Climate Sensitivity of the Arid Scrublands on the Tibetan Plateau Mediated by Plant Nutrient Traits and Soil Nutrient Availability
Abstract
:Highlights
- Principal component regressions revealed the climate sensitivity of Tibetan arid desert scrubs.
- Plant nutrient traits and soil nutrient availability regulate desert scrubs’ climate sensitivity.
- Scrubs’ sensitivity to temperature is mainly regulated by the nitrogen contents of soils and leaves.
- Scrubs’ sensitivity to precipitation is affected by the leaf carbon content of dominant species.
- Neither soil nor plant nutritional properties alone can well explain scrubs’ sensitivity to droughts.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Remote Sensing Data Collection and Processing
2.3. Weather Data Collection and Processing
2.4. Climatic Sensitivity Index of Desert-Scrubs
2.5. Plant Nutrient Traits and Soil Nutrient Availability
2.6. Plant Functional Trait Diversity
2.7. Statistical Analyses
3. Results
3.1. Vegetation Sensitivity Index and Its Contributors
3.2. Effects of Soil and Plant Properties on VSI
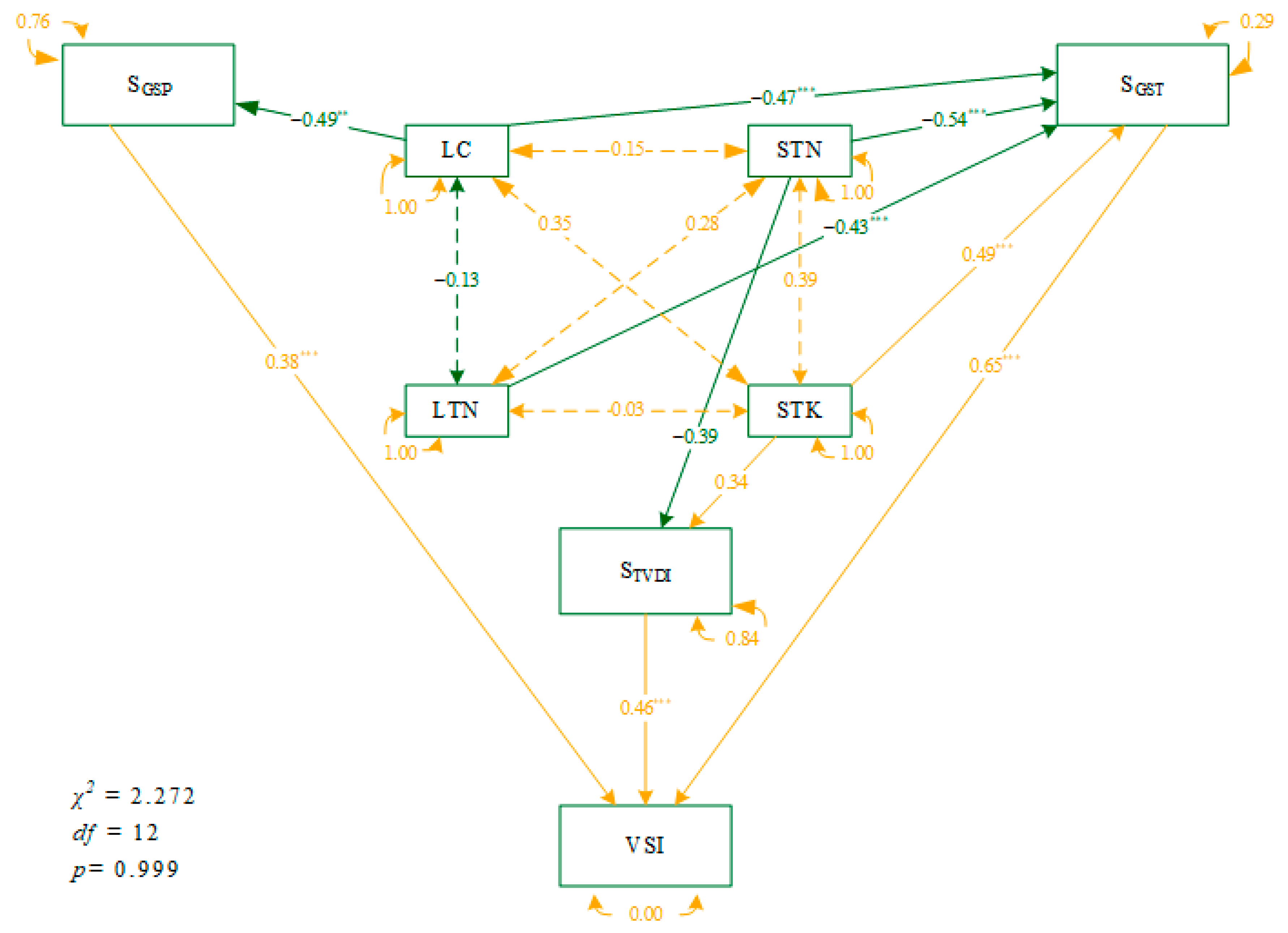
3.3. Causal Network of Plant Traits and Soil Nutrients to VSI under Climate Variability
4. Discussion
4.1. Climate Sensitivity among Different Desert Scrubs
4.2. Desert-Scrubs’ VSI Correlated with Soil and Leaf Nutrients
4.3. The Networks of Direct and Indirect Environmental Influences on VSI
4.4. Limitations and Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reynolds, J.F.; Smith, D.M.; Lambin, E.F.; Turner, B.L., 2nd; Mortimore, M.; Batterbury, S.P.; Downing, T.E.; Dowlatabadi, H.; Fernandez, R.J.; Herrick, J.E.; et al. Global desertification: Building a science for dryland development. Science 2007, 316, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Wetlands and Water; Island Press: Washington, DC, USA, 2005; Volume 5. [Google Scholar]
- Berdugo, M.; Delgado-Baquerizo, M.; Soliveres, S.; Hernandez-Clemente, R.; Zhao, Y.; Gaitan, J.J.; Gross, N.; Saiz, H.; Maire, V.; Lehmann, A.; et al. Global ecosystem thresholds driven by aridity. Science 2020, 367, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, Y.; Fu, C.; Chen, F.; Fu, Q.; Dai, A.; Shinoda, M.; Ma, Z.; Guo, W.; Li, Z.; et al. Dryland climate change: Recent progress and challenges. Rev. Geophys. 2017, 55, 719–778. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Zouber, A.; Lasanta, T.; Pueyo, Y. Dryness is accelerating degradation of vulnerable shrublands in semiarid Mediterranean environments. Ecol. Monogr. 2012, 82, 407–428. [Google Scholar] [CrossRef]
- Huang, J.P.; Yu, H.P.; Guan, X.D.; Wang, G.Y.; Guo, R.X. Accelerated dryland expansion under climate change. Nat. Clim. Chang. 2016, 6, 166–171. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hochard, J.P. Land degradation and poverty. Nat. Sustain. 2018, 1, 623–631. [Google Scholar] [CrossRef]
- Tietjen, B.; Schlaepfer, D.R.; Bradford, J.B.; Lauenroth, W.K.; Hall, S.A.; Duniway, M.C.; Hochstrasser, T.; Jia, G.; Munson, S.M.; Pyke, D.A.; et al. Climate change-induced vegetation shifts lead to more ecological droughts despite projected rainfall increases in many global temperate drylands. Glob. Chang. Biol. 2017, 23, 2743–2754. [Google Scholar] [CrossRef]
- Hantson, S.; Huxman, T.E.; Kimball, S.; Randerson, J.T.; Goulden, M.L. Warming as a driver of vegetation loss in the Sonoran desert of California. J. Geophys. Res.-Biogeo. 2021, 126, e2020JG005942. [Google Scholar] [CrossRef]
- Gherardi, L.A.; Sala, O.E. Effect of interannual precipitation variability on dryland productivity: A global synthesis. Glob. Chang. Biol. 2019, 25, 269–276. [Google Scholar] [CrossRef]
- Hoover, D.L.; Pfennigwerth, A.A.; Duniway, M.C. Drought resistance and resilience: The role of soil moisture-plant interactions and legacies in a dryland ecosystem. J. Ecol. 2021, 109, 3280–3294. [Google Scholar] [CrossRef]
- Pettorelli, N.; Laurance, W.F.; O’Brien, T.G.; Wegmann, M.; Nagendra, H.; Turner, W. Satellite remote sensing for applied ecologists: Opportunities and challenges. J. Appl. Ecol. 2014, 51, 839–848. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.; De Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Seddon, A.W.; Macias-Fauria, M.; Long, P.R.; Benz, D.; Willis, K.J. Sensitivity of global terrestrial ecosystems to climate variability. Nature 2016, 531, 229–232. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Wu, J.; Li, S.; Zhang, B.; Zu, J.; Zhang, H.; Ding, M.; Paudel, B. Increasing sensitivity of alpine grasslands to climate variability along an elevational gradient on the Qinghai-Tibet plateau. Sci. Total Environ. 2019, 678, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, J.S.; Song, C.Q.; He, Y.T.; Niu, B.; Fu, G.; Tarolli, P.; Tietjen, B.; Zhang, X.Z. Temporal variability of precipitation and biomass of alpine grasslands on the northern tibetan plateau. Remote Sens. 2019, 11, 360. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, X.; Li, Y.; Wang, T.; Shen, M.; Du, M.; He, H.; Li, Y.; Luo, W.; Ma, M.; et al. Spatiotemporal pattern of gross primary productivity and its covariation with climate in China over the last thirty years. Glob. Chang. Biol. 2018, 24, 184–196. [Google Scholar] [CrossRef]
- Yuan, Y.; Bao, A.; Liu, T.; Zheng, G.; Jiang, L.; Guo, H.; Jiang, P.; Yu, T.; de Maeyer, P. Assessing vegetation stability to climate variability in central Asia. J. Environ. Manag. 2021, 298, 113330. [Google Scholar] [CrossRef]
- Henn, J.J.; Buzzard, V.; Enquist, B.J.; Halbritter, A.H.; Klanderud, K.; Maitner, B.S.; Michaletz, S.T.; Potsch, C.; Seltzer, L.; Telford, R.J.; et al. Intraspecific trait variation and phenotypic plasticity mediate alpine plant species response to climate change. Front. Plant Sci. 2018, 9, 1548. [Google Scholar] [CrossRef]
- Liu, Y.; Men, M.; Peng, Z.; Houx, J.H., 3rd; Peng, Y. Nitrogen availability determines ecosystem productivity in response to climate warming. Ecology 2022, e3823. [Google Scholar] [CrossRef]
- Palmquist, K.A.; Schlaepfer, D.R.; Renne, R.R.; Torbit, S.C.; Doherty, K.E.; Remington, T.E.; Watson, G.; Bradford, J.B.; Lauenroth, W.K. Divergent climate change effects on widespread dryland plant communities driven by climatic and ecohydrological gradients. Glob. Chang. Biol. 2021, 27, 5169–5185. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- McGill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Kearney, M.; Porter, W.P. Ecologists have already started rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 481–482; author reply 482–483. [Google Scholar] [CrossRef]
- Bruelheide, H.; Dengler, J.; Purschke, O.; Lenoir, J.; Jimenez-Alfaro, B.; Hennekens, S.M.; Botta-Dukat, Z.; Chytry, M.; Field, R.; Jansen, F.; et al. Global trait-environment relationships of plant communities. Nat. Ecol. Evol. 2018, 2, 1906–1917. [Google Scholar] [CrossRef]
- De Bello, F.; Lepš, J.A.N.; Sebastià, M.-T. Predictive value of plant traits to grazing along a climatic gradient in the Mediterranean. J. Appl. Ecol. 2005, 42, 824–833. [Google Scholar] [CrossRef]
- Guo, X.; Liu, H.; Ngosong, C.; Li, B.; Wang, Q.; Zhou, W.; Nie, M. Response of plant functional traits to nitrogen enrichment under climate change: A meta-analysis. Sci. Total Environ. 2022, 834, 155379. [Google Scholar] [CrossRef]
- Midolo, G.; Kuss, P.; Wellstein, C. Land use and water availability drive community-level plant functional diversity of grasslands along a temperature gradient in the Swiss Alps. Sci. Total Environ. 2021, 764, 142888. [Google Scholar] [CrossRef]
- Prieto, I.; Roumet, C.; Cardinael, R.; Dupraz, C.; Jourdan, C.; Kim, J.H.; Maeght, J.L.; Mao, Z.; Pierret, A.; Portillo, N.; et al. Root functional parameters along a land-use gradient: Evidence of a community-level economics spectrum. J. Ecol. 2015, 103, 361–373. [Google Scholar] [CrossRef]
- Wang, H.; Wang, R.; Harrison, S.P.; Prentice, I.C. Leaf morphological traits as adaptations to multiple climate gradients. J. Ecol. 2022, 110, 1344–1355. [Google Scholar] [CrossRef]
- Balachowski, J.A.; Volaire, F.A. Implications of plant functional traits and drought survival strategies for ecological restoration. J. Appl. Ecol. 2018, 55, 631–640. [Google Scholar] [CrossRef]
- Lian, X.; Piao, S.L.; Chen, A.P.; Huntingford, C.; Fu, B.J.; Li, L.Z.X.; Huang, J.P.; Sheffield, J.; Berg, A.M.; Keenan, T.F.; et al. Multifaceted characteristics of dryland aridity changes in a warming world. Nat. Rev. Earth Environ. 2021, 2, 232–250. [Google Scholar] [CrossRef]
- Woods, H.A.; Makino, W.; Cotner, J.B.; Hobbie, S.E.; Harrison, J.F.; Acharya, K.; Elser, J.J. Temperature and the chemical composition of poikilothermic organisms. Funct. Ecol. 2003, 17, 237–245. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, J.; Li, X.; Yang, Z.; Zheng, Y.; Wang, J.; Lin, W.; Xie, J.; Chen, Y.; Yang, Y. Are the combined effects of warming and drought on foliar c:N:P:K stoichiometry in a subtropical forest greater than their individual effects? For. Ecol. Manag. 2019, 448, 256–266. [Google Scholar] [CrossRef]
- Sun, Y.; Liao, J.H.; Zou, X.M.; Xu, X.; Yang, J.Y.; Chen, H.Y.H.; Ruan, H.H. Coherent responses of terrestrial c:N stoichiometry to drought across plants, soil, and microorganisms in forests and grasslands. Agric. For. Meteorol. 2020, 292–293, 108104. [Google Scholar] [CrossRef]
- Niu, D.; Zhang, C.; Ma, P.; Fu, H.; Elser, J.J. Responses of leaf c:N:P stoichiometry to water supply in the desert shrub Zygophyllum xanthoxylum. Plant Biol. 2019, 21, 82–88. [Google Scholar] [CrossRef]
- Castellanos, A.E.; Llano-Sotelo, J.M.; Machado-Encinas, L.I.; Lopez-Pina, J.E.; Romo-Leon, J.R.; Sardans, J.; Penuelas, J. Foliar c, n, and p stoichiometry characterize successful plant ecological strategies in the Sonoran desert. Plant Ecol. 2018, 219, 775–788. [Google Scholar] [CrossRef]
- Huang, J.; Wang, P.; Niu, Y.; Yu, H.; Ma, F.; Xiao, G.; Xu, X. Changes in c:N:P stoichiometry modify n and p conservation strategies of a desert steppe species Glycyrrhiza uralensis. Sci. Rep. 2018, 8, 12668. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Rocha, A.V.; Rastetter, E.B.; Shaver, G.R.; Mishra, U.; Zhuang, Q.L.; Kwiatkowski, B.L. C-n-p interactions control climate driven changes in regional patterns of c storage on the north slope of Alaska. Landsc. Ecol. 2016, 31, 195–213. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Gallardo, A.; Bowker, M.A.; Wallenstein, M.D.; Quero, J.L.; Ochoa, V.; Gozalo, B.; Garcia-Gomez, M.; Soliveres, S.; et al. Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature 2013, 502, 672–676. [Google Scholar] [CrossRef]
- Jiao, F.; Shi, X.R.; Han, F.P.; Yuan, Z.Y. Increasing aridity, temperature and soil pH induce soil c-n-p imbalance in grasslands. Sci. Rep. 2016, 6, 19601. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, S.; Monteiro, J.A.F.; Hulvey, K.B.; Standish, R.J.; Perring, M.P.; Tietjen, B. Global change shifts trade-offs among ecosystem functions in woodlands restored for multifunctionality. J. Appl. Ecol. 2021, 58, 1705–1717. [Google Scholar] [CrossRef]
- Jäschke, Y.; Heberling, G.; Wesche, K. Environmental controls override grazing effects on plant functional traits in Tibetan rangelands. Funct. Ecol. 2020, 34, 747–760. [Google Scholar] [CrossRef]
- Gross, N.; Bagousse-Pinguet, Y.L.; Liancourt, P.; Berdugo, M.; Gotelli, N.J.; Maestre, F.T. Functional trait diversity maximizes ecosystem multifunctionality. Nat. Ecol. Evol. 2017, 1, 132. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Li, Y.; Liu, C.; Xu, L.; Li, M.; Zhang, J.; He, J.; Tang, Z.; Han, X.; Ye, Q.; et al. Plant trait networks: Improved resolution of the dimensionality of adaptation. Trends Ecol. Evol. 2020, 35, 908–918. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Liu, C.; Piao, S.; Sack, L.; Xu, L.; Luo, Y.; He, J.; Han, X.; Zhou, G.; Zhou, X.; et al. Ecosystem traits linking functional traits to macroecology. Trends Ecol. Evol. 2019, 34, 200–210. [Google Scholar] [CrossRef]
- Jin, X.M.; Liu, J.T.; Wang, S.T.; Xia, W. Vegetation dynamics and their response to groundwater and climate variables in Qaidam basin, China. Int. J. Remote Sens. 2016, 37, 710–728. [Google Scholar] [CrossRef]
- Han, J.J.; Wang, J.P.; Chen, L.; Xiang, J.Y.; Ling, Z.Y.; Li, Q.K.; Wang, E.L. Driving factors of desertification in Qaidam basin, China: An 18-year analysis using the geographic detector model. Ecol. Indic. 2021, 124, 107404. [Google Scholar] [CrossRef]
- Shi, X.; Yu, D.; Warner, E.; Pan, X.; Petersen, G.; Gong, Z.; Weindorf, D. Soil database of 1:1,000,000 digital soil survey and reference system of the Chinese genetic soil classification system. Soil Surv. Horiz. 2004, 45, 129–136. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Chen, H.; Fu, Y.; Niu, H.H.; Yang, Y.; Zhang, B.X. Fractional vegetation cover estimation of different vegetation types in the Qaidam basin. Sustainability 2019, 11, 864. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, H.; Niu, H.H.; Zhang, S.Q.; Yang, Y. Spatial and temporal variation of vegetation phenology and its response to climate changes in Qaidam basin from 2000 to 2015. J. Geogr. Sci. 2018, 28, 400–414. [Google Scholar] [CrossRef] [Green Version]
- Savitzky, A.; Golay, M.J. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Holben, B.N. Characteristics of maximum-value composite images from temporal AVHRR data. Int. J. Remote Sens. 1986, 7, 1417–1434. [Google Scholar] [CrossRef]
- Hutchinson, M. ANUSPLIN, version 4.3; Centre for Resource and Environmental Studies, The Australian National University: Canberra, Australia, 2004. [Google Scholar]
- Li, M.; Wu, J.S.; He, Y.T.; Wu, L.; Niu, B.; Song, M.H.; Zhang, X.Z. Dimensionality of grassland stability shifts along with altitudes on the Tibetan plateau. Agric. For. Meteorol. 2020, 291, 108080. [Google Scholar] [CrossRef]
- Sandholt, I.; Rasmussen, K.; Andersen, J. A simple interpretation of the surface temperature/vegetation index space for assessment of surface moisture status. Remote Sens. Environ. 2002, 79, 213–224. [Google Scholar] [CrossRef]
- Kendall, M.G. Rank Correlation Methods, 4th ed.; Charles Grifin: London, UK, 1975. [Google Scholar]
- Mann, H.B. Nonparametric tests against trend. Econometrica 1945, 13, 245–259. [Google Scholar] [CrossRef]
- Akhtar, K.; Wang, W.Y.; Ren, G.X.; Khan, A.; Feng, Y.Z.; Yang, G.H. Changes in soil enzymes, soil properties, and maize crop productivity under wheat straw mulching in Guanzhong, China. Soil Till. Res. 2018, 182, 94–102. [Google Scholar] [CrossRef]
- Wu, J.S.; Song, M.H.; Ma, W.L.; Zhang, X.Z.; Shen, Z.X.; Tarolli, P.; Wurst, S.; Shi, P.L.; Ratzmann, G.; Feng, Y.F.; et al. Plant and soil’s δ n-15 are regulated by climate, soil nutrients, and species diversity in alpine grasslands on the northern Tibetan plateau. Agric. Ecosyst. Environ. 2019, 281, 111–123. [Google Scholar] [CrossRef]
- Gauzere, P.; Doulcier, G.; Devictor, V.; Kefi, S. A framework for estimating species-specific contributions to community indicators. Ecol. Indic. 2019, 99, 74–82. [Google Scholar] [CrossRef]
- Violle, C.; Reich, P.B.; Pacala, S.W.; Enquist, B.J.; Kattge, J. The emergence and promise of functional biogeography. Proc. Natl. Acad. Sci. USA 2014, 111, 13690–13696. [Google Scholar] [CrossRef]
- Wilcoxon, F. Individual comparisons by ranking methods. In Breakthroughs in Statistics; Springer: Berlin/Heidelberg, Germany, 1992; pp. 196–202. [Google Scholar]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.92). 2021. Available online: https://github.com/taiyun/corrplot (accessed on 26 February 2022).
- Niu, W.; Chen, H.; Wu, J. Soil moisture and soluble salt content dominate changes in foliar delta(13)c and delta(15)n of desert communities in the Qaidam basin, Qinghai-Tibetan plateau. Front. Plant Sci. 2021, 12, 675817. [Google Scholar] [CrossRef]
- Wu, J.; Li, M.; Fiedler, S.; Ma, W.; Wang, X.; Zhang, X.; Tietjen, B. Impacts of grazing exclusion on productivity partitioning along regional plant diversity and climatic gradients in Tibetan alpine grasslands. J. Environ. Manag. 2019, 231, 635–645. [Google Scholar] [CrossRef]
- Rosseel, Y. Lavaan: An R package for structural equation modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- ESRI. ArcGIS Desktop: Release 10.2; Environmental Systems Research Institute: Redlands, CA, USA, 2014. [Google Scholar]
- Kusch, E.; Davy, R.; Seddon, A.W.R. Vegetation-memory effects and their association with vegetation resilience in global drylands. J. Ecol. 2022, 110, 1561–1574. [Google Scholar] [CrossRef]
- Ogle, K.; Barber, J.J.; Barron-Gafford, G.A.; Bentley, L.P.; Young, J.M.; Huxman, T.E.; Loik, M.E.; Tissue, D.T. Quantifying ecological memory in plant and ecosystem processes. Ecol. Lett. 2015, 18, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Easterling, D.R.; Wehner, M.F. Is the climate warming or cooling? Geophys. Res. Lett. 2009, 36. [Google Scholar] [CrossRef]
- Zhu, Y.K.; Zhang, J.T.; Zhang, Y.Q.; Qin, S.G.; Shao, Y.Y.; Gao, Y. Responses of vegetation to climatic variations in the desert region of northern China. Catena 2019, 175, 27–36. [Google Scholar] [CrossRef]
- Olson, M.E.; Soriano, D.; Rosell, J.A.; Anfodillo, T.; Donoghue, M.J.; Edwards, E.J.; Leon-Gomez, C.; Dawson, T.; Camarero Martinez, J.J.; Castorena, M.; et al. Plant height and hydraulic vulnerability to drought and cold. Proc. Natl. Acad. Sci. USA 2018, 115, 7551–7556. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Adler, P.B.; Godoy, O.; James, E.C.; Fuller, S.; Levine, J.M. Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 2015, 29, 592–599. [Google Scholar] [CrossRef]
- Aerts, R.; Chapin, F.S. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. In Advances in Ecological Research; Fitter, A.H., Raffaelli, D.G., Eds.; Academic Press: Cambridge, MA, USA, 2000; Volume 30, pp. 1–67. [Google Scholar]
- Hobbie, S.E.; Nadelhoffer, K.J.; Hogberg, P. A synthesis: The role of nutrients as constraints on carbon balances in boreal and arctic regions. Plant Soil 2002, 242, 163–170. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. The temperature-dependence of soil organic-matter decomposition, and the effect of global warming on soil organic-c storage. Soil Biol. Biochem. 1995, 27, 753–760. [Google Scholar] [CrossRef]
- Sparks, D.L.; Liebhardt, W.C. Temperature effects on potassium exchange and selectivity in Delaware soils. Soil Sci. 1982, 133, 10–17. [Google Scholar] [CrossRef]
- Lewis, J.D.; Lucash, M.; Olszyk, D.M.; Tingey, D.T. Relationships between needle nitrogen concentration and photosynthetic responses of douglas-fir seedlings to elevated CO2 and temperature. New Phytol. 2004, 162, 355–364. [Google Scholar] [CrossRef]
- Berry, J.; Bjorkman, O. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Field, C. The photosynthesis-nitrogen relationship in wild plants. In On the Economy of Form and Function; Cambridge University Press: Cambridge, UK, 1986. [Google Scholar]
- Wright, I.J.; Reich, P.B.; Westoby, M. Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Funct. Ecol. 2001, 15, 423–434. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Cornelissen, J.H.; Falster, D.S.; Garnier, E.; Hikosaka, K.; Lamont, B.B.; Lee, W.; Oleksyn, J.; Osada, N.; et al. Assessing the generality of global leaf trait relationships. New Phytol. 2005, 166, 485–496. [Google Scholar] [CrossRef]
- Blackwell, M.S.A.; Brookes, R.C.; de la Fuente-Martinez, N.; Gordon, H.; Murray, P.J.; Snars, K.E.; Williams, J.K.; Bol, R.; Haygarth, P.M. Chapter 1—Phosphorus solubilization and potential transfer to surface waters from the soil microbial biomass following drying–rewetting and freezing–thawing. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 106, pp. 1–35. [Google Scholar]
- Wang, R.Z.; Creamer, C.A.; Wang, X.; He, P.; Xu, Z.W.; Jiang, Y. The effects of a 9-year nitrogen and water addition on soil aggregate phosphorus and sulfur availability in a semi-arid grassland. Ecol. Indic. 2016, 61, 806–814. [Google Scholar] [CrossRef]
- Farooqui, A.; Pillai, S.K.; Agnihotri, D.; Khan, S.; Tewari, R.; Shukla, S.K.; Ali, S.; Trivedi, A.; Pandita, S.K.; Kumar, K.; et al. Impact of climate on the evolution of vegetation in tectonically active Karewa basin, Kashmir Himalayas. J. Earth Syst. Sci. 2021, 130, 93. [Google Scholar] [CrossRef]
- Li, Z.; Tian, D.; Wang, B.; Wang, J.; Wang, S.; Chen, H.Y.H.; Xu, X.; Wang, C.; He, N.; Niu, S. Microbes drive global soil nitrogen mineralization and availability. Glob. Chang. Biol. 2019, 25, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Trenberth, K.E.; Dai, A.G.; van der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Brenner, D.L.; Amundson, R.; Baisden, W.T.; Kendall, C.; Harden, J. Soil n and n-15 variation with time in a California annual grassland ecosystem. Geochim. Cosmochim. Acta 2001, 65, 4171–4186. [Google Scholar] [CrossRef]
- Evans, S.E.; Burke, I.C. Carbon and Nitrogen Decoupling Under an 11-Year Drought in the Shortgrass Steppe. Ecosystems 2013, 16, 704–705. [Google Scholar] [CrossRef]
- Körner, C.; Kèorner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Kuchenbuch, R.; Claassen, N.; Jungk, A. Potassium availability in relation to soil moisture. Plant Soil 1986, 95, 233–243. [Google Scholar] [CrossRef]
- Bargagli, R.; Brown, D.H.; Nelli, L. Metal biomonitoring with mosses: Procedures for correcting for soil contamination. Environ. Pollut. 1995, 89, 169–175. [Google Scholar] [CrossRef]
- Mahlstein, I.; Daniel, J.S.; Solomon, S. Pace of shifts in climate regions increases with global temperature. Nat. Clim. Chang. 2013, 3, 739–743. [Google Scholar] [CrossRef]
- Moles, A.T.; Perkins, S.E.; Laffan, S.W.; Flores-Moreno, H.; Awasthy, M.; Tindall, M.L.; Sack, L.; Pitman, A.; Kattge, J.; Aarssen, L.W.; et al. Which is a better predictor of plant traits: Temperature or precipitation? J. Veg. Sci. 2014, 25, 1167–1180. [Google Scholar] [CrossRef]
- Coban, O.; de Deyn, G.B.; van der Ploeg, M. Soil microbiota as game-changers in restoration of degraded lands. Science 2022, 375, abe0725. [Google Scholar] [CrossRef]
- Watanabe, T.; Broadley, M.R.; Jansen, S.; White, P.J.; Takada, J.; Satake, K.; Takamatsu, T.; Tuah, S.J.; Osaki, M. Evolutionary control of leaf element composition in plants. New Phytol. 2007, 174, 516–523. [Google Scholar] [CrossRef]
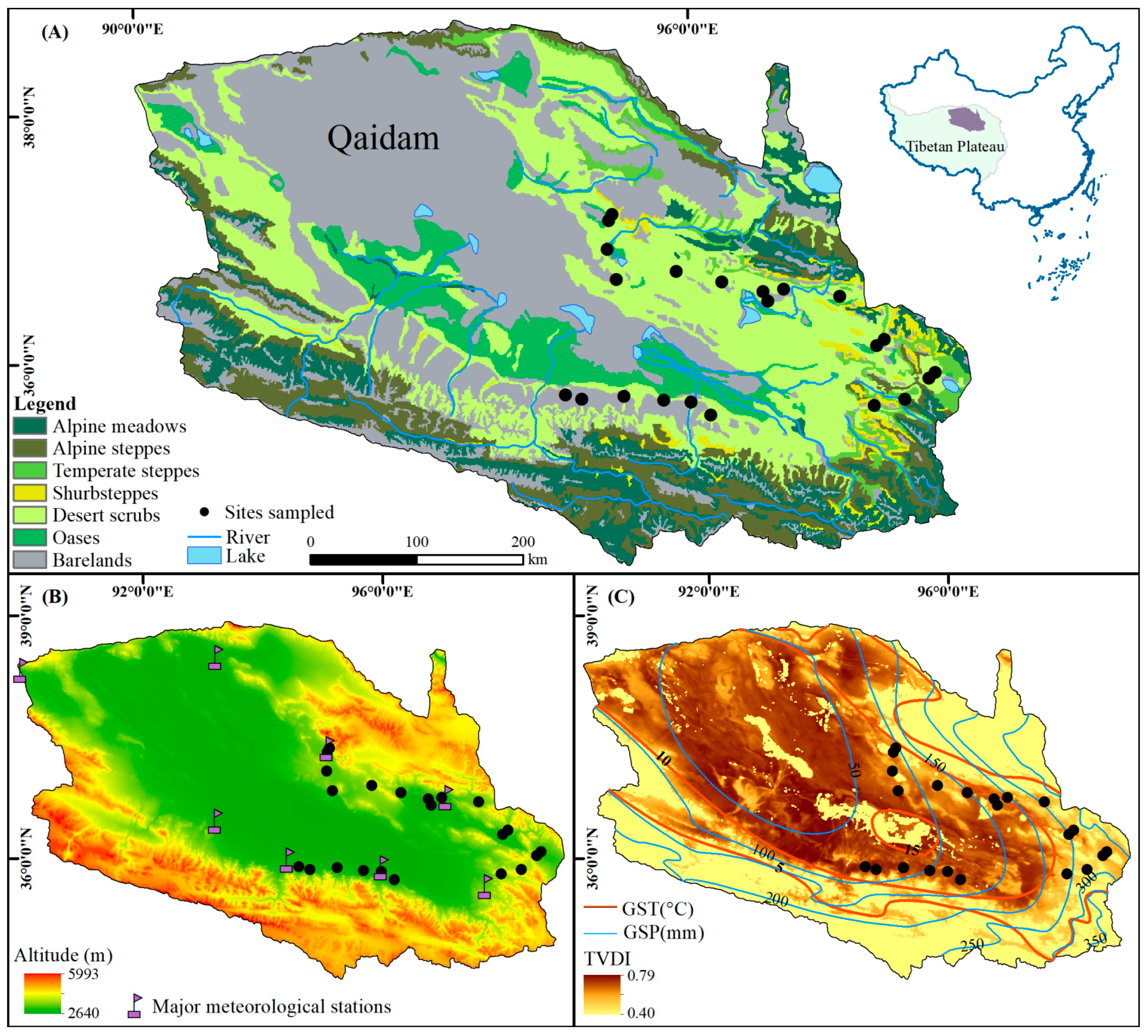

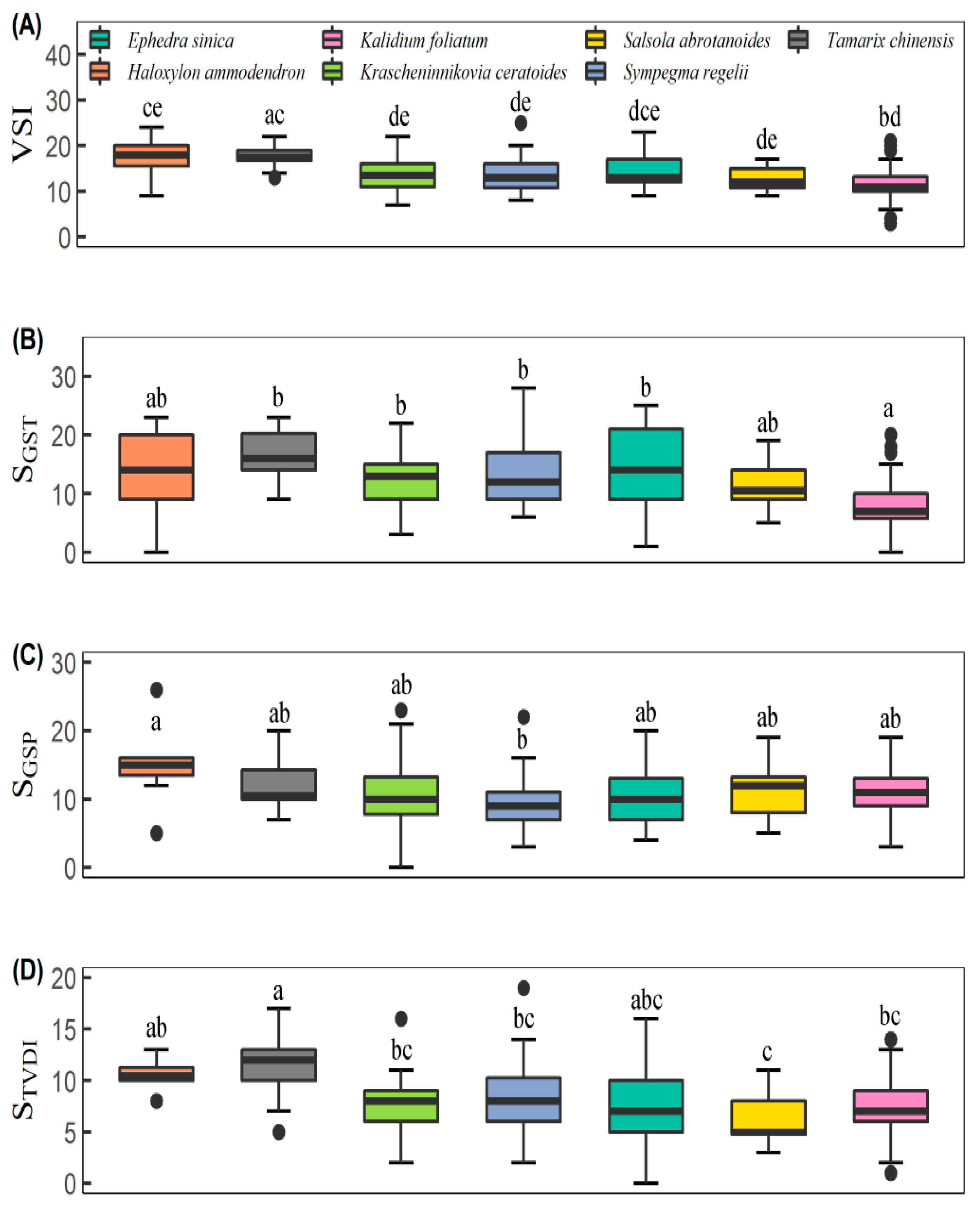
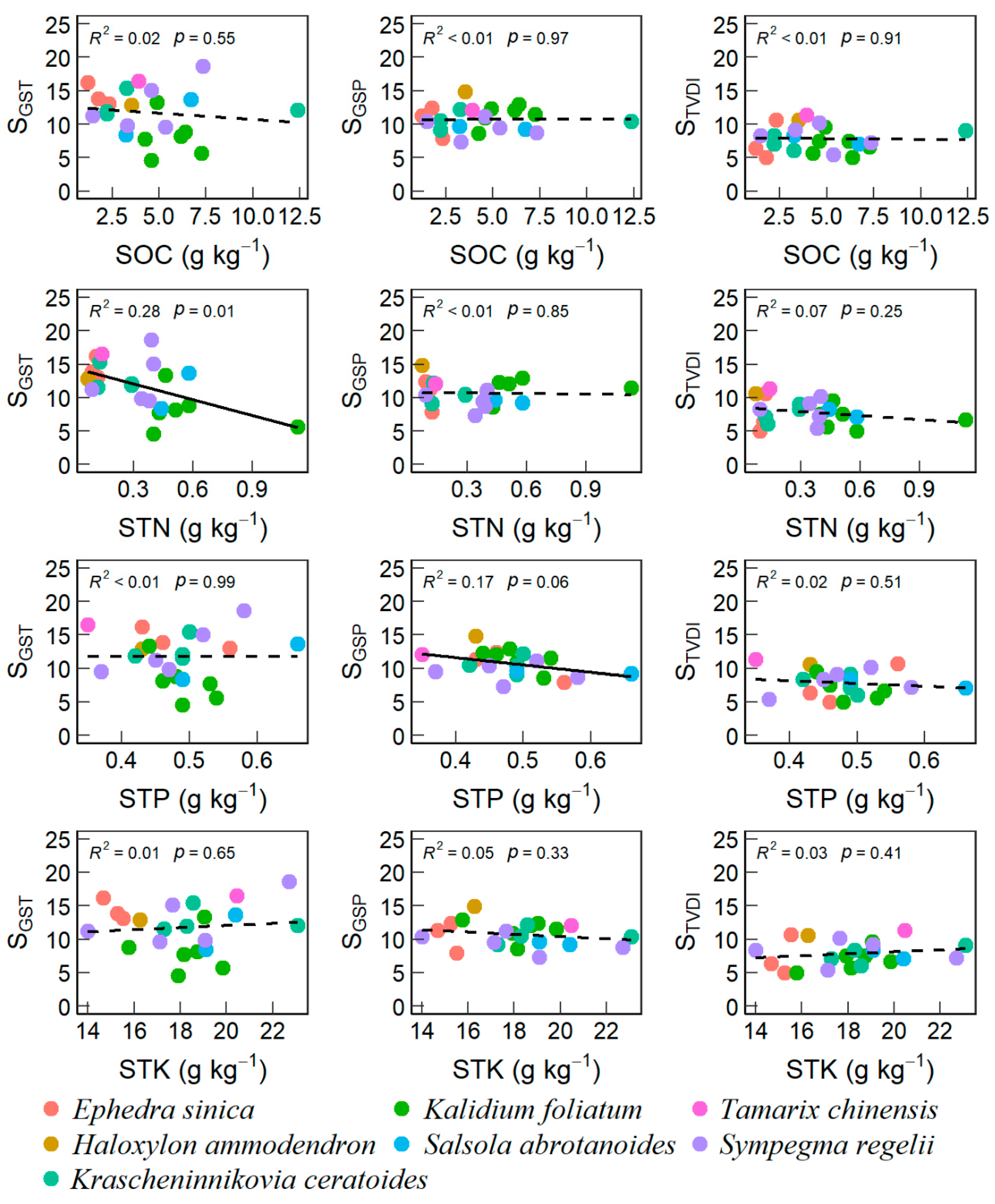
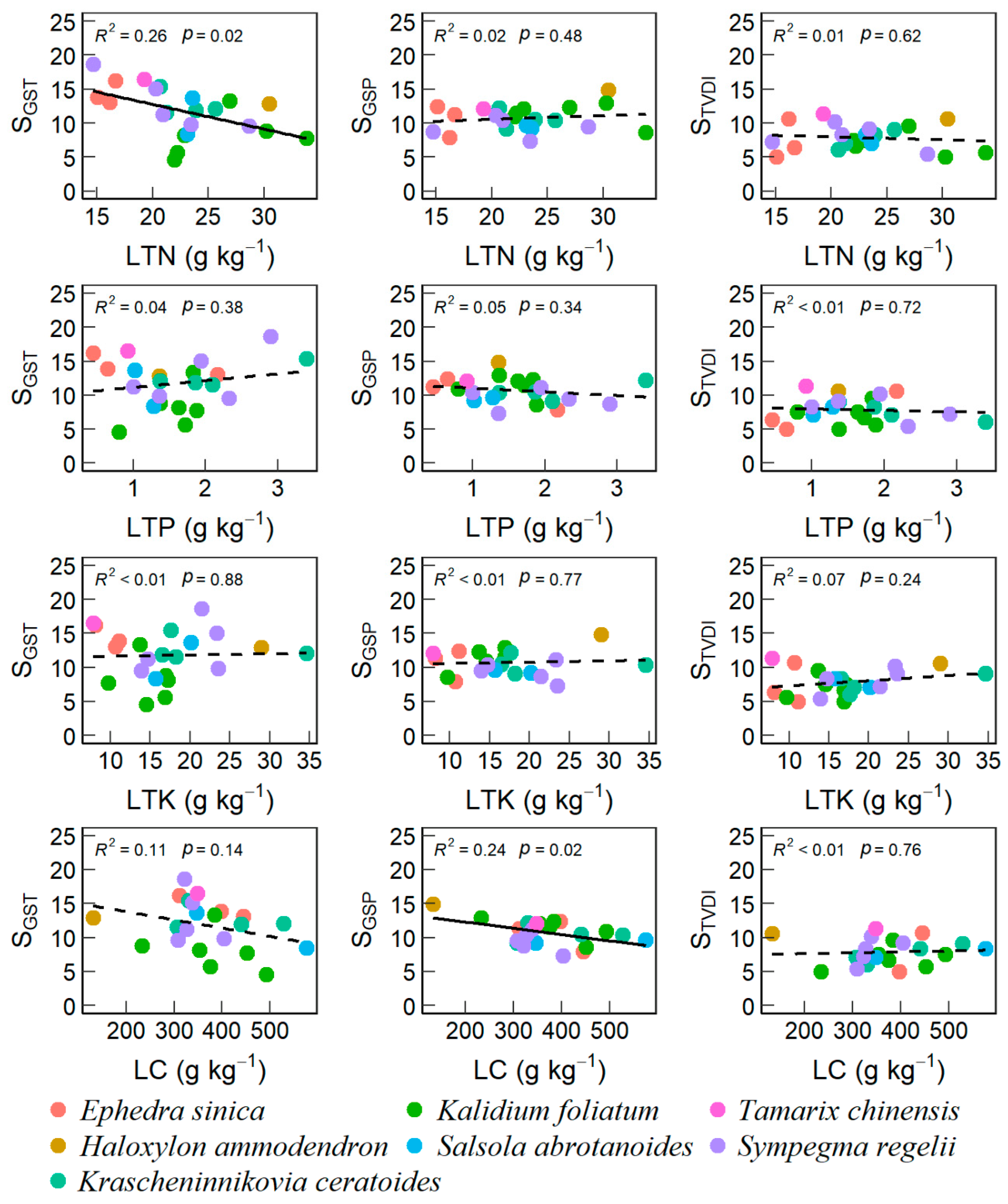
| Site | Long (°E) | Lat. (°N) | Alt. (m) | Dominant Species | DSC (%) | GST (°C) | GSP (mm) | SOC (g kg−1) | STN (g kg−1) | STK (g kg−1) | STP (g kg−1) | LC (g kg−1) | LTN (g kg−1) | LTP (g kg−1) | LTK (g kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site1 | 95.091 | 36.346 | 2890 | Sympegma regelii | 53.0 | 16.4 | 66 | 1.48 | 0.09 | 14.00 | 0.45 | 326.60 | 20.95 | 1.00 | 14.72 |
| Site2 | 95.267 | 36.325 | 2970 | Ephedra sinica | 65.0 | 15.8 | 72 | 1.21 | 0.11 | 14.67 | 0.43 | 311.36 | 16.67 | 0.45 | 8.16 |
| Site3 | 95.703 | 36.381 | 2850 | Ephedra sinica | 48.0 | 16.8 | 73 | 1.76 | 0.09 | 15.27 | 0.46 | 397.40 | 15.09 | 0.65 | 11.13 |
| Site4 | 96.126 | 36.378 | 2765 | Tamarix chinensis | 100.0 | 17.3 | 79 | 3.91 | 0.14 | 20.46 | 0.35 | 348.00 | 19.25 | 0.92 | 7.88 |
| Site5 | 96.413 | 36.382 | 2855 | Krascheninnikovia ceratoides | 50.0 | 16.7 | 92 | 2.20 | 0.12 | 17.29 | 0.49 | 307.20 | 21.28 | 2.10 | 18.18 |
| Site6 | 96.630 | 36.293 | 2850 | Haloxylon ammodendron | 100.0 | 16.4 | 101 | 3.52 | 0.07 | 16.27 | 0.43 | 132.00 | 30.45 | 1.36 | 28.97 |
| Site7 | 98.329 | 36.469 | 3320 | Kalidium foliatum | 80.0 | 12.2 | 224 | 3.23 | 0.44 | 19.09 | 0.49 | 576.00 | 23.10 | 1.28 | 15.68 |
| Site8 | 98.648 | 36.536 | 3530 | Kalidium foliatum | 59.0 | 10.4 | 264 | 6.16 | 0.51 | 18.74 | 0.46 | 353.51 | 22.87 | 1.63 | 17.19 |
| Site9 | 98.883 | 36.723 | 3175 | Kalidium foliatum | 100.0 | 12.3 | 261 | 7.26 | 1.13 | 19.84 | 0.54 | 375.00 | 22.20 | 1.72 | 16.88 |
| Site10 | 98.947 | 36.773 | 3070 | Kalidium foliatum | 65.0 | 13.1 | 257 | 4.25 | 0.43 | 18.14 | 0.53 | 451.50 | 33.84 | 1.88 | 9.71 |
| Site11 | 98.312 | 36.962 | 2945 | Sympegma regelii | 27.0 | 14.5 | 214 | 4.58 | 0.40 | 17.65 | 0.52 | 338.68 | 20.31 | 1.94 | 23.31 |
| Site12 | 98.390 | 37.017 | 3130 | Kalidium foliatum | 61.0 | 13.0 | 236 | 4.58 | 0.40 | 17.92 | 0.49 | 492.66 | 21.99 | 0.80 | 14.55 |
| Site13 | 97.886 | 37.344 | 3205 | Salsola abrotanoides | 56.0 | 12.8 | 222 | 6.38 | 0.58 | 15.77 | 0.48 | 233.33 | 30.24 | 1.37 | 16.91 |
| Site14 | 97.284 | 37.366 | 2970 | Sympegma regelii | 70.0 | 14.6 | 172 | 5.35 | 0.38 | 17.12 | 0.37 | 307.80 | 28.65 | 2.33 | 13.93 |
| Site15 | 97.127 | 37.258 | 2860 | Krascheninnikovia ceratoides | 56.0 | 16.3 | 146 | 2.20 | 0.29 | 18.29 | 0.42 | 440.00 | 23.90 | 1.86 | 16.50 |
| Site16 | 97.068 | 37.333 | 2905 | Kalidium foliatum | 83.33 | 15.8 | 149 | 4.90 | 0.46 | 19.04 | 0.44 | 384.00 | 26.95 | 1.83 | 13.69 |
| Site17 | 96.623 | 37.384 | 2980 | Sympegma regelii | 60.00 | 15.7 | 126 | 3.30 | 0.34 | 19.08 | 0.47 | 403.80 | 23.42 | 1.36 | 23.54 |
| Site18 | 96.135 | 37.440 | 3620 | Salsola abrotanoides | 66.67 | 11.2 | 135 | 6.71 | 0.58 | 20.40 | 0.66 | 346.67 | 23.57 | 1.02 | 20.15 |
| Site19 | 95.395 | 37.855 | 3310 | Sympegma regelii | 40.00 | 13.1 | 91 | 7.33 | 0.39 | 22.71 | 0.58 | 321.60 | 14.70 | 2.90 | 21.44 |
| Site20 | 95.364 | 37.800 | 3190 | Krascheninnikovia ceratoides | 100.00 | 13.8 | 85 | 12.38 | 0.29 | 23.09 | 0.49 | 528.00 | 25.67 | 1.37 | 34.63 |
| Site21 | 95.381 | 37.565 | 3185 | Krascheninnikovia ceratoides | 50.00 | 14.1 | 82 | 3.25 | 0.13 | 18.56 | 0.50 | 330.00 | 20.65 | 3.40 | 17.60 |
| Site22 | 95.506 | 37.328 | 3035 | Ephedra sinica | 40.00 | 15.3 | 76 | 2.31 | 0.12 | 15.52 | 0.56 | 444.60 | 16.19 | 2.17 | 10.66 |
| Haloxylon ammodendron (%) | Tamarix chinensis (%) | Krascheninnikovia ceratoides (%) | Sympegma regelii (%) | Ephedra sinica (%) | Salsola abrotanoides (%) | Kalidium foliatum (%) | Others (%) | Total (%) | |
|---|---|---|---|---|---|---|---|---|---|
| VSI ≤ 10 | 3.24 | 1.10 | 4.57 | 0.32 | 0.69 | 4.07 | 0.83 | 2.45 | 17.32 |
| 10 < VSI ≤ 20 | 8.00 | 6.96 | 16.64 | 1.39 | 2.88 | 20.70 | 5.46 | 7.88 | 70.27 |
| 20 < VSI ≤ 30 | 0.96 | 1.28 | 2.78 | 0.16 | 0.41 | 3.64 | 0.80 | 1.09 | 11.23 |
| VSI > 30 | 0.09 | 0.10 | 0.36 | 0.01 | 0.04 | 0.40 | 0.06 | 0.11 | 1.18 |
| Predictor | d.f. | VSI, R2 = 0.57 | SGST, R2 = 0.74 | SGSP, R2 = 0.45 | STVDI, R2 = 0.21 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | η2 (%) | F | p | η2 (%) | F | p | η2 (%) | F | p | η2 (%) | ||
| SOC | 1 | 0.29 | 0.597 | 0.98 | 0.89 | 0.362 | 1.80 | 0.00 | 0.970 | 0.01 | 0.01 | 0.910 | 0.07 |
| STN | 1 | 7.74 | 0.016 * | 25.88 | 14.87 | 0.002 ** | 29.90 | 0.07 | 0.795 | 0.30 | 1.54 | 0.236 | 8.34 |
| STP | 1 | 0.14 | 0.710 | 0.48 | 2.01 | 0.180 | 4.04 | 4.43 | 0.055 | 18.88 | 0.09 | 0.764 | 0.51 |
| STK | 1 | 1.31 | 0.273 | 4.39 | 3.79 | 0.073 | 7.62 | 1.94 | 0.188 | 8.25 | 1.82 | 0.200 | 9.83 |
| LTN | 1 | 1.35 | 0.266 | 4.53 | 4.53 | 0.053 | 9.11 | 0.04 | 0.850 | 0.16 | 0.00 | 0.963 | 0.01 |
| LTP | 1 | 0.00 | 0.946 | 0.02 | 1.11 | 0.311 | 2.23 | 0.08 | 0.788 | 0.32 | 0.45 | 0.516 | 2.41 |
| LTK | 1 | 0.96 | 0.345 | 3.22 | 0.05 | 0.831 | 0.10 | 0.63 | 0.443 | 2.67 | 1.57 | 0.232 | 8.50 |
| LC | 1 | 5.09 | 0.042 * | 17.02 | 9.48 | 0.009 ** | 19.06 | 3.27 | 0.094 | 13.96 | 0.02 | 0.903 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Chen, H.; Li, M.; Fiedler, S.; Mărgărint, M.C.; Nowak, A.; Wesche, K.; Tietjen, B.; Wu, J. Climate Sensitivity of the Arid Scrublands on the Tibetan Plateau Mediated by Plant Nutrient Traits and Soil Nutrient Availability. Remote Sens. 2022, 14, 4601. https://doi.org/10.3390/rs14184601
Chen B, Chen H, Li M, Fiedler S, Mărgărint MC, Nowak A, Wesche K, Tietjen B, Wu J. Climate Sensitivity of the Arid Scrublands on the Tibetan Plateau Mediated by Plant Nutrient Traits and Soil Nutrient Availability. Remote Sensing. 2022; 14(18):4601. https://doi.org/10.3390/rs14184601
Chicago/Turabian StyleChen, Ben, Hui Chen, Meng Li, Sebastian Fiedler, Mihai Ciprian Mărgărint, Arkadiusz Nowak, Karsten Wesche, Britta Tietjen, and Jianshuang Wu. 2022. "Climate Sensitivity of the Arid Scrublands on the Tibetan Plateau Mediated by Plant Nutrient Traits and Soil Nutrient Availability" Remote Sensing 14, no. 18: 4601. https://doi.org/10.3390/rs14184601






