Early Monitoring of Cotton Verticillium Wilt by Leaf Multiple “Symptom” Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Method of Classifying the Severity of VW
2.2. Method for the Determination of Leaf Reflectance Spectra
2.3. Determination of Physiological Parameters of Cotton Leaves
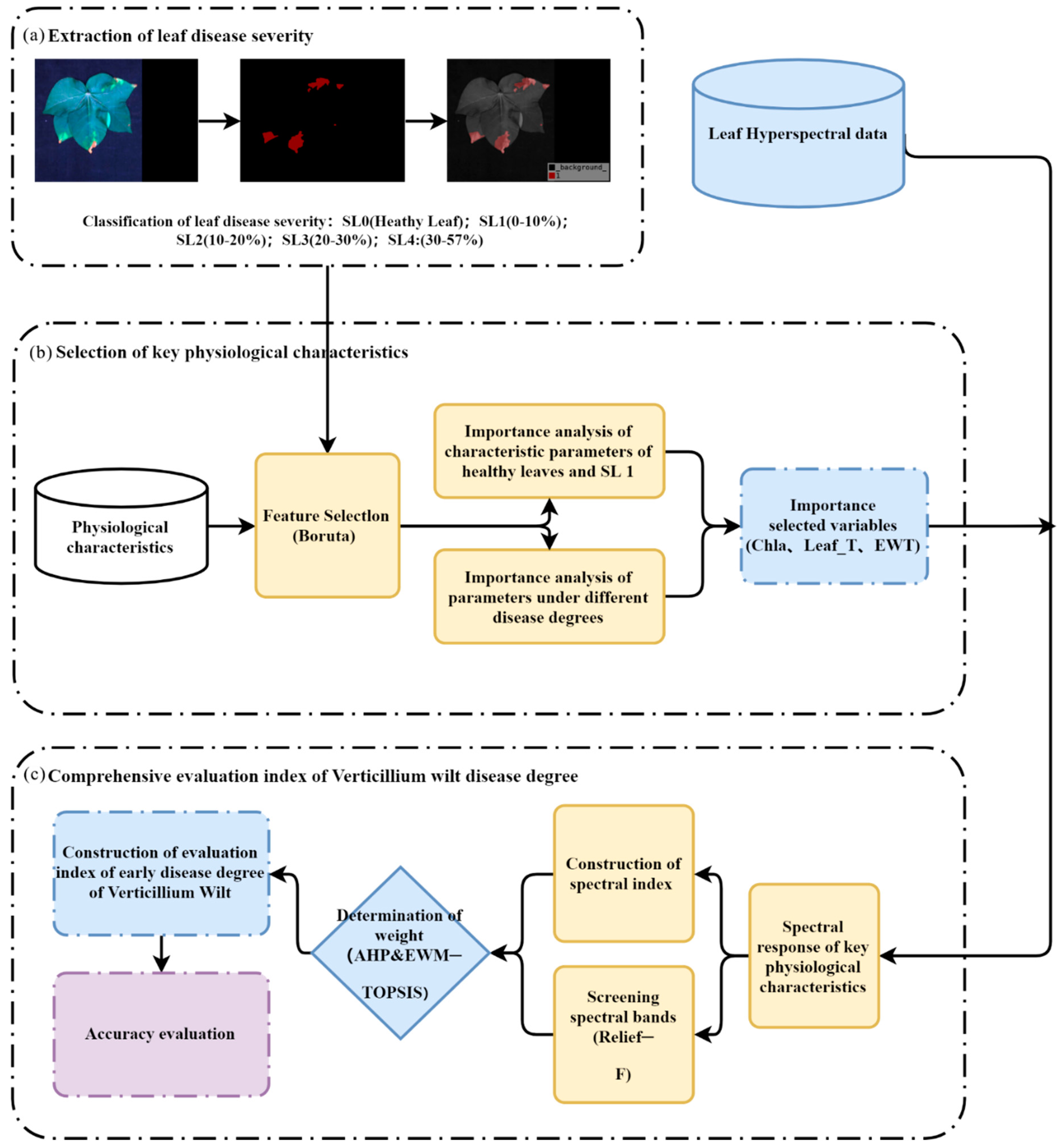
2.4. Screening of Key Physiological Characteristics of Cotton Leaves under VW Stress and Construction of a Spectral Index of Multiple “Symptom” Characteristics
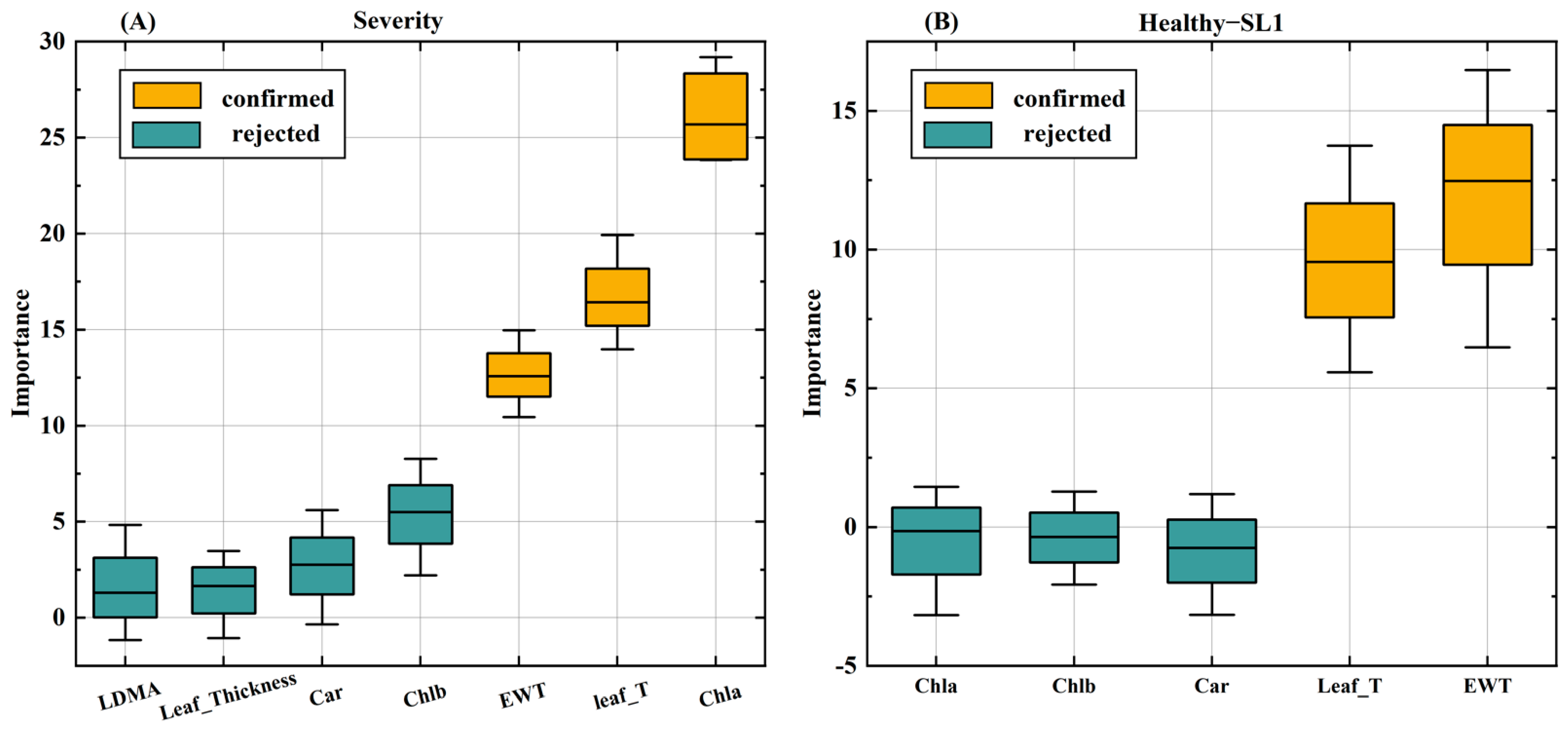
2.4.1. Screening Methods for Key Physiological Traits
2.4.2. Construction of a Spectral Index of Multiple “Symptom” Characteristics
2.4.3. Classic Vegetation Index
2.5. Methodology for the Development of Early Monitoring Indicators for VW in Cotton
2.6. Development of Remote Sensing Indicators for Early VW Monitoring of Multiple ‘Symptoms’ of Cotton
3. Results
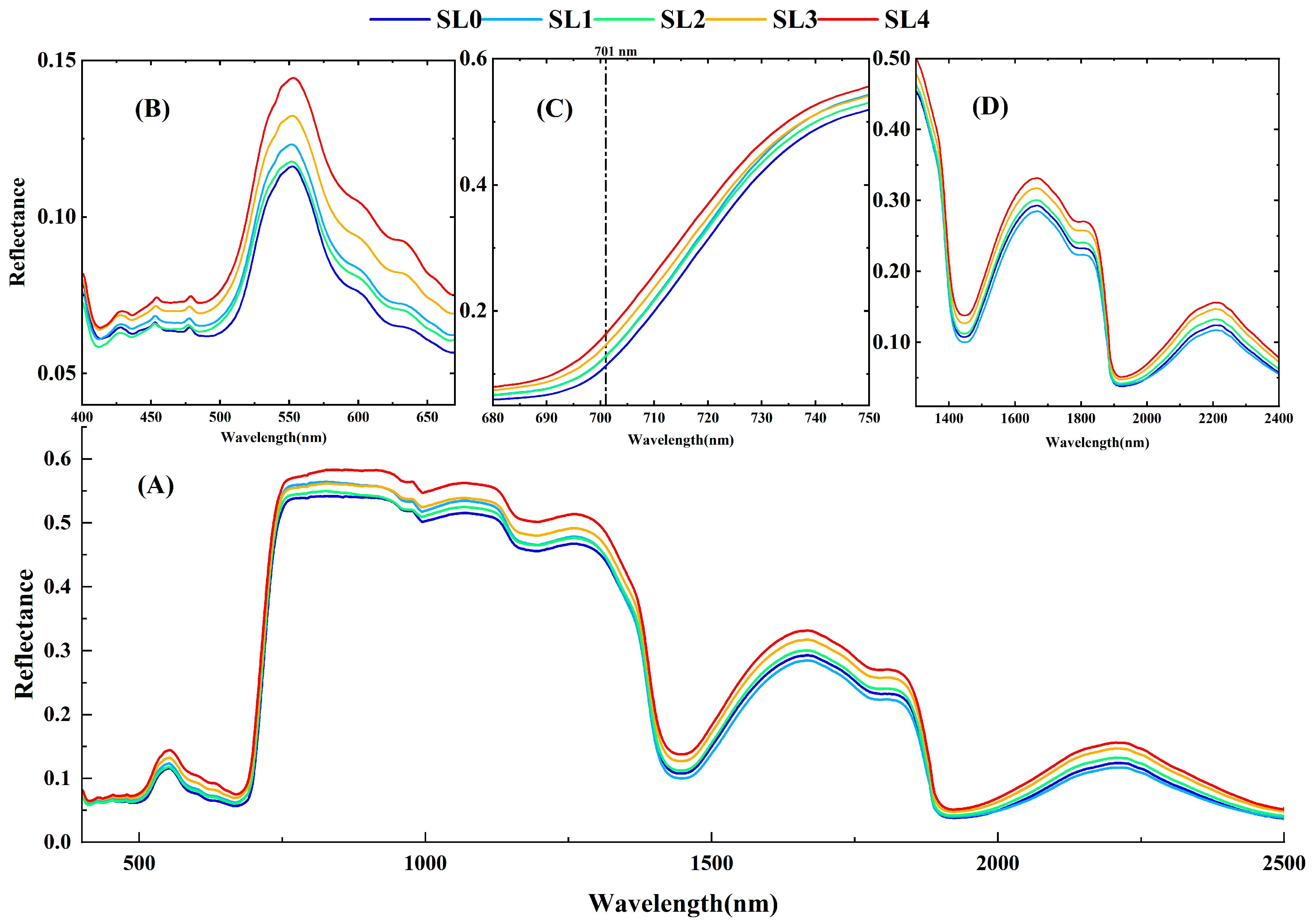
3.1. Spectral Characteristics of Cotton VW Leaves under Different Disease Levels
3.2. Effect of Different Disease Levels on Physiological Indicators of Cotton Leaves
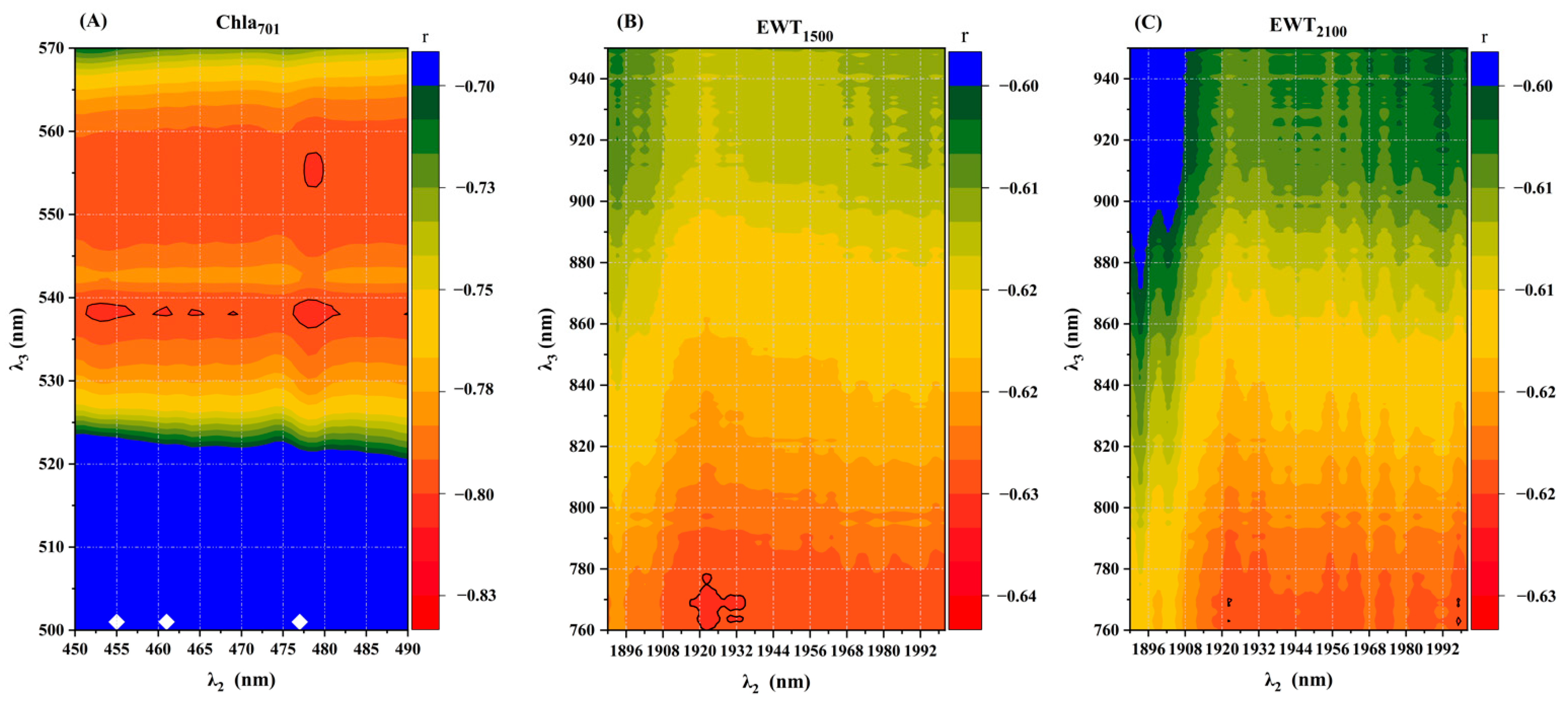
3.3. Selection of Characteristic Bands and Construction of Spectral Indices
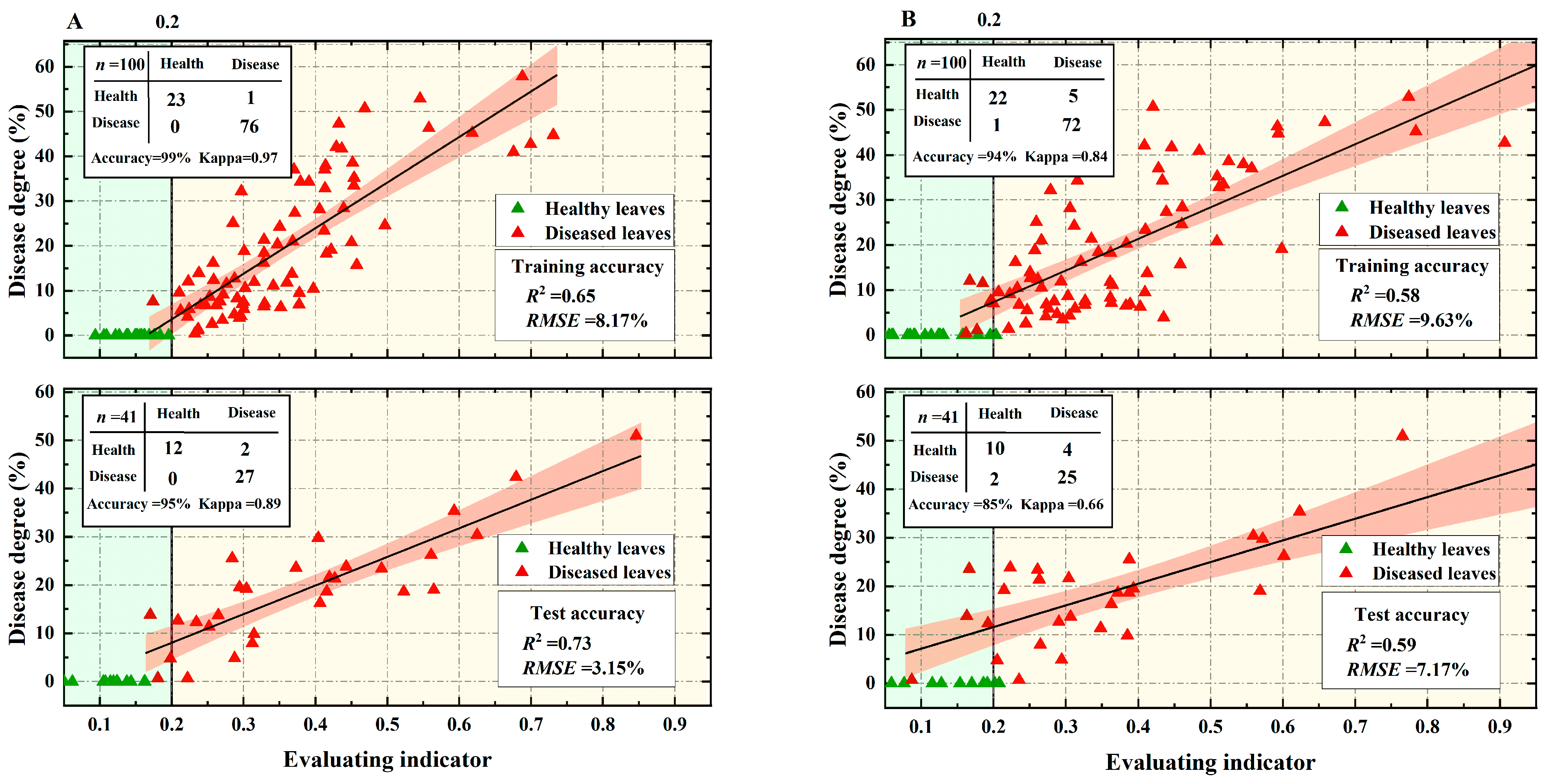
3.4. Constructing Early Monitoring Indicators for VW in Cotton Based on AHP and EWM
4. Discussion
5. Conclusions
- (1)
- When compared to the classical vegetation index, the constructed spectral index of physiological traits of cotton under VW stress correlates more significantly with the severity of cotton diseased leaves. Achieving early and more accurate monitoring of the extent of disease in vegetation requires quantification of the physiological conditions corresponding to plant-specific biotic stresses;
- (2)
- The CVWEI can further improve the early detection and severity monitoring of cotton VW by comparing the constructed early detection index of multiple ‘symptom’ features of cotton VW with the model constructed from a single physiological spectral index. The test set had a classification accuracy of 95%, a Kappa coefficient of 0.89, a linear regression model for disease severity, R2 = 0.73, and an RMSE of 3.15%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, R.; Li, J.; Xie, C.; Jian, W.; Yang, X. An Overview of the Molecular Genetics of Plant Resistance to the Verticillium Wilt Pathogen Verticillium dahliae. Int. J. Mol. Sci. 2020, 21, 1120. [Google Scholar] [CrossRef] [Green Version]
- Fradin, E.F.; Thomma, B.P. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef]
- Calderón, R.; Navas-Cortés, J.A.; Lucena, C.; Zarco-Tejada, P.J. High-resolution airborne hyperspectral and thermal imagery for early detection of Verticillium wilt of olive using fluorescence, temperature and narrow-band spectral indices. Remote Sens. Environ. 2013, 139, 231–245. [Google Scholar] [CrossRef]
- Mahlein, A.K.; Rumpf, T.; Welke, P.; Dehne, H.W.; Plümer, L.; Steiner, U.; Oerke, E.C. Development of spectral indices for detecting and identifying plant diseases. Remote Sens. Environ. 2013, 128, 21–30. [Google Scholar] [CrossRef]
- Galieni, A.; D’Ascenzo, N.; Stagnari, F.; Pagnani, G.; Xie, Q.; Pisante, M. Past and Future of Plant Stress Detection: An Overview from Remote Sensing to Positron Emission Tomography. Front. Plant Sci. 2020, 11, 609155. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y.; Wang, T.; Dong, Q.; Li, J.; Niu, C. Detection of 12 common food-borne bacterial pathogens by TaqMan real-time PCR using a single set of reaction conditions. Front. Microbiol. 2019, 10, 222. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Pu, R.; Gonzalez-Moreno, P.; Yuan, L.; Wu, K.; Huang, W. Monitoring plant diseases and pests through remote sensing technology: A review. Comput. Electron. Agr. 2019, 165, 104943. [Google Scholar] [CrossRef]
- Ortiz, J.C.M.; Carvajal, L.M.H.; Fernandez, V.B. Detection of significant wavelengths for identifying and classifying Fusarium oxysporum during the incubation period and water stress in Solanum lycopersicum plants using reflectance spectroscopy. J. Plant Prot. Res. 2019, 59, 244–254. [Google Scholar]
- Cunniffe, N.J.; Cobb, R.C.; Meentemeyer, R.K.; Rizzo, D.M.; Gilligan, C.A. Modeling when, where, and how to manage a forest epidemic, motivated by sudden oak death in California. Proc. Natl. Acad. Sci. USA 2016, 113, 5640–5645. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Clemente, R.; Hornero, A.; Mottus, M.; Penuelas, J.; González-Dugo, V.; Jiménez, J.C.; Suárez, L.; Alonso, L.; Zarco-Tejada, P.J. Early Diagnosis of Vegetation Health from High-Resolution Hyperspectral and Thermal Imagery: Lessons Learned from Empirical Relationships and Radiative Transfer Modelling. Curr. For. Rep. 2019, 5, 169–183. [Google Scholar] [CrossRef] [Green Version]
- Russell, B.C.; Torralba, A.; Murphy, K.P.; Freeman, W.T. LabelMe: A Database and Web-Based Tool for Image Annotation. Int. J. Comput. Vis. 2008, 77, 157–173. [Google Scholar] [CrossRef]
- Chen, B.; Li, S.; Wang, K.; Zhou, G.; Bai, J. Evaluating the severity level of cotton Verticillium using spectral signature analysis. Int. J. Remote Sens. 2012, 33, 2706–2724. [Google Scholar] [CrossRef]
- Hampton, R.E.; Wullschleger, S.D.; Oosterhuis, D.M. Impact of Verticillium wilt on net photosynthesis, respiration and photorespiration in field-grown cotton (Gossypium hirsutum L.). Physiol. Mol. Plant Pathol. 1990, 37, 271–280. [Google Scholar] [CrossRef]
- Saeed, I.A.M.; MacGuidwin, A.E.; Rouse, D.I.; Sharkey, T.D. Limitation to Photosynthesis in Pratylenchus penetrans- and Verticillium dahliae-Infected Potato. Crop Sci. 1999, 39, 1340–1346. [Google Scholar] [CrossRef]
- Hernández-Clemente, R.; Navarro-Cerrillo, R.M.; Zarco-Tejada, P.J. Carotenoid content estimation in a heterogeneous conifer forest using narrow-band indices and PROSPECT+DART simulations. Remote Sens. Environ. 2012, 127, 298–315. [Google Scholar] [CrossRef]
- Abadia, J. Iron and plant pigments. In Iron Chelation in Plants and Soil Microorganisms; Academic Press: Cambridge, MA, USA, 1993; pp. 327–343. [Google Scholar]
- Li, X.; Sun, Z.; Lu, S.; Omasa, K. A multi-angular invariant spectral index for the estimation of leaf water content across a wide range of plant species in different growth stages. Remote Sens. Environ. 2021, 253, 112230. [Google Scholar] [CrossRef]
- Kuhlgert, S.; Austic, G.; Zegarac, R.; Osei-Bonsu, I.; Hoh, D.; Chilvers, M.I.; Roth, M.G.; Bi, K.; TerAvest, D.; Weebadde, P.; et al. Multispe Q Beta: A tool for large-scale plant phenotyping connected to the open PhotosynQ network. R. Soc. Open Sci. 2016, 3, 160592. [Google Scholar] [CrossRef] [Green Version]
- Kursa, M.B.; Rudnicki, W.R. Feature selection with the Boruta package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Gao, B. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Apan, A.; Held, A.; Phinn, S.; Markley, J. Detecting sugarcane ‘orange rust’disease using EO-1 Hyperion hyperspectral imagery. Int. J. Remote Sens. 2004, 25, 489–498. [Google Scholar] [CrossRef] [Green Version]
- Dash, J.; Curran, P.J. The MERIS terrestrial chlorophyll index. Int. J. Remote Sens. 2004, 25, 5403–5413. [Google Scholar] [CrossRef]
- Gamon, J.; Serrano, L.; Surfus, J.S. The photochemical reflectance index: An optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, G.A. Spectral indices for estimating photosynthetic pigment concentrations: A test using senescent tree leaves. Int. J. Remote Sens. 1998, 19, 657–675. [Google Scholar] [CrossRef]
- Jing, X.; Huang, W.; Wang, J.; Wang, J.; Wang, K. Hyperspectral inversion models on verticillium wilt severity of cotton leaf. Spectrosc. Spect. Anal. 2009, 29, 3348–3352. [Google Scholar]
- Machardy, W.E.; Busch, L.V.; Hall, R. Verticillium wilt of chrysanthemum: Quantitative relationship between increased stomatal resistance and local vascular dysfunction preceding wilt. Can. J. Bot. 1976, 54, 1023–1034. [Google Scholar] [CrossRef]
- Pascual, I.; Azcona, I.; Morales, F.; Aguirreolea, J.; Sánchez-Díaz, M. Photosynthetic response of pepper plants to wilt induced by Verticillium dahliae and soil water deficit. J. Plant Physiol. 2010, 167, 701–708. [Google Scholar] [CrossRef]
- Tzeng, D.D.; de Vay, J.E. Physiological responses of Gossypium hirsutum L. to infection by defoliating and nondefoliating pathotypes of Verticillium dahliae Kleb. Physiol. Plant Pathol. 1985, 26, 57–72. [Google Scholar] [CrossRef]
- Matorin, D.N.; Timofeev, N.P.; Glinushkin, A.P.; Bratkovskaja, L.B.; Zayadan, B.K. Effect of Fungal Infection with Bipolaris sorokiniana on Photosynthetic Light Reactions in Wheat Analyzed by Fluorescence Spectroscopy. Mosc. Univ. Biol. Sci. Bull. 2018, 73, 203–208. [Google Scholar] [CrossRef]
- Zhang, L.; Qiao, N.; Huang, C.; Wang, S. Monitoring Drought Effects on Vegetation Productivity Using Satellite Solar-Induced Chlorophyll Fluorescence. Remote Sens. 2019, 11, 378. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liang, C.; Wu, S.; Zhang, X.; Tang, J.; Jian, G.; Jiao, G.; Li, F.; Chu, C. Significant Improvement of Cotton Verticillium Wilt Resistance by Manipulating the Expression of Gastrodia Antifungal Proteins. Mol. Plant 2016, 9, 1436–1439. [Google Scholar] [CrossRef] [Green Version]
- Berdugo, C.A.; Zito, R.; Paulus, S.; Mahlein, A.K. Fusion of sensor data for the detection and differentiation of plant diseases in cucumber. Plant Pathol. 2014, 63, 1344–1356. [Google Scholar] [CrossRef]
- López-López, M.; Calderón, R.; González-Dugo, V.; Zarco-Tejada, P.J.; Fereres, E. Early detection and quantification of almond red leaf blotch using high-resolution hyperspectral and thermal imagery. Remote Sens. 2016, 8, 276. [Google Scholar] [CrossRef]
- Gold, K.M.; Townsend, P.A.; Chlus, A.; Herrmann, I.; Couture, J.J.; Larson, E.R.; Gevens, A.J. Hyperspectral Measurements Enable Pre-Symptomatic Detection and Differentiation of Contrasting Physiological Effects of Late Blight and Early Blight in Potato. Remote Sens. 2020, 12, 286. [Google Scholar] [CrossRef] [Green Version]
- Zarco-Tejada, P.J.; Camino, C.; Beck, P.S.A.; Calderon, R.; Hornero, A.; Hernández-Clemente, R.; Kattenborn, T.; Montes-Borrego, M.; Susca, L.; Morelli, M.; et al. Previsual symptoms of Xylella fastidiosa infection revealed in spectral plant-trait alterations. Nat. Plants 2018, 4, 432–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camino, C.; Calderón, R.; Parnell, S.; Dierkes, H.; Chemin, Y.; Román-Écija, M.; Montes-Borrego, M.; Landa, B.B.; Navas-Cortes, J.A.; Zarco-Tejada, P.J.; et al. Detection of Xylella fastidiosa in almond orchards by synergic use of an epidemic spread model and remotely sensed plant traits. Remote Sens. Environ. 2021, 260, 112420. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Su, B. Significant remote sensing vegetation indices: A review of developments and applications. J. Sens. 2017, 2017, 1353691. [Google Scholar] [CrossRef] [Green Version]
- Carter, G.A.; Miller, R.L. Early detection of plant stress by digital imaging within narrow stress-sensitive wavebands. Remote Sens. Environ. 1994, 50, 295–302. [Google Scholar] [CrossRef]
- Chen, B.; Han, H.; Wang, F.; Liu, Z.; Deng, F.; Lin, H.; Yu, Y.; Li, S.; Wang, K.; Xiao, C. Monitoring chlorophyll and nitrogen contents in cotton leaf infected by verticillium wilt with spectra red edge parameters. Acta Agron. Sinica 2013, 39, 319–329. [Google Scholar] [CrossRef]
- Czarnik-Matusewicz, B.; Pilorz, S. Study of the temperature-dependent near-infrared spectra of water by two-dimensional correlation spectroscopy and principal components analysis. Vib. Spectrosc. 2006, 40, 235–245. [Google Scholar] [CrossRef]
- Khan, H.A.; Nakamura, Y.; Furbank, R.T.; Evans, J.R. Effect of leaf temperature on the estimation of photosynthetic and other traits of wheat leaves from hyperspectral reflectance. J. Exp. Bot. 2021, 72, 1271–1281. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.N.; Ding, C.; Xu, W.; Wang, X. Temporal patterns of cotton Fusarium and Verticillium wilt in Jiangsu coastal areas of China. Sci. Rep. 2017, 7, 12581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulridha, J.; Ampatzidis, Y.; Ehsani, R.; de Castro, A.I. Evaluating the performance of spectral features and multivariate analysis tools to detect laurel wilt disease and nutritional deficiency in avocado. Comput. Electron. Agr. 2018, 155, 203–211. [Google Scholar] [CrossRef]
- Palma, D.; Blanchini, F.; Montessoro, P.L. A system-theoretic approach for image-based infectious plant disease severity estimation. PLoS ONE 2022, 17, e272002. [Google Scholar] [CrossRef]
- Abdulridha, J.; Ampatzidis, Y.; Kakarla, S.C.; Roberts, P. Detection of target spot and bacterial spot diseases in tomato using UAV-based and benchtop-based hyperspectral imaging techniques. Precis. Agric. 2020, 21, 955–978. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E. Spectranomics: Emerging science and conservation opportunities at the interface of biodiversity and remote sensing. Glob. Ecol. Conserv. 2016, 8, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Gamon, J.A.; Somers, B.; Malenovský, Z.; Middleton, E.M.; Rascher, U.; Schaepman, M.E. Assessing vegetation function with imaging spectroscopy. Surv. Geophys. 2019, 40, 489–513. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, A.P.; DeVay, J.E.; Pullman, G.S.; Friebertshauser, G.E. A model of Verticillium wilt in relation to cotton growth and development. Phytopathology 1983, 73, 89–95. [Google Scholar] [CrossRef]




| DS Level | Severity | Disease Spot | Sample Size |
|---|---|---|---|
| SL0 | Healthy | 0% | 41 |
| SL1 | Mild severity | 0–10% | 24 |
| SL2 | Low to moderate severity | 10–20% | 36 |
| SL3 | Moderate severity | 20–30% | 31 |
| SL4 | Medium severity | 30–57% | 18 |
| Vegetation Index | Formula | Meaning | Source |
|---|---|---|---|
| NDWI | NDWI = (R830 − R1205)/(R830 + R1205) | Normalized difference water index | [20] |
| DSWI1 | R800/R1600 | Disease stress water index | [21] |
| DSWI2 | R1600/R550 | ||
| HI | (R534 − R698)/(R534 + R698) − 0.5 ∗ R704 | Healthy index | [4] |
| Red_edge | R850/R750 − 1 | Red-edge chlorophyll index | [22] |
| PRI | (R570 − R531)/(R570 + R531) | Photochemical reflectance index | [23] |
| PSSRa | R800/R675 | Pigment-specific simple ratio | [24] |
| PSSRb | R800/R650 | ||
| PSSRc | R800/R500 |
| Vegetation Index | Formula | Meaning | Correlation Coefficient | Source |
|---|---|---|---|---|
| NDWI | NDWI = (R830 − R1205)/(R830 + R1205) | Normalized difference water index | 0.44 ** | [21] |
| DSWI1 | R800/R1600 | Disease stress water index | −0.62 ** | [22] |
| DSWI2 | R1600/R550 | 0.54 ** | ||
| HI | (R534 − R698)/(R534 + R698) − 0.5 ∗ R704 | Healthy index | 0.66 ** | [4] |
| Red_edge | R850/R750 − 1 | Red_edge chlorophyll index | 0.25 | [23] |
| PRI | (R570 − R531)/(R570 + R531) | Photochemical reflectance index | 0.45 ** | [24] |
| PSSRa | R800/R675 | Pigment-specific simple ratio | 0.21 | [25] |
| PSSRb | R800/R650 | 0.32 ** | ||
| PSSRc | R800/R500 | 0.25 | ||
| EWT1500 | (R770 − R1500)/(R770 − R1922) | Water differential ratio index under verticillium wilt stress | 0.66 ** | Proposed in this study |
| EWT2100 | (R765 − R2100)/(R765 − R1922) | 0.62 ** | Proposed in this study | |
| Chla701 | (R538 − R701)/(R538 − R478) | Chlorophyll a difference ratio index under verticillium wilt stress | 0.81 ** | Proposed in this study |
| Target Layer | Index Layer | AHP | EWM | AHP and EWM TOPSIS |
|---|---|---|---|---|
| CVWEI | (R538 − R701)/(R538 − R478) | 0.55 | 0.16 | 0.33 |
| (R770 − R1500)/(R770 − R1922) | 0.10 | 0.31 | 0.26 | |
| (R765 − R2100)/(R765 − R1922) | 0.04 | 0.19 | 0.15 | |
| R1610 | 0.06 | 0.09 | 0.09 | |
| R1892 | 0.07 | 0.14 | 0.10 | |
| R1425 | 0.19 | 0.11 | 0.08 |
| Input | Evaluation Indicator | Training Set | Validation Set |
|---|---|---|---|
| DSWI1 | Accuracy Kappa | 79% | 83% |
| 0.19 | 0.50 | ||
| R2 RMSE | 0.31 | 0.35 | |
| 12.50% | 20.20% | ||
| HI | Accuracy Kappa | 66% | 63% |
| 0.19 | 0.34 | ||
| R2 RMSE | 0.43 | 0.47 | |
| 13.41% | 8.33% | ||
| EWT1500 | Accuracy Kappa | 97% | 85% |
| 0.91 | 0.67 | ||
| R2 RMSE | 0.26 | 0.38 | |
| 13.41% | 9.07% | ||
| Chla701 | Accuracy Kappa | 94% | 85% |
| 0.84 | 0.66 | ||
| R2 RMSE | 0.58 | 0.59 | |
| 9.63% | 7.17% | ||
| CVWEI | Accuracy Kappa | 99% | 95% |
| 0.97 | 0.89 | ||
| R2 RMSE | 0.65 | 0.73 | |
| 8.17% | 3.15% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Huang, C.; Kang, X.; Qin, S.; Ma, L.; Wang, J.; Zhou, X.; Lv, X.; Zhang, Z. Early Monitoring of Cotton Verticillium Wilt by Leaf Multiple “Symptom” Characteristics. Remote Sens. 2022, 14, 5241. https://doi.org/10.3390/rs14205241
Yang M, Huang C, Kang X, Qin S, Ma L, Wang J, Zhou X, Lv X, Zhang Z. Early Monitoring of Cotton Verticillium Wilt by Leaf Multiple “Symptom” Characteristics. Remote Sensing. 2022; 14(20):5241. https://doi.org/10.3390/rs14205241
Chicago/Turabian StyleYang, Mi, Changping Huang, Xiaoyan Kang, Shizhe Qin, Lulu Ma, Jin Wang, Xiaoting Zhou, Xin Lv, and Ze Zhang. 2022. "Early Monitoring of Cotton Verticillium Wilt by Leaf Multiple “Symptom” Characteristics" Remote Sensing 14, no. 20: 5241. https://doi.org/10.3390/rs14205241







