Developing Hyperspectral Indices for Assessing Seasonal Variations in the Ratio of Chlorophyll to Carotenoid in Deciduous Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Composite Dataset
2.2. Spectral Preprocessing Transformations
2.3. Published Spectral Indices
2.4. Development of New Hyperspectral Indices
2.5. Statistical Criteria
3. Results
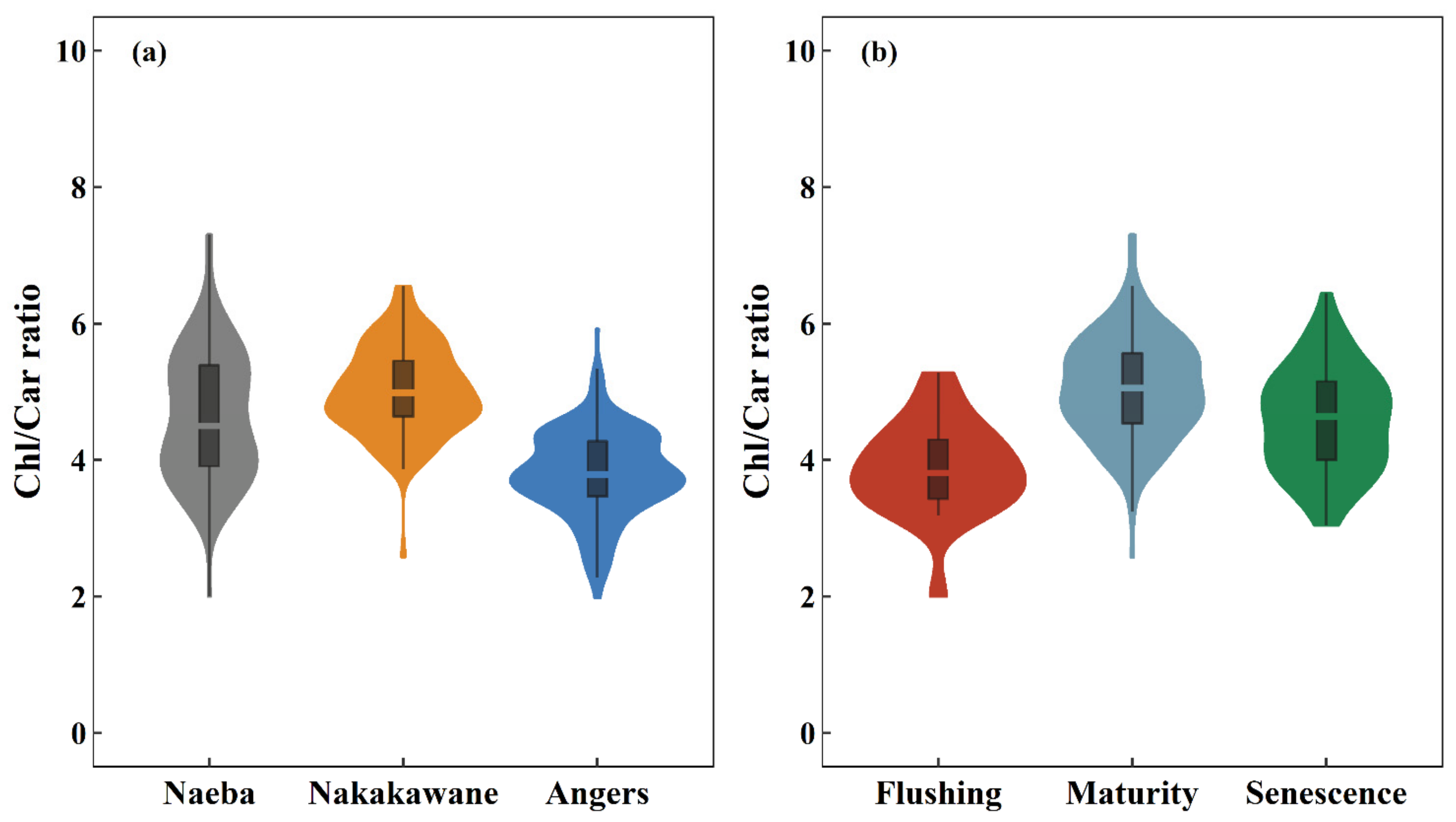
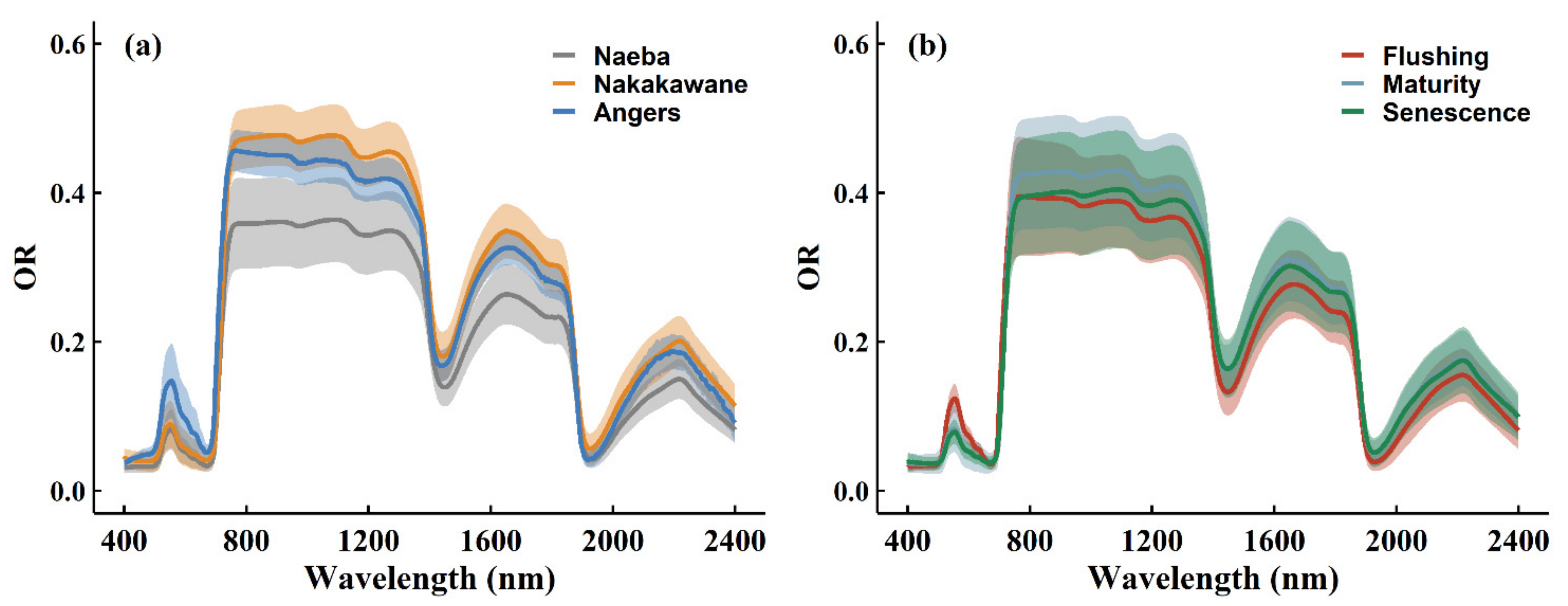
3.1. Leaf Chlorophyll/Carotenoid Ratio of the Composite Dataset
3.2. Evaluation of the Reported Spectral Indices Using the Composite Dataset
3.3. Establishment of New Spectral Indices
4. Discussion
4.1. Inapplicability of Published Indices Suggest the Ratio Should Be Investigated Independently
4.2. Evaluations of Developed Indices for Quantifying and Tracking the Ratio of Chlorophyll to Carotenoid
4.3. Performance of Different Preprocessing and Transformations for Tracing the Ratio of Chlorophyll to Carotenoid
4.4. Future Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef] [Green Version]
- Ritz, T.; Damjanović, A.; Schulten, K.; Zhang, J.P.; Koyama, Y. Efficient Light Harvesting through Carotenoids. Photosynth. Res. 2000, 66, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Drewry, D.T.; Slattery, R.A.; VanLoocke, A.; Cho, Y.B.; Ort, D.R. Chlorophyll Can Be Reduced in Crop Canopies with Little Penalty to Photosynthesis. Plant Physiol. 2018, 176, 1215–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackburn, G.A. Hyperspectral Remote Sensing of Plant Pigments. J. Exp. Bot. 2007, 58, 855–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demmig-Adams, B. Survey of Thermal Energy Dissipation and Pigment Composition in Sun and Shade Leaves. Plant Cell Physiol. 1998, 39, 474–482. [Google Scholar] [CrossRef]
- Fang, Z.; Bouwkamp, J.C.; Solomos, T. Chlorophyllase Activities and Chlorophyll Degradation during Leaf Senescence in Non-Yellowing Mutant and Wild Type of Phaseolus Vulgaris L. J. Exp. Bot. 1998, 49, 503–510. [Google Scholar]
- Asner, G.P.; Martin, R.E.; Ford, A.J.; Metcalee, D.J.; Liddell, M.J. Leaf Chemical and Spectral Diversity in Australian Tropical Forests. Ecol. Appl. 2009, 19, 236–253. [Google Scholar] [CrossRef]
- Curran, P.J.; Windham, W.R.; Gholz, H.L. Exploring the Relationship between Reflectance Red Edge and Chlorophyll Concentration in Slash Pine Leaves. Tree Physiol. 1990, 7, 33–48. [Google Scholar] [CrossRef]
- Ustin, S.L.; Gitelson, A.A.; Jacquemoud, S.; Schaepman, M.; Asner, G.P.; Gamon, J.A.; Zarco-Tejada, P. Retrieval of Foliar Information about Plant Pigment Systems from High Resolution Spectroscopy. Remote Sens. Environ. 2009, 113, S67–S77. [Google Scholar] [CrossRef] [Green Version]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-Destructive Optical Detection of Pigment Changes during Leaf Senescence and Fruit Ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Filimon, R.V.; Rotaru, L.; Filimon, R.M. Quantitative Investigation of Leaf Photosynthetic Pigments during Annual Biological Cycle of Vitis Vinifera L. Table Grape Cultivars. S. Afr. J. Enol. Vitic. 2016, 37, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Solovchenko, A. Photoprotection in Plants: Optical Screening-Based Mechanisms; Springer: New York, NY, USA, 2010. [Google Scholar]
- Croft, H.; Chen, J.M.; Froelich, N.J.; Chen, B.; Staebler, R.M. Seasonal Controls of Canopy Chlorophyll Content on Forest Carbon Uptake: Implications for GPP Modeling. J. Geophys. Res. Biogeosci. 2015, 120, 1576–1586. [Google Scholar] [CrossRef] [Green Version]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-Band Model for Noninvasive Estimation of Chlorophyll, Carotenoids, and Anthocyanin Contents in Higher Plant Leaves. Geophys. Res. Lett. 2006, 33, 2–6. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, C.; Zhang, J.; Yang, H.; Xu, L.; Wang, Q.; Sack, L.; Wu, X.; Hou, J.; He, N. Variation in Leaf Chlorophyll Concentration from Tropical to Cold-Temperate Forests: Association with Gross Primary Productivity. Ecol. Indic. 2018, 85, 383–389. [Google Scholar] [CrossRef]
- Ustin, S.L.; Roberts, D.A.; Gamon, J.A.; Asner, G.P.; Green, R.O. Using Imaging Spectroscopy to Study Ecosystem Processes and Properties. Bioscience 2004, 54, 523–534. [Google Scholar] [CrossRef]
- Ormrod, D.P.; Lesser, V.M.; Olszyk, D.M.; Tingey, D.T. Elevated Temperature and Carbon Dioxide Affect Chlorophylls and Carotenoids in Douglas-Fir Seedlings. Int. J. Plant Sci. 1999, 160, 529–534. [Google Scholar] [CrossRef]
- Qiu, T.; Jiang, L.L.; Li, S.Z.; Yang, Y.F. Small-Scale Habitat-Specific Variation and Adaptive Divergence of Photosynthetic Pigments in Different Alkali Soils in Reed Identified by Common Garden and Genetic Tests. Front. Plant Sci. 2017, 7, 2016. [Google Scholar] [CrossRef] [Green Version]
- Rosevear, M.J.; Young, A.J.; Johnson, G.N. Growth Conditions Are More Important than Species Origin in Determining Leaf Pigment Content of British Plant Species. Funct. Ecol. 2001, 15, 474–480. [Google Scholar] [CrossRef]
- Ivanov, L.A.; Ivanova, L.A.; Ronzhina, D.A.; Yudina, P.K. Changes in the Chlorophyll and Carotenoid Contents in the Leaves of Steppe Plants along a Latitudinal Gradient in South Ural. Russ. J. Plant Physiol. 2013, 60, 812–820. [Google Scholar] [CrossRef]
- Casierra-posada, F.; Peña-olmos, J.E.; Zapata-casierra, E. Pigment Content in Strawberry Leaves (Fragaria sp.) Exposed to Different Light Quality. Rev. UDCA Actual. Divulg. Cient. 2014, 17, 87–94. [Google Scholar]
- Lichtenthaler, H.K. Biosynthesis, Accumulation and Emission of Carotenoids, α-Tocopherol, Plastoquinone, and Isoprene in Leaves under High Photosynthetic Irradiance. Photosynth. Res. 2007, 92, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Babani, F.; Navrátil, M.; Buschmann, C. Chlorophyll Fluorescence Kinetics, Photosynthetic Activity, and Pigment Composition of Blue-Shade and Half-Shade Leaves as Compared to Sun and Shade Leaves of Different Trees. Photosynth. Res. 2013, 117, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Babani, F.; Langsdorf, G. Chlorophyll Fluorescence Imaging of Photosynthetic Activity in Sun and Shade Leaves of Trees. Photosynth. Res. 2007, 93, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Ač, A.; Marek, M.V.; Kalina, J.; Urban, O. Differences in Pigment Composition, Photosynthetic Rates and Chlorophyll Fluorescence Images of Sun and Shade Leaves of Four Tree Species. Plant Physiol. Biochem. 2007, 45, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Sarijeva, G.; Knapp, M.; Lichtenthaler, H.K. Differences in Photosynthetic Activity, Chlorophyll and Carotenoid Levels, and in Chlorophyll Fluorescence Parameters in Green Sun and Shade Leaves of Ginkgo and Fagus. J. Plant Physiol. 2007, 164, 950–955. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Babani, F. Contents of Photosynthetic Pigments and Ratios of Chlorophyll a/b and Chlorophylls to Carotenoids (z+b)/(x+c) in C4 Plants as Compared to C3 Plants. Photosynthetica 2022, 60, 1–7. [Google Scholar]
- Villa, P.; Bolpagni, R.; Pinardi, M.; Tóth, V.R. Leaf Reflectance Can Surrogate Foliar Economics Better than Physiological Traits across Macrophyte Species. Plant Methods 2021, 17, 115. [Google Scholar] [CrossRef]
- Yang, X.; Tang, J.; Mustard, J.F.; Wu, J.; Zhao, K.; Serbin, S.; Lee, J.E. Seasonal Variability of Multiple Leaf Traits Captured by Leaf Spectroscopy at Two Temperate Deciduous Forests. Remote Sens. Environ. 2016, 179, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Cheng, R.; Magney, T.S.; Dutta, D.; Bowling, D.R.; Logan, B.A.; Burns, S.P.; Blanken, P.D.; Grossmann, K.; Lopez, S.; Richardson, A.D.; et al. Decomposing Reflectance Spectra to Track Gross Primary Production in a Subalpine Evergreen Forest. Biogeosciences 2020, 17, 4523–4544. [Google Scholar] [CrossRef]
- Gamon, J.A.; Huemmrich, K.F.; Wong, C.Y.S.; Ensminger, I.; Garrity, S.; Hollinger, D.Y.; Noormets, A.; Peñuelask, J. A Remotely Sensed Pigment Index Reveals Photosynthetic Phenology in Evergreen Conifers. Proc. Natl. Acad. Sci. USA 2016, 113, 13087–13092. [Google Scholar] [CrossRef] [Green Version]
- Seyednasrollah, B.; Bowling, D.R.; Cheng, R.; Logan, B.A.; Magney, T.S.; Frankenberg, C.; Yang, J.C.; Young, A.M.; Hufkens, K.; Arain, M.A.; et al. Seasonal Variation in the Canopy Color of Temperate Evergreen Conifer Forests. New Phytol. 2021, 229, 2586–2600. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Merzlyak, M.N. Signature Analysis of Leaf Reflectance Spectra: Algorithm Development for Remote Sensing of Chlorophyll. J. Plant Physiol. 1996, 148, 494–500. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing Carotenoid Content in Plant Leaves with Reflectance Spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar] [CrossRef]
- Curran, P.J. Remote Sensing of Foliar Chemistry. Remote Sens. Environ. 1989, 30, 271–278. [Google Scholar] [CrossRef]
- Kattenborn, T.; Schiefer, F.; Zarco-tejada, P.; Schmidtlein, S. Advantages of Retrieving Pigment Content [μg/Cm2] versus Concentration [%] from Canopy Re Fl Ectance. Remote Sens. Environ. 2019, 230, 111195. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between Leaf Pigment Content and Spectral Reflectance across a Wide Range of Species, Leaf Structures and Developmental Stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Gamon, J.A.; Penuelas, J.; Field, C.B. A Narrow-Waveband Spectral Index That Tracks Diurnal Changes in Photosynthetic Efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Gamon, J.A.; Surfus, J.S. Assessing Leaf Pigment Content and Activity with a Reflectometer. New Phytol. 1999, 143, 105–117. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote Estimation of Chlorophyll Content in Higher Plant Leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Le Maire, G.; François, C.; Dufrêne, E. Towards Universal Broad Leaf Chlorophyll Indices Using PROSPECT Simulated Database and Hyperspectral Reflectance Measurements. Remote Sens. Environ. 2004, 89, 1–28. [Google Scholar] [CrossRef]
- Zarco-tejada, P.J.; Hornero, A.; Beck, P.S.A.; Kattenborn, T.; Kempeneers, P.; Hernández-clemente, R. Chlorophyll Content Estimation in an Open-Canopy Conifer Forest with Sentinel-2A and Hyperspectral Imagery in the Context of Forest Decline. Remote Sens. Environ. 2019, 223, 320–335. [Google Scholar] [CrossRef] [PubMed]
- Main, R.; Cho, M.A.; Mathieu, R.; O’Kennedy, M.M.; Ramoelo, A.; Koch, S. An Investigation into Robust Spectral Indices for Leaf Chlorophyll Estimation. ISPRS J. Photogramm. Remote Sens. 2011, 66, 751–761. [Google Scholar] [CrossRef]
- Hernández-Clemente, R.; Navarro-Cerrillo, R.M.; Zarco-Tejada, P.J. Carotenoid Content Estimation in a Heterogeneous Conifer Forest Using Narrow-Band Indices and PROSPECT+DART Simulations. Remote Sens. Environ. 2012, 127, 298–315. [Google Scholar] [CrossRef]
- Peñuelas, J.; Gamon, J.A.; Fredeen, A.L.; Merino, J.; Field, C.B. Reflectance Indices Associated with Physiological Changes in Nitrogen- and Water-Limited Sunflower Leaves. Remote Sens. Environ. 1994, 48, 135–146. [Google Scholar] [CrossRef]
- Penuelas, J.; Baret, F.; Filella, I. Semi-Empirical Indices to Assess Carotenoids/Chlorophyll a Ratio from Leaf Spectral Reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Zhou, X.; Huang, W.; Zhang, J.; Kong, W.; Casa, R.; Huang, Y. A Novel Combined Spectral Index for Estimating the Ratio of Carotenoid to Chlorophyll Content to Monitor Crop Physiological and Phenological Status. Int. J. Appl. Earth Obs. Geoinf. 2019, 76, 128–142. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, W.; Kong, W.; Ye, H.; Dong, Y.; Casa, R. Assessment of Leaf Carotenoids Content with a New Carotenoid Index: Development and Validation on Experimental and Model Data. Int. J. Appl. Earth Obs. Geoinf. 2017, 57, 24–35. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote Estimation of Canopy Chlorophyll Content in Crops. Geophys. Res. Lett. 2005, 32, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Garrity, S.R.; Eitel, J.U.H.; Vierling, L.A. Disentangling the Relationships between Plant Pigments and the Photochemical Reflectance Index Reveals a New Approach for Remote Estimation of Carotenoid Content. Remote Sens. Environ. 2011, 115, 628–635. [Google Scholar] [CrossRef]
- Filella, I.; Porcar-Castell, A.; Munné-Bosch, S.; Bäck, J.; Garbulsky, M.F.; Peñuelas, J. PRI Assessment of Long-Term Changes in Carotenoids/Chlorophyll Ratio and Short-Term Changes in de-Epoxidation State of the Xanthophyll Cycle. Int. J. Remote Sens. 2009, 30, 4443–4455. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gamon, J.A.; Solovchenko, A. Multiple Drivers of Seasonal Change in PRI: Implications for Photosynthesis 1. Leaf Level. Remote Sens. Environ. 2017, 191, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Muraoka, H.; Saigusa, N.; Nasahara, K.N.; Noda, H.; Yoshino, J.; Saitoh, T.M.; Nagai, S.; Murayama, S.; Koizumi, H. Effects of Seasonal and Interannual Variations in Leaf Photosynthesis and Canopy Leaf Area Index on Gross Primary Production of a Cool-Temperate Deciduous Broadleaf Forest in Takayama, Japan. J. Plant Res. 2010, 123, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Wang, Q.; Jin, J. Exploring the Instability of the Relationship between Maximum Potential Electron Transport Rate and Maximum Carboxylation Rate in Cool-Temperate Deciduous Forests. Agric. For. Meteorol. 2021, 308–309, 108614. [Google Scholar] [CrossRef]
- Wang, Q.; IIo, A.; Tenhunen, J.; Kakubari, Y. Annual and Seasonal Variations in Photosynthetic Capacity of Fagus Crenata along an Elevation Gradient in the Naeba Mountains, Japan. Tree Physiol. 2008, 28, 277–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldocchi, D. “Breathing” of the Terrestrial Biosphere: Lessons Learned from a Global Network of Carbon Dioxide Flux Measurement Systems. Aust. J. Bot. 2008, 56, 1–26. [Google Scholar] [CrossRef]
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting Plant Phenology in Response to Global Change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef]
- Richardson, A.D.; Hollinger, D.Y.; Dail, D.B.; Lee, J.T.; Munger, J.W.; O’Keefe, J. Influence of Spring Phenology on Seasonal and Annual Carbon Balance in Two Contrasting New England Forests. Tree Physiol. 2009, 29, 321–331. [Google Scholar] [CrossRef]
- Dillen, S.Y.; de Beeck, M.O.; Hufkens, K.; Buonanduci, M.; Phillips, N.G. Seasonal Patterns of Foliar Reflectance in Relation to Photosynthetic Capacity and Color Index in Two Co-Occurring Tree Species, Quercus Rubra and Betula Papyrifera. Agric. For. Meteorol. 2012, 160, 60–68. [Google Scholar] [CrossRef]
- Ito, A.; Muraoka, H.; Koizumi, H.; Saigusa, N.; Murayama, S.; Yamamoto, S. Seasonal Variation in Leaf Properties and Ecosystem Carbon Budget in a Cool-Temperate Deciduous Broad-Leaved Forest: Simulation Analysis at Takayama Site, Japan. Ecol. Res. 2006, 21, 137–149. [Google Scholar] [CrossRef]
- Croft, H.C. Remote Sensing of Leaf Pigments. In Comprehensive Remote Sensing; Elsevier: Oxford, UK, 2018; pp. 117–142. [Google Scholar]
- Marien, B.; Balzarolo, M.; Dox, I.; Leys, S.; Lor, M.J.; Geron, C.; Portillo-estrada, M.; Abdelgawad, H.; Asard, H.; Campioli, M. Detecting the Onset of Autumn Leaf Senescence in Deciduous Forest Trees of the Temperate Zone. New Phytol. 2019, 224, 166–176. [Google Scholar] [CrossRef]
- Jin, J.; Wang, Q. Informative Bands Used by Efficient Hyperspectral Indices to Predict Leaf Biochemical Contents Are Determined by Their Relative Absorptions. Int. J. Appl. Earth Obs. Geoinf. 2018, 73, 616–626. [Google Scholar] [CrossRef]
- Sonobe, R.; Wang, Q. Nondestructive Assessments of Carotenoids Content of Broadleaved Plant Species Using Hyperspectral Indices. Comput. Electron. Agric. 2018, 145, 18–26. [Google Scholar] [CrossRef]
- Jin, J.; Pratama, B.A.; Wang, Q. Tracing Leaf Photosynthetic Parameters Using Hyperspectral Indices in an Alpine Deciduous Forest. Remote Sens. 2020, 12, 1124. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Jin, J.; Sonobe, R.; Chen, J. Derivative Hyperspectral Vegetation Indices in Characterizing Forest Biophysical and Biochemical Quantities. In Hyperspectral Indices and Image Classifications for Agriculture and Vegetation; Thenkabail, P.S., Lyon, J.G., Huete, A., Eds.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Burger, J.; Geladi, P. Spectral Pre-Treatments of Hyperspectral near Infrared Images: Analysis of Diffuse Reflectance Scattering. J. Near Infrared Spectrosc. 2007, 15, 29–37. [Google Scholar] [CrossRef]
- Demetriades-Shah, T.H.; Steven, M.D.; Clark, J.A. High Resolution Derivative Spectra in Remote Sensing. Remote Sens. Environ. 1990, 33, 55–64. [Google Scholar] [CrossRef]
- Imanishi, J.; Sugimoto, K.; Morimoto, Y. Detecting Drought Status and LAI of Two Quercus Species Canopies Using Derivative Spectra. Comput. Electron. Agric. 2004, 43, 109–129. [Google Scholar] [CrossRef]
- Rinnan, Å.; Van Den Berg, F.; Engelsen, S.B. Review of the Most Common Pre-Processing Techniques for near-Infrared Spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Dhanoa, M.S.; Lister, S.J.; Sanderson, R.; Barnes, R.J. The Link between Multiplicative Scatter Correction (MSC) and Standard Normal Variate (SNV) Transformations of NIR Spectra. J. Near Infrared Spectrosc. 1994, 2, 43–47. [Google Scholar] [CrossRef]
- Martens, H.; Stark, E. Extended Multiplicative Signal Correction and Spectral Interference Subtraction: New Preprocessing Methods for near Infrared Spectroscopy. J. Pharm. Biomed. Anal. 1991, 9, 625–635. [Google Scholar] [CrossRef]
- Iio, A.; Yokoyama, A.; Takano, M.; Nakamura, T.; Fukasawa, H.; Nose, Y.; Kakubari, Y. Interannual Variation in Leaf Photosynthetic Capacity during Summer in Relation to Nitrogen, Leaf Mass per Area and Climate within a Fagus Crenata Crown on Naeba Mountain, Japan. Tree Physiol. 2008, 28, 1421–1429. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Feret, J.-B.; François, C.; Asner, G.P.; Gitelson, A.A.; Martin, R.E.; Bidel, L.P.R.; Ustin, S.L.; le Maire, G.; Jacquemoud, S. PROSPECT-4 and 5: Advances in the Leaf Optical Properties Model Separating Photosynthetic Pigments. Remote Sens. Environ. 2008, 112, 3030–3043. [Google Scholar] [CrossRef]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard Normal Variate Transformation and De-Trending of near-Infrared Diffuse Reflectance Spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Geladi, P.; MacDougall, D.; Martens, H. Linearization and Scatter-Correction for near-Infrared Reflectance Spectra of Meat. Appl. Spectrosc. 1985, 39, 491–500. [Google Scholar] [CrossRef]
- Wong, C.Y.S.; D’Odorico, P.; Arain, M.A.; Ensminger, I. Tracking the Phenology of Photosynthesis Using Carotenoid-Sensitive and near-Infrared Reflectance Vegetation Indices in a Temperate Evergreen and Mixed Deciduous Forest. New Phytol. 2020, 226, 1682–1695. [Google Scholar] [CrossRef]
- Chang, C.W.; Laird, D.A.; Mausbach, M.J.; Hurburgh, C.R. Near-Infrared Reflectance Spectroscopy-Principal Components Regression Analyses of Soil Properties. Soil Sci. Soc. Am. J. 2001, 65, 480–490. [Google Scholar] [CrossRef] [Green Version]
- Stylinski, C.D.; Gamon, J.A.; Oechel, W.C. Seasonal Patterns of Reflectance Indices, Carotenoid Pigments and Photosynthesis of Evergreen Chaparral Species. Oecologia 2002, 131, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.M.; Trotter, C.M. Estimating Photosynthetic Light-Use Efficiency Using the Photochemical Reflectance Index: Variations among Species. Funct. Plant Biol. 2004, 31, 255–265. [Google Scholar] [CrossRef]
- Garbulsky, M.F.; Peñuelas, J.; Gamon, J.; Inoue, Y.; Filella, I. The Photochemical Reflectance Index (PRI) and the Remote Sensing of Leaf, Canopy and Ecosystem Radiation Use Efficiencies. A Review and Meta-Analysis. Remote Sens. Environ. 2011, 115, 281–297. [Google Scholar] [CrossRef]
- Wong, C.Y.S.; Gamon, J.A. Three Causes of Variation in the Photochemical Reflectance Index (PRI) in Evergreen Conifers. New Phytol. 2015, 206, 187–195. [Google Scholar] [CrossRef]
- Middleton, E.M.; Huemmrich, K.F.; Landis, D.R.; Black, T.A.; Barr, A.G.; McCaughey, J.H. Photosynthetic Efficiency of Northern Forest Ecosystems Using a MODIS-Derived Photochemical Reflectance Index (PRI). Remote Sens. Environ. 2016, 187, 345–366. [Google Scholar] [CrossRef]
- Wong, C.Y.S.; Gamon, J.A. The Photochemical Reflectance Index Provides an Optical Indicator of Spring Photosynthetic Activation in Evergreen Conifers. New Phytol. 2015, 206, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Gamon, J.A. Reviews and Syntheses: Optical Sampling of the Flux Tower Footprint. Biogeosciences 2015, 12, 4509–4523. [Google Scholar] [CrossRef] [Green Version]
- Gitelson, A.; Solovchenko, A. Non-Invasive Quantification of Foliar Pigments: Possibilities and Limitations of Reflectance- and Absorbance-Based Approaches. J. Photochem. Photobiol. B Biol. 2018, 178, 537–544. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Chivkunova, O.B. Light-Stress-Induced Pigment Changes and Evidence for Anthocyanin Photoprotection in Apples. J. Photochem. Photobiol. B Biol. 2000, 55, 155–163. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Solovchenko, A.E.; Gitelson, A.A. Reflectance Spectral Features and Non-Destructive Estimation of Chlorophyll, Carotenoid and Anthocyanin Content in Apple Fruit. Postharvest Biol. Technol. 2003, 27, 197–211. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, Q.; Zheng, C. Best Hyperspectral Indices for Tracing Leaf Water Status as Determined from Leaf Dehydration Experiments. Ecol. Indic. 2015, 54, 96–107. [Google Scholar] [CrossRef]
- Junttila, S.; Hölttä, T.; Saarinen, N.; Kankare, V.; Yrttimaa, T.; Hyyppä, J.; Vastaranta, M. Close-Range Hyperspectral Spectroscopy Reveals Leaf Water Content Dynamics Research Highlights. Preprint 2021, 2021080497. [Google Scholar]
- Morley, P.J.; Jump, A.S.; West, M.D.; Donoghue, D.N.M. Spectral Response of Chlorophyll Content during Leaf Senescence in European Beech Trees. Environ. Res. Commun. 2020, 2, 071002. [Google Scholar] [CrossRef]
- Decker, M.; Nielsen, P.V.; Martens, H. Near-Infrared Spectra of Penicillium Camemberti Strains Separated by Extended Multiplicative Signal Correction Improved Prediction of Physical and Chemical Variations. Appl. Spectrosc. 2005, 59, 56–68. [Google Scholar] [CrossRef]
- Martens, H.; Nielsen, J.P.; Engelsen, S.B. Light Scattering and Light Absorbance Separated by Extended Multiplicative Signal Correction. Application to near-Infrared Transmission Analysis of Powder Mixtures. Anal. Chem. 2003, 75, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Goodarzi, M.; Ramon, H.; Saeys, W. Performance Evaluation of Preprocessing Techniques Utilizing Expert Information in Multivariate Calibration. Talanta 2014, 121, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jin, J. Leaf Transpiration of Drought Tolerant Plant Can Be Captured by Hyperspectral Reflectance Using PLSR Analysis. IForest 2016, 9, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Wang, Q. Hyperspectral Indices Based on First Derivative Spectra Closely Trace Canopy Transpiration in a Desert Plant. Ecol. Inform. 2016, 35, 1–8. [Google Scholar] [CrossRef]
- Gamon, J.A.; Somers, B.; Malenovský, Z.; Middleton, E.M.; Rascher, U.; Schaepman, M.E. Assessing Vegetation Function with Imaging Spectroscopy. Surv. Geophys. 2019, 40, 489–513. [Google Scholar] [CrossRef] [Green Version]



| Index | Variable | Formula | Reference |
|---|---|---|---|
| Photochemical reflectance index (PRI) | LUE | Gamon et al. [38] | |
| Normalized pigment chlorophyll ratio index (NPCI) | Total pigments/Chl | Peñuelas et al. [45] | |
| Structure insensitive pigment index (SIPI) | Car/Chla | Peñuelas et al. [45] | |
| Plant senescence reflectance index (PSRI) | Car/Chl | Merzlyak et al. [46] | |
| Carotenoid/chlorophyll ratio index (CCRI) | Car/Chl | Zhou et al. [47] | |
| Chlorophyll/carotenoid index (CCI) | Car/Chl | Wong et al. [78] | |
| Normalized difference vegetation index (NDVI) | Car/Chl |
| Index Type | Wavelength | Formula |
|---|---|---|
| R | ||
| SR | , | |
| D | , | |
| ND | , | |
| ID | , | ) |
| DDn | , | |
| mSR1 | , | |
| mSR2 | , | |
| mND | , | |
| mID | , |
| Dataset | Criteria | PRI | PSRI | SIPI | NPCI | CCRI | CCI | NDVI |
|---|---|---|---|---|---|---|---|---|
| Naeba | R2 | 0.28 | 0.00 | 0.00 | 0.02 | 0.43 | 0.04 | 0.03 |
| RMSE | 0.79 | 0.93 | 0.93 | 0.92 | 0.70 | 0.91 | 0.92 | |
| RPD | 1.19 | 1.01 | 1.01 | 1.02 | 1.33 | 1.03 | 1.02 | |
| Nakakawane | R2 | 0.13 | 0.02 | 0.00 | 0.04 | 0.02 | 0.00 | 0.05 |
| RMSE | 0.60 | 0.64 | 0.65 | 0.63 | 0.64 | 0.65 | 0.63 | |
| RPD | 1.08 | 1.01 | 1.00 | 1.02 | 1.01 | 1.00 | 1.03 | |
| Angers | R2 | 0.36 | 0.37 | 0.24 | 0.31 | 0.29 | 0.14 | 0.45 |
| RMSE | 0.50 | 0.50 | 0.55 | 0.52 | 0.53 | 0.58 | 0.47 | |
| RPD | 1.25 | 1.26 | 1.15 | 1.21 | 1.19 | 1.08 | 1.35 | |
| All | R2 | 0.17 | 0.12 | 0.22 | 0.19 | 0.36 | 0.01 | 0.25 |
| RMSE | 0.82 | 0.84 | 0.79 | 0.81 | 0.72 | 0.90 | 0.78 | |
| RPD | 1.10 | 1.07 | 1.14 | 1.11 | 1.25 | 1.00 | 1.16 |
| Form | Criteria | Index Types | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R | SR | D | ND | ID | DDn | mSR1 | mSR2 | mND | mID | ||
| OR | R2 | 0.35 | 0.51 | 0.50 | 0.53 | 0.50 | 0.44 | 0.48 | 0.51 | 0.47 | 0.49 |
| RMSE | 0.73 | 0.63 | 0.64 | 0.61 | 0.64 | 0.67 | 0.65 | 0.63 | 0.66 | 0.64 | |
| RPD | 1.24 | 1.43 | 1.42 | 1.47 | 1.41 | 1.34 | 1.39 | 1.42 | 1.37 | 1.41 | |
| SNV | R2 | 0.46 | 0.50 | 0.51 | 0.44 | 0.46 | 0.46 | 0.48 | 0.51 | 0.47 | 0.47 |
| RMSE | 0.66 | 0.64 | 0.63 | 0.68 | 0.66 | 0.66 | 0.65 | 0.63 | 0.66 | 0.65 | |
| RPD | 1.36 | 1.42 | 1.43 | 1.33 | 1.37 | 1.36 | 1.39 | 1.42 | 1.37 | 1.38 | |
| MSC | R2 | 0.46 | 0.50 | 0.51 | 0.52 | 0.51 | 0.46 | 0.48 | 0.51 | 0.47 | 0.47 |
| RMSE | 0.66 | 0.64 | 0.63 | 0.62 | 0.63 | 0.66 | 0.65 | 0.63 | 0.66 | 0.65 | |
| RPD | 1.36 | 1.41 | 1.43 | 1.44 | 1.42 | 1.37 | 1.38 | 1.42 | 1.37 | 1.38 | |
| EMSC | R2 | 0.42 | 0.48 | 0.45 | 0.48 | 0.47 | 0.47 | 0.46 | 0.45 | 0.44 | 0.48 |
| RMSE | 0.69 | 0.65 | 0.67 | 0.65 | 0.66 | 0.66 | 0.66 | 0.67 | 0.67 | 0.65 | |
| RPD | 1.31 | 1.39 | 1.35 | 1.39 | 1.37 | 1.37 | 1.36 | 1.35 | 1.34 | 1.39 | |
| Log | R2 | 0.33 | 0.53 | 0.53 | 0.53 | 0.49 | 0.43 | 0.46 | 0.51 | 0.47 | 0.42 |
| RMSE | 0.74 | 0.62 | 0.62 | 0.62 | 0.64 | 0.68 | 0.66 | 0.63 | 0.66 | 0.69 | |
| RPD | 1.22 | 1.45 | 1.46 | 1.45 | 1.40 | 1.33 | 1.36 | 1.43 | 1.38 | 1.31 | |
| 1st derivative | R2 | 0.44 | 0.50 | 0.54 | 0.46 | 0.37 | 0.51 | 0.52 | 0.53 | 0.53 | 0.49 |
| RMSE | 0.68 | 0.64 | 0.61 | 0.66 | 0.71 | 0.63 | 0.62 | 0.62 | 0.62 | 0.64 | |
| RPD | 1.33 | 1.42 | 1.47 | 1.36 | 1.26 | 1.43 | 1.44 | 1.46 | 1.46 | 1.41 | |
| 2nd derivative | R2 | 0.39 | 0.47 | 0.45 | 0.49 | 0.45 | 0.45 | 0.46 | 0.46 | 0.47 | 0.33 |
| RMSE | 0.70 | 0.66 | 0.67 | 0.64 | 0.67 | 0.67 | 0.66 | 0.66 | 0.66 | 0.74 | |
| RPD | 1.28 | 1.38 | 1.35 | 1.40 | 1.36 | 1.35 | 1.37 | 1.36 | 1.37 | 1.22 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, G.; Wang, Q. Developing Hyperspectral Indices for Assessing Seasonal Variations in the Ratio of Chlorophyll to Carotenoid in Deciduous Forests. Remote Sens. 2022, 14, 1324. https://doi.org/10.3390/rs14061324
Song G, Wang Q. Developing Hyperspectral Indices for Assessing Seasonal Variations in the Ratio of Chlorophyll to Carotenoid in Deciduous Forests. Remote Sensing. 2022; 14(6):1324. https://doi.org/10.3390/rs14061324
Chicago/Turabian StyleSong, Guangman, and Quan Wang. 2022. "Developing Hyperspectral Indices for Assessing Seasonal Variations in the Ratio of Chlorophyll to Carotenoid in Deciduous Forests" Remote Sensing 14, no. 6: 1324. https://doi.org/10.3390/rs14061324
APA StyleSong, G., & Wang, Q. (2022). Developing Hyperspectral Indices for Assessing Seasonal Variations in the Ratio of Chlorophyll to Carotenoid in Deciduous Forests. Remote Sensing, 14(6), 1324. https://doi.org/10.3390/rs14061324






