reflectance using the inverted PROSAIL model. Int. J. Appl. Earth Obs. Geoinf. 2007, 9, 360–374. [Google Scholar] [CrossRef]
- Casa, R.; Baret, F.; Buis, S.; Lopez-Lozano, R.; Pascucci, S.; Palombo, A.; Jones, H.G. Estimation of maize canopy properties from remote sensing by inversion of 1-D and 4-D models. Precis. Agric. 2010, 11, 319–334. [Google Scholar] [CrossRef]
- Si, Y.; Schlerf, M.; Zurita-Milla, R.; Skidmore, A.; Wang, T. Mapping spatio-temporal variation of grassland quantity and quality using MERIS data and the PROSAIL model. Remote Sens. Environ. 2012, 121, 415–425. [Google Scholar] [CrossRef]
- Danner, M.; Berger, K.; Wocher, M.; Mauser, W.; Hank, T. Fitted PROSAIL Parameterization of Leaf Inclinations, Water Content and Brown Pigment Content for Winter Wheat and Maize Canopies. Remote Sens. 2019, 11, 1150. [Google Scholar] [CrossRef]
- Rivera, J.P.; Verrelst, J.; Leonenko, G.; Moreno, J.; Rivera, J.P.; Verrelst, J.; Leonenko, G.; Moreno, J. Multiple Cost Functions and Regularization Options for Improved Retrieval of Leaf Chlorophyll Content and LAI through Inversion of the PROSAIL Model. Remote Sens. 2013, 5, 3280–3304. [Google Scholar] [CrossRef]
- Jay, S.; Maupas, F.; Bendoula, R.; Gorretta, N. Retrieving LAI, chlorophyll and nitrogen contents in sugar beet crops from multi-angular optical remote sensing: Comparison of vegetation indices and PROSAIL inversion for field phenotyping. Field Crop. Res. 2017, 210, 33–46. [Google Scholar] [CrossRef]
- Roosjen, P.P.; Brede, B.; Suomalainen, J.M.; Bartholomeus, H.M.; Kooistra, L.; Clevers, J.G. Improved estimation of leaf area index and leaf chlorophyll content of a potato crop using multi-angle spectral data – potential of unmanned aerial vehicle imagery. Int. J. Appl. Earth Obs. Geoinf. 2018, 66, 14–26. [Google Scholar] [CrossRef]


| Model Parameters | Abbreviations | Units | Ranges | Classes |
|---|---|---|---|---|
| Leaf Structure Parameter | N | unitless | 1.0–2.5 | 5 |
| Leaf Chlorophyll Content | g cm | 20–50 | 15 | |
| Leaf Carotenoid Content | g cm | 0–20 | 15 | |
| Leaf Brown-Pigment Content | 0 | 1 | ||
| Leaf Equivalent Thickness | g cm | 0.0107 | 1 | |
| Leaf Dry-Matter Content | g cm | 0.0034 | 1 | |
| Leaf Area Index | m m | 0.5–7.0 | 15 | |
| Leaf Average Angle | 20–85 | 10 | ||
| Hotspot Parameter | 0.01 | 1 | ||
| Solar Zenith Angle | 35 | 1 | ||
| Observer Zenith Angle | 0 | 1 | ||
| Relative Azimuth Angle | 70 | 1 |
| Growth Stages | Variable (Unit) | Sample Numbers | Mean | SD | Range |
|---|---|---|---|---|---|
| Tillering | LAI (unitless) | 14 | 1.50 | 0.30 | 0.971~1.861 |
| LCC g cm) | 14 | 44.13 | 2.50 | 40.545~47.956 | |
| CCC g cm) | 14 | 66.52 | 15.99 | 43.289~87.091 | |
| Jointing | LAI (unitless) | 14 | 3.87 | 0.91 | 2.334~5.303 |
| LCC (g cm) | 14 | 35.33 | 1.99 | 32.262~39.018 | |
| CCC (g cm) | 14 | 137.86 | 37.68 | 79.422~189.494 | |
| Booting | LAI (unitless) | 28 | 4.33 | 0.75 | 2.793~5.377 |
| LCC (g cm) | 28 | 31.25 | 2.64 | 27.528~38.853 | |
| CCC (g cm) | 28 | 136.60 | 32.08 | 86.157~205.630 | |
| Heading | LAI (unitless) | 14 | 3.86 | 0.67 | 2.630~4.826 |
| LCC (g cm) | 14 | 33.52 | 1.83 | 30.213~37.741 | |
| CCC (g cm) | 14 | 129.98 | 26.92 | 79.456~174.296 |
| LAI | LCC | CCC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Growth Stage | Rsoil | |||||||||
| Tillering | Bare | 0.88 | 1.58 | 94.08 | 0.46 | 18.95 | 42.70 | 0.90 | 15.44 | 18.08 |
| Flooded | 0.92 | 1.11 | 66.32 | 0.14 | 14.34 | 31.90 | 0.90 | 13.64 | 16.10 | |
| Bare+Flooded | 0.89 | 1.23 | 72.20 | 0.00 | 14.46 | 31.81 | 0.91 | 13.52 | 16.07 | |
| Jointing | Bare | 0.64 | 0.63 | 13.10 | 0.64 | 3.79 | 8.78 | 0.78 | 25.52 | 15.66 |
| Flooded | 0.71 | 0.64 | 12.45 | 0.81 | 2.58 | 6.01 | 0.79 | 21.61 | 11.93 | |
| Bare+Flooded | 0.74 | 0.61 | 12.93 | 0.72 | 2.45 | 5.97 | 0.79 | 21.57 | 12.07 | |
| Booting | Bare | 0.64 | 0.62 | 10.79 | 0.62 | 6.12 | 17.79 | 0.77 | 21.50 | 12.19 |
| Flooded | 0.63 | 0.60 | 10.54 | 0.64 | 6.57 | 19.63 | 0.76 | 23.09 | 13.12 | |
| Bare+Flooded | 0.61 | 0.66 | 11.73 | 0.67 | 6.58 | 19.12 | 0.78 | 23.05 | 13.15 | |
| Heading | Bare | 0.75 | 0.34 | 7.62 | 0.30 | 5.18 | 14.69 | 0.83 | 20.97 | 12.83 |
| Flooded | 0.72 | 0.48 | 9.53 | 0.40 | 2.29 | 4.20 | 0.82 | 15.33 | 8.35 | |
| Bare+Flooded | 0.80 | 0.34 | 7.73 | 0.26 | 3.84 | 10.63 | 0.86 | 19.12 | 11.07 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Chen, S.; Peng, Z.; Huang, J.; Wang, C.; Jiang, H.; Zheng, Q.; Li, D. Phenology Effects on Physically Based Estimation of Paddy Rice Canopy Traits from UAV Hyperspectral Imagery. Remote Sens. 2021, 13, 1792. https://doi.org/10.3390/rs13091792
Wang L, Chen S, Peng Z, Huang J, Wang C, Jiang H, Zheng Q, Li D. Phenology Effects on Physically Based Estimation of Paddy Rice Canopy Traits from UAV Hyperspectral Imagery. Remote Sensing. 2021; 13(9):1792. https://doi.org/10.3390/rs13091792
Chicago/Turabian StyleWang, Li, Shuisen Chen, Zhiping Peng, Jichuan Huang, Chongyang Wang, Hao Jiang, Qiong Zheng, and Dan Li. 2021. "Phenology Effects on Physically Based Estimation of Paddy Rice Canopy Traits from UAV Hyperspectral Imagery" Remote Sensing 13, no. 9: 1792. https://doi.org/10.3390/rs13091792
APA StyleWang, L., Chen, S., Peng, Z., Huang, J., Wang, C., Jiang, H., Zheng, Q., & Li, D. (2021). Phenology Effects on Physically Based Estimation of Paddy Rice Canopy Traits from UAV Hyperspectral Imagery. Remote Sensing, 13(9), 1792. https://doi.org/10.3390/rs13091792









