Quantification of Foraging Areas for the Northern Bald Ibis (Geronticus eremita) in the Northern Alpine Foothills: A Random Forest Model Fitted with Optical and Actively Sensed Earth Observation Data
Abstract
:1. Introduction
2. Methods
2.1. Study Species and Study Area
2.2. Biologging
2.3. Environmental Indices
2.4. Species Distribution Modeling
2.5. Variable Importance
2.6. Ethical Standards
3. Results
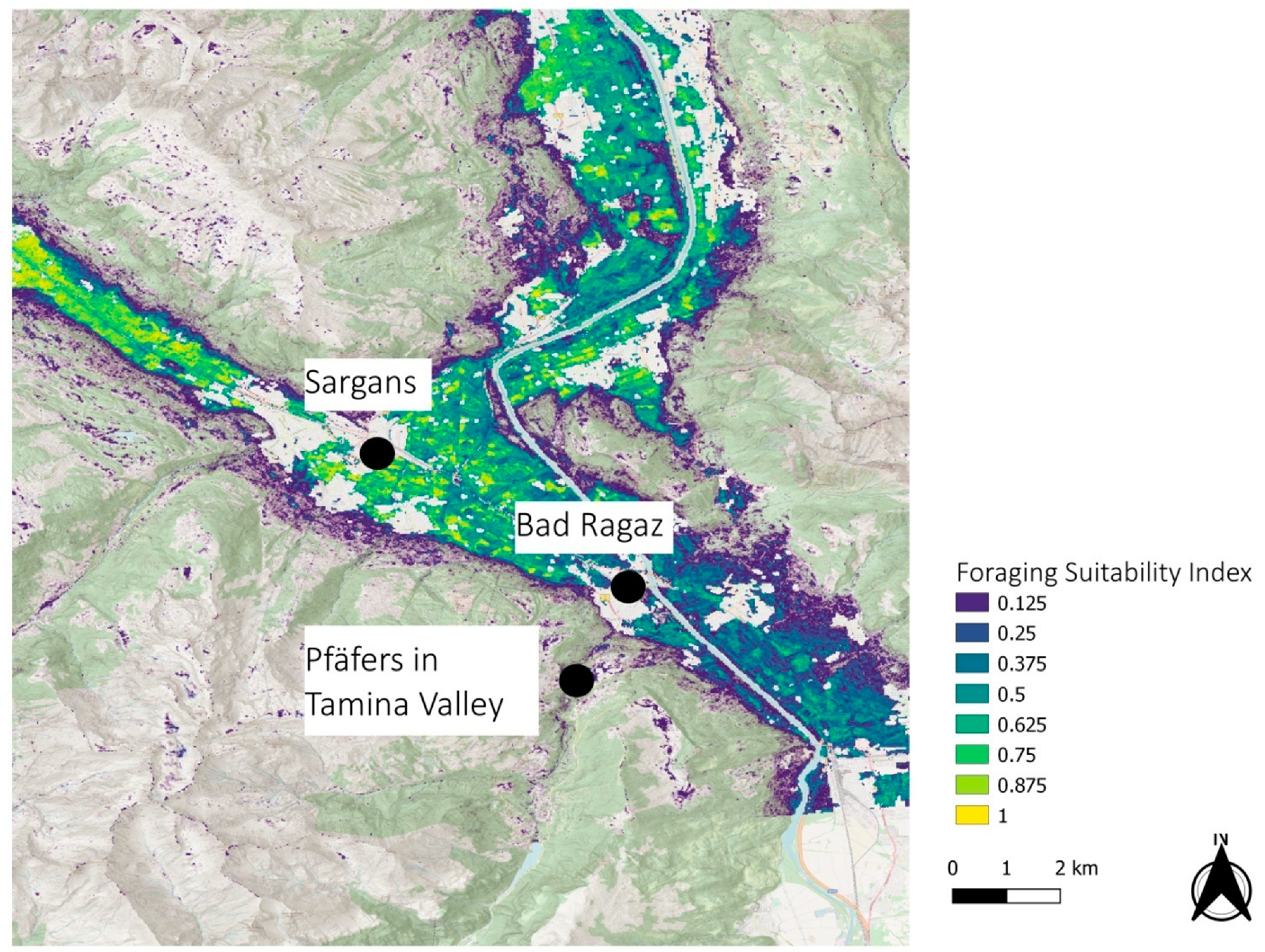
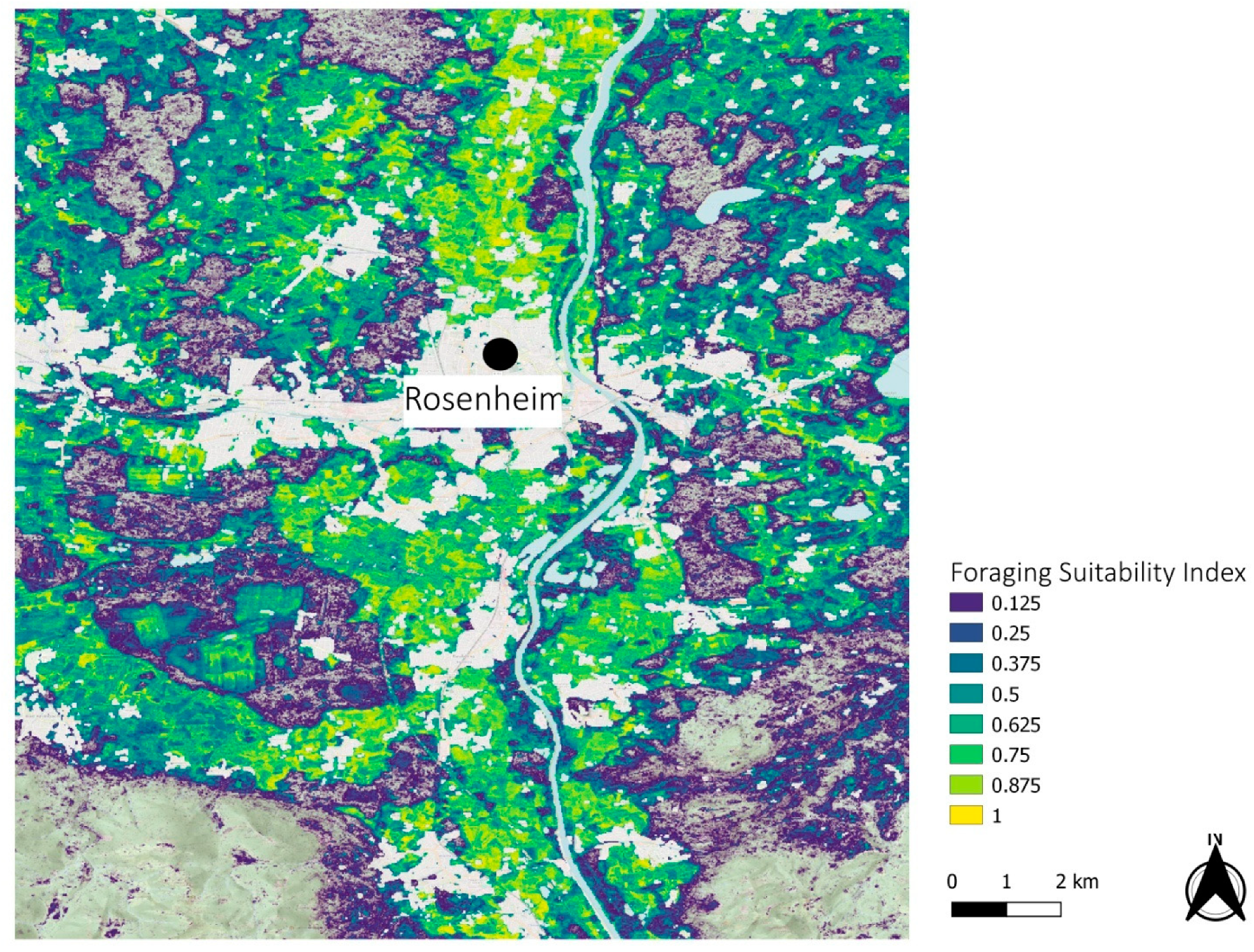
3.1. Overall Study Area
3.2. Regional Examples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Böhm, C.; Bowden, C.G.R.; Seddon, P.J.; Hatipoǧlu, T.; Oubrou, W.; El Bekkay, M.; Quevedo, M.A.; Fritz, J.; Yeniyurt, C.; Lopez, J.M.; et al. The northern bald ibis Geronticus eremita: History, current status and future perspectives. Oryx 2020, 55, 934–946. [Google Scholar] [CrossRef]
- Lindsell, J.A.; Serra, G.; Peške, L.; Abdullah, M.S.; Al Qaim, G.; Kanani, A.; Wondafrash, M. Satellite tracking reveals the migration route and wintering area of the Middle East population of Critically Endangered northern bald ibis Geronticus eremita. Oryx 2009, 43, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Schenker, A. Das ehemalige Verbreitunsgebiet des Waldrapps Geronticus eremita in Europa. Ornithol. Beob. 1977, 74, 13–30. [Google Scholar]
- Schenker, A.; Cahenzli, F.; Gutbrod, K.G.; Thevenot, M.; Erhardt, A. The Northern Bald Ibis Geronticus eremita in Morocco since 1900: Analysis of ecological requirements. Bird Conserv. Int. 2019, 30, 117–138. [Google Scholar] [CrossRef]
- Schenker, A.N.D.R.E.; Serra, G. Review of historical breeding sites of the Northern Bald Ibis Geronticus eremita in Syria and south-eastern Turkey. Bird Conserv. Int. 2021, 1, 1–10. [Google Scholar] [CrossRef]
- Serra, G.; Lindsell, J.A.; Peske, L.; Fritz, J.; Bowden, C.G.R.; Bruschini, C.; Welch, G.; Tavares, J.; Wondafrash, M. Accounting for the low survival of the Critically Endangered northern bald ibis Geronticus eremita on a major migratory flyway. Oryx 2015, 49, 312–320. [Google Scholar] [CrossRef] [Green Version]
- Fritz, J.; Janák, J. How human intervention and climate change shaped the fate of the Northern Bald Ibis from ancient Egypt to the presence: An interdisciplinary approach to extinction and recovery of an iconic bird species. bioRxiv, 2020; preprint. [Google Scholar] [CrossRef]
- Unsöld, M.; Fritz, J. Der Waldrapp-ein Vogel zwischen Ausrottung und Wiederkehr. Wildbiologie 2011, 2, 1–16. [Google Scholar]
- Bowden, C.G.R.; Smith, K.W.; ElBekkay, M.; Oubrou, W.; Aghnaj, A.; Jimenez-Armesto, M. Contribution of research to conservation action for the Northern Bald Ibis Geronticus eremita in Morocco. Bird Conserv. Int. 2008, 18, S74–S90. [Google Scholar] [CrossRef] [Green Version]
- Böhm, C.; Fritz, J.; Asmus, J. Koordination Und Kooperation Von Zoo-Und Freilandarbeit Bis Zur Wiederansiedlung: Vier Fallbeispiele. In Wildvogelhaltung; Lantermann, W., Asmus, J., Eds.; Springer Spektrum: Berlin/Heidelberg, Germany. [CrossRef]
- Fritz, J. The European LIFE+ northern bald ibis reintroduction project. Oryx 2021, 55, 809–810. [Google Scholar] [CrossRef]
- Fritz, J.; Kramer, R.; Hoffmann, W.; Trobe, D.; Unsöld, M. Back into the wild: Establishing a migratory Northern bald ibis Geronticus eremita population in Europe. Int. Zoo Yearb. 2017, 51, 107–123. [Google Scholar] [CrossRef]
- Serra, G.; Bruschini, C.; Peske, L.; Kubsa, A.; Wondafrash, M.; Lindsell, J.A. An assessment of ecological conditions and threats at the Ethiopian wintering site of the last known eastern colony of Critically Endangered Northern Bald Ibis Geronticus eremita. Undefined 2013, 23, 399–413. [Google Scholar] [CrossRef] [Green Version]
- Zoufal, K.; Fritz, J.; Bichler, M.; Kirbauer, M.; Markut, T.; Meran, I.; Wolf, A.; Kotrschal, K. Feeding Ecology of the Northern Bald Ibis in Different Habitat Types: An Experiment Field Study with Handraised Individuals. J. Ornithol. 2006, 147, 1–279. [Google Scholar]
- Serra, G.; Abdallah, M.S.; Quaim, G. Feeding ecology and behaviour of the last known surviving Northern Bald Ibises, Geronticus eremita, at their breeding quarter in Syria. Zool. Middle East 2008, 43, 55–68. [Google Scholar] [CrossRef]
- Yeniyurt, C.; Oppel, S.; Isfendiyaroglu, S.; Ozkinaci, G.; Erkol, I.L.; Bowden, C.G.R. Influence of feeding ecology on breeding success of a semi-wild population of the critically endangered northern bald ibis geronticus eremita in southern Turkey. Bird Conserv. Int. 2017, 27, 537–549. [Google Scholar] [CrossRef]
- Fritz, J.; Wirtz, S.; Unsöld, M. Aspects of the biology and genetic of the Northern Bald Ibis: Reply to Bauer et al. (2016) Bird Neozoa in Germany–Revision of the national status rating. Vogelwarte 2017, 55, 141–145. [Google Scholar]
- Gesner, C. Historiae Animalium liber III, Qui Est De Avium Natura; zurich: Zürich, Switzerland, 1557. [Google Scholar]
- Schenker, A. Der Waldrapp-ein histroisches Wildbret. Wildbiologie 1981, 4, 1–12. [Google Scholar]
- Drenske, S.; Radchuk, V.; Scherer, C.; Esterer, C.; Kowarik, I.; Fritz, J.; Kramer-Schadt, S. Halfway to self-sustainability: Reintroduced migratory European Northern Bald Ibises (Geronticus eremita) still need management interventions for population viability. bioRxiv 2021. [Google Scholar] [CrossRef]
- Osborne, P.E.; Seddon, P.J. Selecting suitable habitats for reintroductions: Variation, change and the role of species distribution modelling. Reintroduct. Biol. Integr. Sci. Manag. 2012, 1, 73–104. [Google Scholar]
- Armstrong, D.P.; Seddon, P.J. Directions in reintroduction biology. Trends Ecol. Evol. 2008, 23, 20–25. [Google Scholar] [CrossRef]
- Parker, K.A.; Ewen, J.G.; Seddon, P.J.; Armstrong, D.P. Post-release monitoring of bird translocations: Why is it important and how we do it? Notornis 2013, 60, 85–92. [Google Scholar]
- Hunter-Ayad, J.; Ohlemüller, R.; Recio, M.R.; Seddon, P.J. Reintroduction modelling: A guide to choosing and combining models for species reintroductions. J. Appl. Ecol. 2020, 57, 1233–1243. [Google Scholar] [CrossRef]
- Soorae, P.S. (Ed.) Global Re-introduction Perspectives, 2013: Further Case Studies from Around the Globe; IUCN/SSC Re-Introduction Specialist Group & Environment Agency-Abu Dhabi: Gland, Switzerland, 2013. [Google Scholar]
- He, K.S.; Bradley, B.A.; Cord, A.F.; Rocchini, D.; Tuanmu, M.N.; Schmidtlein, S.; Turner, W.; Wegmann, M.; Pettorelli, N. Will remote sensing shape the next generation of species distribution models? Remote Sens. Ecol. Conserv. 2015, 1, 4–18. [Google Scholar] [CrossRef] [Green Version]
- Pettorelli, N.; Schulte to Bühne, H.; Tulloch, A.; Dubois, G.; Macinnis-Ng, C.; Queirós, A.M.; Keith, D.A.; Wegmann, M.; Schrodt, F.; Stellmes, M.; et al. Satellite remote sensing of ecosystem functions: Opportunities, challenges and way forward. Remote Sens. Ecol. Conserv. 2018, 4, 71–93. [Google Scholar] [CrossRef]
- Pettorelli, N.; Safi, K.; Turner, W. Satellite remote sensing, biodiversity research and conservation of the future. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130190. [Google Scholar] [CrossRef]
- Remelgado, R.; Leutner, B.; Safi, K.; Sonnenschein, R.; Kuebert, C.; Wegmann, M. Linking animal movement and remote sensing–mapping resource suitability from a remote sensing perspective. Remote Sens. Ecol. Conserv. 2018, 4, 211–224. [Google Scholar] [CrossRef] [Green Version]
- Rocchini, D.; Boyd, D.S.; Féret, J.B.; Foody, G.M.; He, K.S.; Lausch, A.; Nagendra, H.; Wegmann, M.; Pettorelli, N. Satellite remote sensing to monitor species diversity: Potential and pitfalls. Remote Sens. Ecol. Conserv. 2016, 2, 25–36. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Fritz, J.; Eberhard, B.; Esterer, C.; Goenner, B.; Trobe, D.; Unsoeld, M.; Voelkl, B.; Wehner, H.; Scope, A. Biologging is suspect to cause corneal opacity in two populations of wild living Northern Bald Ibises (Geronticus eremita). Avian Res. 2020, 11, 38. [Google Scholar] [CrossRef]
- Schenker, A. Replik zum Beitrag von Armin Landmann betreffend den Waldrapp Geronticus eremita. Vogelwarte 2017, 55, 129–138. [Google Scholar]
- QGIS Development Team QGIS Geographic Information System. 2020. Available online: https://qgis.org/de/site/ (accessed on 7 August 2020).
- Bauer-Marschallinger, B.; Cao, S.; Navacchi, C.; Freeman, V.; Reuß, F.; Geudtner, D.; Rommen, B.; Vega, F.C.; Snoeij, P.; Attema, E.; et al. The normalised Sentinel-1 Global Backscatter Model, mapping Earth’s land surface with C-band microwaves. Sci. Data 2021, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Bousbih, S.; Zribi, M.; Lili Chabaane, Z.; Baghdadi, N.; El Hajj, M.; Gao, Q.; Mougenot, B.; Lili-Chabaane, Z.; Baghdadi, N. Potential of Sentinel-1 radar datA for the assessment of soil and cereal cover parameters. Sensors 2015, 17, 2617. [Google Scholar] [CrossRef] [Green Version]
- Vreugdenhil, M.; Wagner, W.; Bauer-Marschallinger, B.; Pfeil, I.; Teubner, I.; Rüdiger, C.; Strauss, P. Sensitivity of Sentinel-1 backscatter to vegetation dynamics: An Austrian case study. Remote Sens. 2018, 10, 1396. [Google Scholar] [CrossRef] [Green Version]
- Buchhorn, M.; Lesiv, M.; Tsendbazar, N.E.; Herold, M.; Bertels, L.; Smets, B. Copernicus global land cover layers-collection 2. Remote Sens. 2020, 12, 1044. [Google Scholar] [CrossRef] [Green Version]
- Farr, T.G.; Rosen, P.A.; Caro, E.; Crippen, R.; Duren, R.; Hensley, S.; Kobrick, M.; Paller, M.; Rodriguez, E.; Roth, L.; et al. The Shuttle Radar Topography Mission. Rev. Geophys. 2007, 45, 2004. [Google Scholar] [CrossRef] [Green Version]
- Muñoz Sabater, J. ERA5-Land Monthly Averaged Data from 1981 to Present. Copernic. Clim. Chang. Serv. (C3S) Clim. Data Store (CDS) 2019, 146, 1999–2049. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-resolution global maps of 21st-century forest cover change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marconcini, M.; Metz-Marconcini, A.; Üreyen, S.; Palacios-Lopez, D.; Hanke, W.; Bachofer, F.; Zeidler, J.; Esch, T.; Gorelick, N.; Kakarla, A.; et al. Outlining where humans live, the World Settlement Footprint 2015. Sci. Data 2020, 7, 242. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.rstudio.com/products/rstudio/download/ (accessed on 25 July 2020).
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Cerasoli, F.; Iannella, M.; D’Alessandro, P.; Biondi, M. Comparing pseudo-absences generation techniques in Boosted Regression Trees models for conservation purposes: A case study on amphibians in a protected area. PLoS ONE 2017, 12, e0187589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hijams, R.J.; Phillips, S.; Elith, J.L. Dismo: Species Distribution Modeling. 2021. Available online: https://cran.r-project.org/web/packages/dismo/dismo.pdf (accessed on 24 October 2021).
- Zlonis, E.J.; Deo, R.; Berdeen, J.B. LiDAR and multispectral imagery predict the occurrence of tree cavities suitable for a cavity-nesting duck. Remote Sens. Ecol. Conserv. 2021. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Thuiller, W.; Georges, D.; Engler, R.; Lafourcade, B. BIOMOD: Tutorial. Available online: http://www.ecochange-project.eu (accessed on 14 November 2021).
- Wolf, M.; Griffith, B.; Reed, C.S.A.T. Avian and mamalian translocations: Update and reanalysis of 1987 survey data. Conserv. Biol. 1996, 10, 1142–1154. [Google Scholar] [CrossRef]
- Rutz, C.; Hays, G.C. New frontiers in biologging science. Biol. Lett. 2009, 5, 289–292. [Google Scholar] [CrossRef] [Green Version]
- Wikelski, M.; Tertitski, G. Living sentinels for climate change effects. Science 2016, 352, 775–776. [Google Scholar] [CrossRef] [Green Version]
- Portugal, S.J.; White, C.R. Miniaturization of biologgers is not alleviating the 5% rule. Methods Ecol. Evol. 2018, 9, 1662–1666. [Google Scholar] [CrossRef]
- Flack, A.; Nagy, M.; Fiedler, W.; Couzin, I.D.; Wikelski, M. From local collective behavior to global migratory patterns in white storks. Science 2018, 360, 911–914. [Google Scholar] [CrossRef] [Green Version]
- Thaxter, C.B.; Ross-Smith, V.H.; Clark, J.A.; Clark, N.A.; Conway, G.J.; Masden, E.A.; Wade, H.M.; Leat, E.H.K.; Gear, S.C.; Marsh, M.; et al. Contrasting effects of GPS device and harness attachment on adult survival of Lesser Black-backed Gulls Larus fuscus and Great Skuas Stercorarius skua. Ibis 2016, 158, 279–290. [Google Scholar] [CrossRef]
- Bodey, T.W.; Cleasby, I.R.; Bell, F.; Parr, N.; Schultz, A.; Votier, S.C.; Bearhop, S. A phylogenetically controlled metaanalysis of biologging device effects on birds: Deleterious effects and a call for more standardized reporting of study data. Methods Ecol. Evol. 2018, 9, 946–955. [Google Scholar] [CrossRef] [Green Version]
- Geen, G.R.; Robinson, R.A.; Baillie, S.R. Effects of tracking devices on individual birds–A review of the evidence. J. Avian Biol. 2019, 50, 2. [Google Scholar] [CrossRef]
- Hötker, H.; Bernady, P.; Cimiotti, D.; Dziewiaty, K.; Joest, R.; Rasran, L. Maisanbau für Biogasanlagen—CO2-Bilanz und Wirkung auf die Vogelwelt. Ber. Zum Vogelschutz 2009, 46, 107–125. [Google Scholar]
- Plenk, A. Landwirtschaft, Lebensmittel und Veterinärmedizin—Zukunft der Forschung in Österreich. In Proceedings of the ALVA–Jahrestagung 2011, Graz, Austria, 23–24 May 2011. [Google Scholar]
- Muller, A.; Schader, C.; El-Hage Scialabba, N.; Brüggemann, J.; Isensee, A.; Erb, K.H.; Smith, P.; Klocke, P.; Leiber, F.; Stolze, M.; et al. Strategies for feeding the world more sustainably with organic agriculture. Nat. Commun. 2017, 8, 1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meemken, E.M.; Qaim, M. Organic Agriculture, Food Security, and the Environment. Annu. Rev. Resour. Econ. 2018, 10, 39–63. [Google Scholar] [CrossRef] [Green Version]
- Bowden, C.G.R. International Single Species Action Plan for the Conservation of the Northern Bald Ibis (Geronticus eremita); The Royal Society for the Protection of Birds (RSPB), United Kingdom BirdLife International: Sandy, UK, 2015; Volume 55. [Google Scholar]



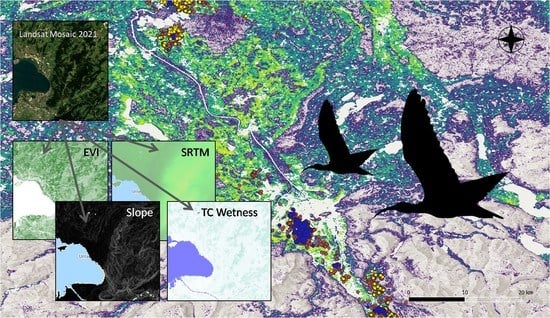
| Index | Full Name | Satellite/Source Mission | Resolution (Meters) | Year of Data Collection |
|---|---|---|---|---|
| EVI | Enhanced vegetation index | Landsat-8 | 30 m | 2021 |
| TC Brightness | Tasseled cap brightness | Landsat-8 | 30 m | 2021 |
| TC Wetness | Tasseled cap wetness | Landsat-8 | 30 m | 2021 |
| NDWI | Normalized difference water index | Landsat-8 | 30 m | 2021 |
| Grass cover | Grass cover fraction | CORINE Landcover Inventory | 100 m | 2018 |
| Elevation | Elevation | Shuttle Radar Topography Mission | 30 m | 2000 |
| Slope | Slope | Shuttle Radar Topography Mission | 30 m | 2000 |
| VV intensity | Intensity of vertical send and vertical received radar data | Sentinel-1 | 10 m | 2021 |
| Surface Temperature | Surface Temperature | [40] | 1000 m | 2021 |
| Water | Water Mask | [41] | 30 m | 2015 |
| World Settlement Footprint | Impervious/urban areas | DLR | 10 m | 2015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wehner, H.; Huchler, K.; Fritz, J. Quantification of Foraging Areas for the Northern Bald Ibis (Geronticus eremita) in the Northern Alpine Foothills: A Random Forest Model Fitted with Optical and Actively Sensed Earth Observation Data. Remote Sens. 2022, 14, 1015. https://doi.org/10.3390/rs14041015
Wehner H, Huchler K, Fritz J. Quantification of Foraging Areas for the Northern Bald Ibis (Geronticus eremita) in the Northern Alpine Foothills: A Random Forest Model Fitted with Optical and Actively Sensed Earth Observation Data. Remote Sensing. 2022; 14(4):1015. https://doi.org/10.3390/rs14041015
Chicago/Turabian StyleWehner, Helena, Katharina Huchler, and Johannes Fritz. 2022. "Quantification of Foraging Areas for the Northern Bald Ibis (Geronticus eremita) in the Northern Alpine Foothills: A Random Forest Model Fitted with Optical and Actively Sensed Earth Observation Data" Remote Sensing 14, no. 4: 1015. https://doi.org/10.3390/rs14041015
APA StyleWehner, H., Huchler, K., & Fritz, J. (2022). Quantification of Foraging Areas for the Northern Bald Ibis (Geronticus eremita) in the Northern Alpine Foothills: A Random Forest Model Fitted with Optical and Actively Sensed Earth Observation Data. Remote Sensing, 14(4), 1015. https://doi.org/10.3390/rs14041015