A Spectral Library Study of Mixtures of Common Lunar Minerals and Glass
Abstract
1. Introduction
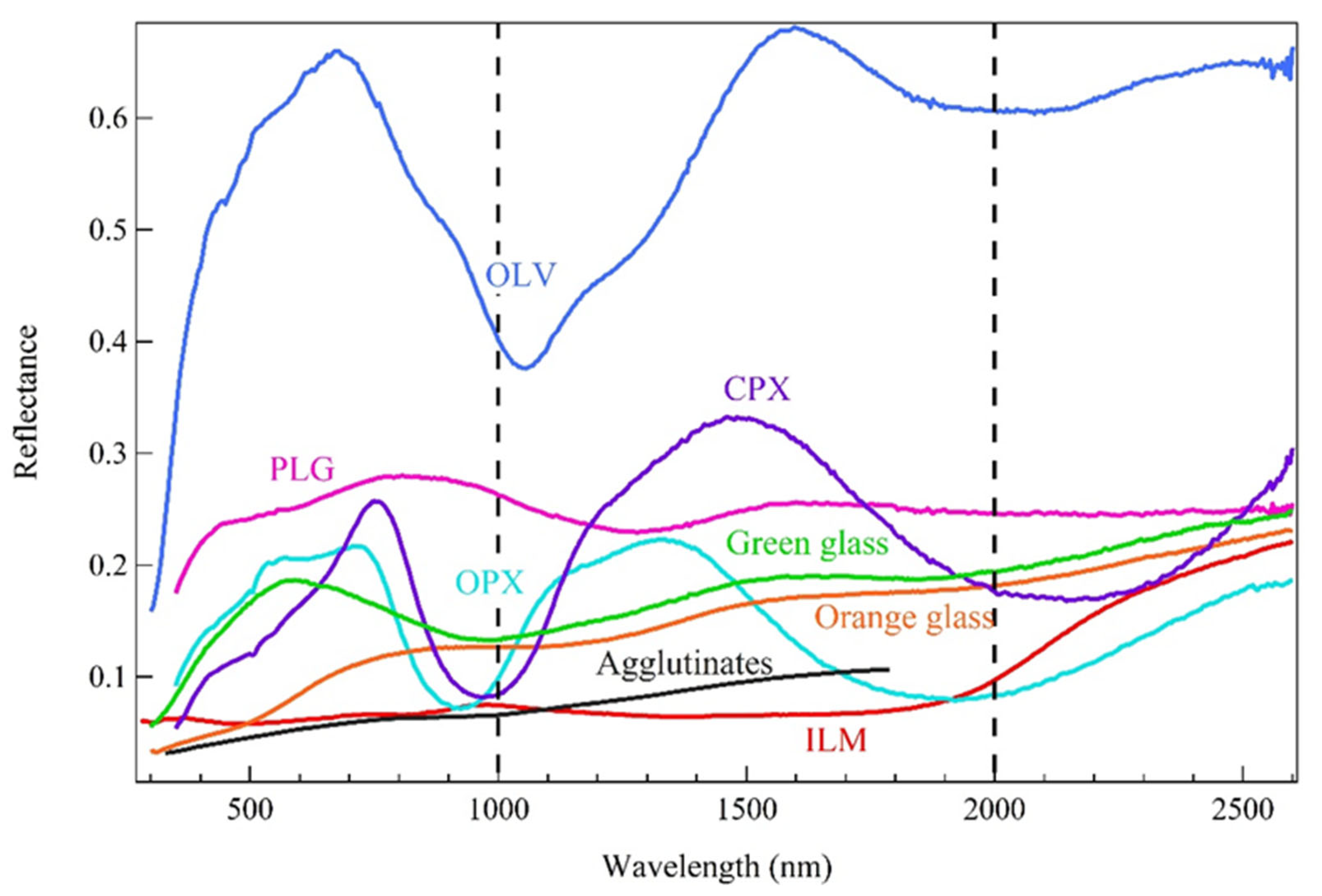
2. Method and Data
2.1. Model Descriptions
2.2. Synthetic Glass Spectra
2.3. Spectral Library Construction
2.4. Spectral Matching Algorithms
3. Results
3.1. Test the Accuracy of the MSL by the LSCC Lunar Soil Samples
3.2. Test the Accuracy of the MSL by the RELAB Samples
4. Applications to CE-3 In Situ Lunar Measurements
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucey, P.G. Radiative transfer modeling of the effect of mineralogy on some empirical methods for estimating iron concentration from multispectral imaging of the moon. J. Geophys. Res. Planets 2006, 111, E08003. [Google Scholar] [CrossRef]
- Shkuratov, Y.; Kaydash, V.; Korokhin, V.; Velikodsky, Y.; Opanasenko, N.; Videen, G. Optical measurements of the Moon as a tool to study its surface. Planet. Space Sci. 2011, 59, 1326–1371. [Google Scholar] [CrossRef]
- Adams, J.B.; McCord, T.B. Optical properties of mineral separates, glass, and anorthositic fragments from Apollo mare samples. In Lunar and Planetary Science Conference Proceedings; Pergamon Press: New York, NY, USA, 1971; Volume 2, p. 2183. [Google Scholar]
- Farr, T.G.; Bates, B.A.; Ralph, R.L.; Adams, J.B. Effects of overlapping optical absorption bands of pyroxene and glass on the reflectance spectra of lunar soils. In Lunar and Planetary Science Conference Proceedings; Pergamon Press: New York, NY, USA, 1980; Volume 11, pp. 719–729. [Google Scholar]
- Pieters, C.M.; Noble, S.K. Space weathering on airless bodies. J. Geophys. Res. Planets 2016, 121, 1865–1884. [Google Scholar] [CrossRef]
- Clénet, H.; Pinet, P.; Daydou, Y.; Heuripeau, F.; Rosemberg, C.; Baratoux, D.; Chevrel, S. A new systematic approach using the modified gaussian model: Insight for the characterization of chemical composition of olivines, pyroxenes and olivine–pyroxene mixtures. Icarus 2011, 213, 404–422. [Google Scholar] [CrossRef]
- Klima, R.L.; Dyar, M.D.; Pieters, C.M. Near-infrared spectra of clinopyroxenes: Effects of calcium content and crystal structure. Meteorit. Planet. Sci. 2011, 46, 379–395. [Google Scholar] [CrossRef]
- Pieters, C.M. Strength of mineral absorption features in the transmitted component of near-infrared reflected light: First results from RELAB. J. Geophys. Res. Solid Earth 1983, 88, 9534–9544. [Google Scholar] [CrossRef]
- Besse, S.; Sunshine, J.M.; Gaddis, L.R. Volcanic glass signatures inspectroscopic survey of newly proposedlunar pyroclastic deposits. J. Geophys. Res. Planets 2014, 119, 355–372. [Google Scholar] [CrossRef]
- Taylor, K.E. Summarizing multiple aspects of model performance in a single diagram. J. Geophys. Res. 2001, 106, 7183–7192. [Google Scholar] [CrossRef]
- McKay, D.S.; Heiken, G.H.; Taylor, R.M.; Clanton, U.S.; Morrison, D.A.; Ladle, G.H. Apollo 14 soils: Size distribution and particle types. In Lunar and Planetary Science Conference Proceedings; Pergamon Press: New York, NY, USA, 1972; Volume 3, p. 983. [Google Scholar]
- McKay, D.S.; Basu, A. The production curve for agglutinates in planetary regoliths. J. Geophys. Res. Solid Earth 1983, 88, B193–B199. [Google Scholar] [CrossRef]
- Pieters, C.M.; Fischer, E.M.; Rode, O.; Basu, A. Optical effects of space weathering: The role of the finest fraction. J. Geophys. Res. Planets 1993, 98, 20817–20824. [Google Scholar] [CrossRef]
- Wetzel, D.; Hauri, E.; Saal, A.; Rutherford, M. Carbon content and degassing history of the lunar volcanic glasses. Nat. Geosci. 2015, 8, 755–758. [Google Scholar] [CrossRef]
- Scudder, N.A.; Horgan, B.H.; Rampe, E.B.; Smith, R.J.; Rutledge, A.M. The effects of magmatic evolution, crystallinity, and microtexture on the visible/near-infrared and thermal-infrared spectra of volcanic rocks. Icarus 2021, 359, 114344. [Google Scholar] [CrossRef]
- Wilson, L.; Head, J.W., III. Ascent and eruption of basaltic magma on the Earth and Moon. J. Geophys. Res. Solid Earth 1981, 86, 2971–3001. [Google Scholar] [CrossRef]
- Saal, A.E.; Hauri, E.H.; Cascio, M.L.; van Orman, J.A.; Rutherford, M.C.; Cooper, R.F. Volatile content of lunar volcanic glasses and the presence of water in the Moon’s interior. Nature 2008, 454, 192–195. [Google Scholar] [CrossRef]
- Arndt, J.; Engelhardt, W.V.; Gonzalez-Cabeza, I.; Meier, B. Formation of apollo 15 green glass beads. J. Geophys. Res. Solid Earth 1984, 89, C225–C232. [Google Scholar] [CrossRef]
- Cannon, K.M.; Mustard, J.F.; Parman, S.W.; Sklute, E.C.; Dyar, M.D.; Cooper, R.F. Spectral properties of Martian and other planetary glasses and their detection in remotely sensed data. J. Geophys. Res. Planets 2017, 122, 249–268. [Google Scholar] [CrossRef]
- Hapke, B. Bidirectional reflectance spectroscopy: 1. theory. J. Geophys. Res. Planets 1981, 86, 3039–3054. [Google Scholar] [CrossRef]
- Lemelin, M.; Lucey, P.G.; Song, E.; Taylor, G.J. Lunar central peak mineralogy and iron content using the kaguya multiband imager: Reassessment of the compositional structure of the lunar crust. J. Geophys. Res. Planets 2015, 120, 869–887. [Google Scholar] [CrossRef]
- Sun, L.; Lucey, P.G. Unmixing Mineral Abundance and Mg# With Radiative Transfer Theory: Modeling and Applications. J. Geophys. Res. Planets 2021, 126, e2020JE006691. [Google Scholar]
- Cannon, K.; Mustard, J. Preserved glass-rich impactites on Mars. Geology 2015, 43, 635–638. [Google Scholar] [CrossRef]
- Pieters, C.M.; Hanna, K.D.; Cheek, L.; Dhingra, D.; Prissel, T.; Jackson, C.; Taylor, L.A. The distribution of mg-spinel across the moon and constraints on crustal origin. Am. Miner. 2014, 99, 1893–1910. [Google Scholar] [CrossRef]
- Denevi, B.W.; Yasanayake, C.N.; Jolliff, B.L.; Lawrence, S.J.; Hiroi, T.; Martin, A.C. The Spectral Properties of Lunar Agglutinates. In Lunar and Planetary Science Conference; Pergamon Press: New York, NY, USA, 2021; Volume 2548, p. 2368. [Google Scholar]
- Zhang, H.; Yang, Y.; Yuan, Y.; Jin, W.; Lucey, P.G.; Zhu, M.-H.; Kaydash, V.G.; Shkuratov, Y.G.; Di, K.; Wan, W.; et al. In situ optical measurements of Chang’E-3 landing site in Mare Imbrium: 1. Mineral abundances inferred from spectral reflectance. Geophys. Res. Lett. 2015, 42, 6945–6950. [Google Scholar] [CrossRef]
- Hu, X.; Ma, P.; Yang, Y.; Zhu, M.H.; Jiang, T.; Lucey, P.G.; Sun, L.; Zhang, H.; Li, C.; Xu, R.; et al. Mineral abundances inferred from in situ reflectance measurements of Chang’E-4 landing site in South Pole-Aitken basin. Geophys. Res. Lett. 2019, 46, 9439–9447. [Google Scholar] [CrossRef]
- Morris, R.V. The surface exposure (maturity) of lunar soils: Some concepts and Is/FeO complication. In Lunar and Planetary Science Conference Proceedings; Pergamon Press: New York, NY, USA, 1978; Volume 9, pp. 2287–2297. [Google Scholar]
- Taylor, L.; Cahill, J.; Patchen, A.; Pieters, C.; Morris, R.; Keller, L.; McKay, D. Mineralogical and chemical characterization of lunar highland regolith: Lessons learned from mare soils. In Lunar and Planetary Science Conference; Pergamon Press: New York, NY, USA, 2001; Volume 32, p. 2196. [Google Scholar]
- Wang, Z.; Wu, Y.; Blewett, D.T.; Cloutis, E.A.; Zheng, Y.; Chen, J. Submicroscopic metallic iron in lunar soils estimated from the in situ spectra of the Chang’E-3 mission. Geophys. Res. Lett. 2017, 44, 3485–3492. [Google Scholar] [CrossRef]
- Ling, Z.; Jolliff, B.L.; Wang, A.; Li, C.; Liu, J.; Zhang, J.; Li, B.; Sun, L.; Chen, J.; Xiao, L.; et al. Correlated compositional and mineralogical investigations at the Chang’e-3 landing site. Nat. Commun. 2015, 6, 8880. [Google Scholar] [CrossRef]
- Hapke, B. Theory of Reflectance and Emittance Spectroscopy; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Mustard, J.F.; Pieters, C.M. Photometric phase functions of common geologic minerals and applications to quantitative analysis of mineral mixture reflectance spectra. J. Geophys. Res. Planets 1989, 94, 13619–13634. [Google Scholar] [CrossRef]
- Lucey, P.G. Mineral maps of the moon. Geophys. Res. Lett. 2004, 31, L08701. [Google Scholar] [CrossRef]
- Lucey, P.G.; Blewett, D.T.; Jolliff, B.L. Lunar iron and titanium abundance algorithms based on final processing of Clementine ultraviolet-visible images. J. Geophys. Res. Planets 2000, 105, 20297–20305. [Google Scholar] [CrossRef]
- Hapke, B. Space weathering from mercury to the asteroid belt. J. Geophys. Res. Planets 2001, 106, 10039–10073. [Google Scholar] [CrossRef]
- Taylor, L.; Patchen, A.; Taylor, D.; Chambers, J.; McKay, D. X-ray digital imaging and petrography of lunar mare soils: Data input for remote sensing calibrations. Icarus 1996, 124, 500–512. [Google Scholar] [CrossRef]
- Sunshine, J.M.; Pieters, C.M. Estimating modal abundances from the spectra of natural and laboratory pyroxene mixtures using the modified Gaussian model. J. Geophys. Res. Planets 1993, 98, 9075–9087. [Google Scholar] [CrossRef]
- Zeng, X.; Li, X.; Martin, D.; Tang, H.; Yu, W.; Liu, J.; Wang, S. Micro-FTIR spectroscopy of lunar pyroclastic and impact glasses as a new diagnostic tool to discern them. J. Geophys. Res. Planets 2019, 124, 3267–3282. [Google Scholar] [CrossRef]
- Salisbury, J.W.; Basu, A.; Fischer, E.M. Thermal infrared spectra of lunar soils. Icarus 1997, 130, 125–139. [Google Scholar] [CrossRef]






| Compositions | SiO2 | TiO2 | Al2O3 | Cr2O3 | FeO | MnO | MgO | CaO | Na2O | K2O |
|---|---|---|---|---|---|---|---|---|---|---|
| Synthetic green glass (wt.%) | 25.58 | 0.55 | 7.89 | 0.51 | 19.49 | 0.28 | 17.1 | 8.43 | 0.19 | 0.01 |
| Synthetic orange glass (wt.%) | 38.39 | 9.98 | 6.01 | 0.66 | 22.86 | 0.31 | 13.87 | 7.44 | 0.42 | 0.06 |
| Soil ID | Measurement (wt.%) | MSL (without Glass) (wt.%) | MSL (with Green Glass) (wt.%) | MSL (with Orange Glass) (wt.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OLV | PYX | PLG | OLV | PYX | PLG | OLV | PYX | PLG | OLV | PYX | PLG | |
| X12001-56 | 11.7 | 49.7 | 38.6 | 17.9 | 52.4 | 29.7 | 13.5 | 57.3 | 29.5 | 15.18 | 55.8 | 29.0 |
| X12030-14 | 9.5 | 54.7 | 35.8 | 14.4 | 58.9 | 26.7 | 2.4 | 63.9 | 33.8 | 15.6 | 57.1 | 27.3 |
| X15041-94 | 6.9 | 47.7 | 45.5 | 19.1 | 45.3 | 35.7 | 8.1 | 56.4 | 34.3 | 15.9 | 56.1 | 28.0 |
| X15071-52 | 7.2 | 42.9 | 49.9 | 18.5 | 53.8 | 27.7 | 6.5 | 59.0 | 34.5 | 18.3 | 58.0 | 23.8 |
| X70181-47 | 12.4 | 27.8 | 59.8 | 21.2 | 32.8 | 46.0 | 13.1 | 23.4 | 63.5 | 16.8 | 37.8 | 45.5 |
| X71061-14 | 14.0 | 38.8 | 47.2 | 23.5 | 16.2 | 60.3 | 11.8 | 22.7 | 65.5 | 22.6 | 25.1 | 52.3 |
| X71501-35 | 9.2 | 37.1 | 53.7 | 19.4 | 28.6 | 51.0 | 8.5 | 48.3 | 43.3 | 9.6 | 50.7 | 39.8 |
| X79221-81 | 11.7 | 33.3 | 55.0 | 28.3 | 28.0 | 43.7 | 24.6 | 35.4 | 40.0 | 19.0 | 38.4 | 42.5 |
| X10084-78 | 3.6 | 40.1 | 56.3 | 24.8 | 22.9 | 52.3 | 17.0 | 37.3 | 45.8 | 8.6 | 52.1 | 49.2 |
| X14141-5.7 | 4.0 | 26.9 | 69.1 | 10.5 | 33.1 | 56.3 | 9.2 | 32.1 | 58.8 | 10.4 | 29.2 | 60.5 |
| X14163-57 | 6.1 | 40.4 | 53.5 | 6.8 | 36.5 | 47.3 | 1.7 | 36.8 | 61.5 | 9.7 | 38.3 | 52.0 |
| X14259-85 | 5.4 | 35.1 | 59.5 | 4.4 | 25.3 | 70.3 | 5.7 | 28.3 | 66.0 | 7.7 | 25.1 | 63.3 |
| X14260-72 | 5.1 | 40.7 | 54.2 | 6.6 | 26.4 | 67.0 | 7.3 | 29.0 | 63.8 | 4.8 | 28.2 | 63.8 |
| X61141-56 | 3.5 | 7.3 | 89.2 | 3.8 | 13.2 | 83.0 | 1.4 | 19.7 | 79.0 | 5.3 | 21.0 | 67.0 |
| X61221-9.2 | 3.0 | 8.0 | 89.1 | 1.2 | 35.5 | 63.3 | 0.4 | 27.6 | 72.0 | 3.6 | 23.2 | 73.8 |
| X62231-91 | 3.9 | 10.0 | 86.1 | 4.8 | 22.3 | 73.7 | 2.8 | 14.3 | 83.0 | 3.4 | 11.8 | 73.3 |
| X64801-82 | 2.6 | 7.3 | 90.1 | 1.3 | 16.3 | 82.3 | 1.0 | 14.8 | 84.3 | 4.0 | 9.8 | 85.8 |
| X67461-25 | 2.3 | 6.2 | 91.6 | 3.9 | 10.3 | 86.3 | 0.8 | 12.5 | 84.5 | 4.1 | 15.3 | 80.5 |
| X67481-31 | 4.1 | 7.9 | 87.9 | 3.1 | 6.6 | 90.3 | 3.8 | 8.4 | 87.8 | 3.8 | 8.4 | 87.8 |
| Value (wt.%) | Soil ID (Highland) | X14141-5.7 | X14163-57 | X14259-85 | X14260-72 | X61141-56 | X61221-9.2 | X62231-91 | X64801-82 | X67461-25 | X67481-31 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Meas. | Agglutinates * | 48.6 | 58.5 | 68.7 | 65.2 | 53.9 | 32.6 | 55.0 | 61.0 | 32.4 | 28.6 |
| MSL | Orange glass | 5.0 | 6.3 | 2.5 | 3.8 | 8.8 | 10.0 | 3.8 | 3.8 | 2.5 | 2.5 |
| Green glass | 1.3 | 5.0 | 2.5 | 2.5 | 5.0 | 5.0 | 2.5 | 2.5 | 3.8 | 1.3 | |
| Value (wt.%) | Soil ID (Mare) | X12001-56 | X12030-14 | X15041-94 | X15071-52 | X70181-47 | X71061-41 | X71501-35 | X79221-81 | X10084-78 | |
| Meas. | Agglutinates * | 56.8 | 49.8 | 56.7 | 49.2 | 51.7 | 37.9 | 44.8 | 54.3 | 57.0 | |
| Volcanic glass | 1.3 | 1.5 | 2.6 | 4.1 | 9.2 | 18.8 | 7.5 | 9.2 | 2.9 | ||
| MSL | Orange glass | 6.3 | 12.5 | 7.5 | 2.5 | 6.8 | 18.8 | 13.8 | 12.5 | 17.5 | |
| Green glass | 2.5 | 11.3 | 5.0 | 6.3 | 5.0 | 8.8 | 8.8 | 5.0 | 11.3 |
| Sample ID | RELAB Data | Retrieved Abundance by MSL with Glass | |||
|---|---|---|---|---|---|
| Endmember of the Samples | Real Abundance (wt.%) | Endmember of the MSL | MSL Result (with Orange Glass) (wt.%) | MSL Result (with Green Glass) (wt.%) | |
| XS-JFM-006 | OLV | 21 | OLV | 9.2 | 12.3 |
| OPX | 11 | OPX | 3.5 | 6 | |
| CPX | 42 | CPX | 48.2 | 49.4 | |
| PLG | 23 | PLG | 39.1 | 31 | |
| ILM | 3 | glass | 0 | 1.3 | |
| XS-JFM-009 | OLV | 30 | OLV | 2.9 | 8 |
| OPX | 49 | OPX | 47.9 | 32.9 | |
| CPX | 10 | CPX | 10.1 | 25.5 | |
| PLG | 4 | PLG | 35.3 | 27.4 | |
| ILM | 7 | glass | 3.8 | 6.3 | |
| XS-JFM-012 | OLV | 5 | OLV | 2 | 3.1 |
| OPX | 5 | OPX | 0 | 0 | |
| CPX | 47 | CPX | 66.5 | 65.9 | |
| PLG | 15 | PLG | 30.5 | 31 | |
| ILM | 28 | glass | 0 | 0 | |
| XS-JFM-015 | OLV | 23 | OLV | 11.2 | 13.3 |
| OPX | 0 | OPX | 0 | 0 | |
| CPX | 0 | CPX | 16.1 | 20.1 | |
| PLG | 69 | PLG | 70.2 | 64.5 | |
| ILM | 8 | glass | 2.5 | 1.3 | |
| MSL with Orange Glass (wt.%) | MSL with Green Glass (wt.%) | MSL without Glass (wt.%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Data | OLV | OPX | CPX | PLG | glass | OLV | OPX | CPX | PLG | glass | OLV | OPX | CPX | PLG |
| Node N203 | 17.8 | 3.5 | 27.8 | 46.0 | 5.0 | 16.8 | 0.0 | 36.0 | 43.3 | 3.8 | 23.8 | 6.0 | 46.9 | 23.3 |
| Node N205 | 25.1 | 4.3 | 32.6 | 28.1 | 10.0 | 21.3 | 1.0 | 41.7 | 30.9 | 5.0 | 28.9 | 5.8 | 43.8 | 21.5 |
| Node E | 29.9 | 2.1 | 27.8 | 33.6 | 6.6 | 26.2 | 0.0 | 28.6 | 36.4 | 8.8 | 30.5 | 0.0 | 28.6 | 40.9 |
| Node S3 | 19.7 | 1.8 | 25.5 | 39.3 | 13.8 | 20.2 | 3.1 | 32.2 | 39.5 | 5.0 | 23.1 | 13.1 | 44.6 | 19.2 |
| (N205 + S3)/2 | 22.4 | 3.1 | 29.1 | 33.7 | 11.9 | 20.8 | 2.1 | 37.0 | 35.2 | 5.1 | 26.0 | 9.5 | 44.2 | 20.3 |
| Average | 23.1 | 2.9 | 28.4 | 36.8 | 8.9 | 21.4 | 1.0 | 34.4 | 37.5 | 5.7 | 26.5 | 6.2 | 41.0 | 26.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Jiang, T.; Ma, P.; Zhang, H.; Lucey, P.; Zhu, M. A Spectral Library Study of Mixtures of Common Lunar Minerals and Glass. Remote Sens. 2023, 15, 2195. https://doi.org/10.3390/rs15082195
Hu X, Jiang T, Ma P, Zhang H, Lucey P, Zhu M. A Spectral Library Study of Mixtures of Common Lunar Minerals and Glass. Remote Sensing. 2023; 15(8):2195. https://doi.org/10.3390/rs15082195
Chicago/Turabian StyleHu, Xiaoyi, Te Jiang, Pei Ma, Hao Zhang, Paul Lucey, and Menghua Zhu. 2023. "A Spectral Library Study of Mixtures of Common Lunar Minerals and Glass" Remote Sensing 15, no. 8: 2195. https://doi.org/10.3390/rs15082195
APA StyleHu, X., Jiang, T., Ma, P., Zhang, H., Lucey, P., & Zhu, M. (2023). A Spectral Library Study of Mixtures of Common Lunar Minerals and Glass. Remote Sensing, 15(8), 2195. https://doi.org/10.3390/rs15082195






