Impact of Fragmentation on Carbon Uptake in Subtropical Forest Landscapes in Zhejiang Province, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Forest Map Data and Fragmentation Levels Classification
2.3. Time Series GPP Dataset
2.4. Data Analysis
2.4.1. Comparison of GPP Trend among Different Fragmentation Levels
2.4.2. Detecting the Relative Importance of the Control Pathways of GPP
2.4.3. Comparison of GPP Control Pathways among Different Fragmentation Levels
2.4.4. Statistical Analysis and Data Processing Methods
3. Results
3.1. GPP Dynamics of Different Fragmentation Levels
3.2. Relative Importance of Different GPP Control Pathways
3.3. GPP Control Pathways of Different Fragmentation Levels
4. Discussion
4.1. Impacts of Forest Fragmentation on Carbon Uptake
4.2. Impacts of Forest Fragmentation on Control Pathways of Carbon Uptake
4.3. Insights on Local Forest Management under Forest Fragmentation
4.4. Research Uncertainties and Prospects
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Canadell, J.G.; Raupach, M.R. Managing forests for climate change mitigation. Science 2008, 320, 1456–1457. [Google Scholar] [CrossRef]
- Fang, J.Y.; Guo, Z.D.; Hu, H.F.; Kato, T.; Muraoka, H.; Son, Y. Forest biomass carbon sinks in East Asia, with special reference to the relative contributions of forest expansion and forest growth. Glob. Change Biol. 2014, 20, 2019–2030. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Pirjola, L.; Ketzel, M.; Harrison, R.M. Nanoparticle emissions from 11 non-vehicle exhaust sources—A review. Atmos. Environ. 2013, 67, 252–277. [Google Scholar] [CrossRef]
- Hansen, M.C.; Stehman, S.V.; Potapov, P.V. Quantification of global gross forest cover loss. Proc. Natl. Acad. Sci. USA 2010, 107, 8650–8655. [Google Scholar] [CrossRef] [PubMed]
- Arora, V.K.; Boer, G.J. Uncertainties in the 20th century carbon budget associated with land use change. Glob. Change Biol. 2010, 16, 3327–3348. [Google Scholar] [CrossRef]
- Curtis, P.G.; Slay, C.M.; Harris, N.L.; Tyukavina, A.; Hansen, M.C. Classifying drivers of global forest loss. Science 2018, 361, 1108–1111. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, J.; Wu, W.; Liu, J. Global forest fragmentation change from 2000 to 2020. Nat. Commun. 2023, 14, 3752. [Google Scholar] [CrossRef] [PubMed]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef]
- Collins, C.D.; Banks-Leite, C.; Brudvig, L.A.; Foster, B.L.; Cook, W.M.; Damschen, E.I.; Andrade, A.; Austin, M.; Camargo, J.L.; Driscoll, D.A.; et al. Fragmentation affects plant community composition over time. Ecography 2017, 40, 119–130. [Google Scholar] [CrossRef]
- Bello, C.; Galetti, M.; Pizo, M.A.; Magnago, L.F.S.; Rocha, M.F.; Lima, R.A.F.; Peres, C.A.; Ovaskainen, O.; Jordano, P. Defaunation affects carbon storage in tropical forests. Sci. Adv. 2015, 1, e1501105. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Shen, C.; Lou, D.; Fu, S.; Guan, D. Ecosystem carbon storage in forest fragments of differing patch size. Sci. Rep. 2017, 7, 13173. [Google Scholar] [CrossRef]
- Smith, I.A.; Hutyra, L.R.; Reinmann, A.B.; Marrs, J.K.; Thompson, J.R. Piecing together the fragments: Elucidating edge effects on forest carbon dynamics. Front. Ecol. Environ. 2018, 16, 213–221. [Google Scholar] [CrossRef]
- Chaplin-Kramer, R.; Ramler, I.; Sharp, R.; Haddad, N.M.; Gerber, J.S.; West, P.C.; Mandle, L.; Engstrom, P.; Baccini, A.; Sim, S.; et al. Degradation in carbon stocks near tropical forest edges. Nat. Commun. 2015, 6, 10158. [Google Scholar] [CrossRef] [PubMed]
- Ordway, E.M.; Asner, G.P. Carbon declines along tropical forest edges correspond to heterogeneous effects on canopy structure and function. Proc. Natl. Acad. Sci. USA 2020, 117, 7863–7870. [Google Scholar] [CrossRef] [PubMed]
- Reinmann, A.B.; Hutyra, L.R. Edge effects enhance carbon uptake and its vulnerability to climate change in temperate broadleaf forests. Proc. Natl. Acad. Sci. USA 2017, 114, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Morreale, L.L.; Thompson, J.R.; Tang, X.; Reinmann, A.B.; Hutyra, L.R. Elevated growth and biomass along temperate forest edges. Nat. Commun. 2021, 12, 7181. [Google Scholar] [CrossRef]
- Remy, E.; Wuyts, K.; Boeckx, P.; Ginzburg, S.; Gundersen, P.; Demey, A.; Van Den Bulcke, J.; Van Acker, J.; Verheyen, K. Strong gradients in nitrogen and carbon stocks at temperate forest edges. For. Ecol. Manag. 2016, 376, 45–58. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Riitters, K.; Wickham, J.; O’Neill, R.; Jones, B.; Smith, E. Global-scale patterns of forest fragmentation. Conserv. Ecol. 2000, 4, 30. [Google Scholar] [CrossRef]
- Riitters, K.H.; Wickham, J.D.; O’Neill, R.V.; Jones, K.B.; Smith, E.R.; Coulston, J.W.; Wade, T.G.; Smith, J.H. Fragmentation of continental United States forests. Ecosystems 2002, 5, 815–822. [Google Scholar] [CrossRef]
- Hermosilla, T.; Wulder, M.A.; White, J.C.; Coops, N.C.; Pickell, P.D.; Bolton, D.K. Impact of time on interpretations of forest fragmentation: Three-decades of fragmentation dynamics over Canada. Remote Sens. Environ. 2019, 222, 65–77. [Google Scholar] [CrossRef]
- Monteith, J.L. Solar-radiation and productivity in tropical ecosystems. J. Appl. Ecol. 1972, 9, 747–766. [Google Scholar] [CrossRef]
- Xia, J.; Niu, S.; Ciais, P.; Janssens, I.A.; Chen, J.; Ammann, C.; Arain, A.; Blanken, P.D.; Cescatti, A.; Bonal, D.; et al. Joint control of terrestrial gross primary productivity by plant phenology and physiology. Proc. Natl. Acad. Sci. USA 2015, 112, 2788–2793. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhang, Y.; Ciais, P.; Xiao, X.; Luo, Y.; Caylor, K.K.; Huang, Y.; Wang, G. Dominant role of plant physiology in trend and variability of gross primary productivity in North America. Sci. Rep. 2017, 7, 41366. [Google Scholar] [CrossRef]
- Keenan, T.F.; Gray, J.; Friedl, M.A.; Toomey, M.; Bohrer, G.; Hollinger, D.Y.; Munger, J.W.; O’Keefe, J.; Schmid, H.P.; SueWing, I.; et al. Net carbon uptake has increased through warming-induced changes in temperate forest phenology. Nat. Clim. Change 2014, 4, 598–604. [Google Scholar] [CrossRef]
- Arroyo-Rodriguez, V.; Melo, F.P.L.; Martinez-Ramos, M.; Bongers, F.; Chazdon, R.L.; Meave, J.A.; Norden, N.; Santos, B.A.; Leal, I.R.; Tabarelli, M. Multiple successional pathways in human-modified tropical landscapes: New insights from forest succession, forest fragmentation and landscape ecology research. Biol. Rev. 2017, 92, 326–340. [Google Scholar] [CrossRef]
- Grilli, G.; Urcelay, C.; Galetto, L. Forest fragment size and nutrient availability: Complex responses of mycorrhizal fungi in native-exotic hosts. Plant Ecol. 2012, 213, 155–165. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-Resolution Global Maps of 21st-Century Forest Cover Change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef]
- Qin, Y.W.; Xiao, X.M.; Dong, J.W.; Zhang, G.L.; Shimada, M.; Liu, J.Y.; Li, C.G.; Kou, W.L.; Moore, B. Forest cover maps of China in 2010 from multiple approaches and data sources: PALSAR, Landsat, MODIS, FRA, and NFI. ISPRS J. Photogramm. Remote Sens. 2015, 109, 1–16. [Google Scholar] [CrossRef]
- Dong, J.; Xiao, X.; Sheldon, S.; Biradar, C.; Zhang, G.; Nguyen Dinh, D.; Hazarika, M.; Wikantika, K.; Takeuhci, W.; Moore, B., III. A 50-m Forest Cover Map in Southeast Asia from ALOS/PALSAR and Its Application on Forest Fragmentation Assessment. PLoS ONE 2014, 9, e85801. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, X.; Li, X.; Ma, J.; Chen, B.; Qin, Y.; Dong, J.; Zhao, B. Multi-scale assessments of forest fragmentation in China. Biodiversity 2017, 25, 372–381. (In Chinese) [Google Scholar] [CrossRef]
- Ma, J.; Xiao, X.M.; Zhang, Y.; Doughty, R.; Chen, B.Q.; Zhao, B. Spatial-temporal consistency between gross primary productivity and solar-induced chlorophyll fluorescence of vegetation in China during 2007-2014. Sci. Total Environ. 2018, 639, 1241–1253. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, X.M.; Wu, X.C.; Zhou, S.; Zhang, G.L.; Qin, Y.W.; Dong, J.W. Data Descriptor: A global moderate resolution dataset of gross primary production of vegetation for 2000-2016. Sci. Data 2017, 4, 13. [Google Scholar] [CrossRef]
- Zhao, J.-J.; Liu, L.-Y. Extraction of temperate vegetation phenology thresholds in North America based on flux tower observation data. Chin. J. Appl. Ecol. 2013, 24, 311–318. [Google Scholar]
- White, M.A.; Thornton, P.E.; Running, S.W. A continental phenology model for monitoring vegetation responses to interannual climatic variability. Glob. Biogeochem. Cycles 1997, 11, 217–234. [Google Scholar] [CrossRef]
- Groemping, U. Relative importance for linear regression in R: The package relaimpo. J. Stat. Softw. 2006, 17, 1–27. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Berenguer, E.; Ferreira, J.; Gardner, T.A.; Aragao, L.; De Camargo, P.B.; Cerri, C.E.; Durigan, M.; De Oliveira, R.C.; Vieira, I.C.G.; Barlow, J. A large-scale field assessment of carbon stocks in human-modified tropical forests. Glob. Change Biol. 2014, 20, 3713–3726. [Google Scholar] [CrossRef] [PubMed]
- Silverio, D.V.; Brando, P.M.; Bustamante, M.M.C.; Putz, F.E.; Marra, D.M.; Levick, S.R.; Trumbore, S.E. Fire, fragmentation, and windstorms: A recipe for tropical forest degradation. J. Ecol. 2019, 107, 656–667. [Google Scholar] [CrossRef]
- Zeng, Z.Z.; Gower, D.B.; Wood, E.F. Accelerating forest loss in Southeast Asian Massif in the 21st century: A case study in Nan Province, Thailand. Glob. Change Biol. 2018, 24, 4682–4695. [Google Scholar] [CrossRef]
- Barber, C.P.; Cochrane, M.A.; Souza, C.M.; Laurance, W.F. Roads, deforestation, and the mitigating effect of protected areas in the Amazon. Biol. Conserv. 2014, 177, 203–209. [Google Scholar] [CrossRef]
- Briber, B.M.; Hutyra, L.R.; Reinmann, A.B.; Raciti, S.M.; Dearborn, V.K.; Holden, C.E.; Dunn, A.L. Tree Productivity Enhanced with Conversion from Forest to Urban Land Covers. PLoS ONE 2015, 10, e0136237. [Google Scholar] [CrossRef] [PubMed]
- Martin-Benito, D.; Pederson, N. Convergence in drought stress, but a divergence of climatic drivers across a latitudinal gradient in a temperate broadleaf forest. J. Biogeogr. 2015, 42, 925–937. [Google Scholar] [CrossRef]
- Tang, G.P.; Beckage, B.; Smith, B.; Miller, P.A. Estimating potential forest NPP, biomass and their climatic sensitivity in New England using a dynamic ecosystem model. Ecosphere 2010, 1, 1–20. [Google Scholar] [CrossRef]
- Campbell, M.J.; Edwards, W.; Magrach, A.; Alamgir, M.; Porolak, G.; Mohandass, D.; Laurance, W.F. Edge disturbance drives liana abundance increase and alteration of liana-host tree interactions in tropical forest fragments. Ecol. Evol. 2018, 8, 4237–4251. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Bohn, F.; de Paula, M.D.; Dislich, C.; Groeneveld, J.; Gutierrez, A.G.; Kazmierczak, M.; Knapp, N.; Lehmann, S.; Paulick, S.; et al. Lessons learned from applying a forest gap model to understand ecosystem and carbon dynamics of complex tropical forests. Ecol. Model. 2016, 326, 124–133. [Google Scholar] [CrossRef]
- Malhi, Y.; Aragao, L.; Galbraith, D.; Huntingford, C.; Fisher, R.; Zelazowski, P.; Sitch, S.; McSweeney, C.; Meir, P. Exploring the likelihood and mechanism of a climate-change-induced dieback of the Amazon rainforest. Proc. Natl. Acad. Sci. USA 2009, 106, 20610–20615. [Google Scholar] [CrossRef]
- Qie, L.; Lewis, S.L.; Sullivan, M.J.P.; Lopez-Gonzalez, G.; Pickavance, G.C.; Sunderland, T.; Ashton, P.; Hubau, W.; Abu Salim, K.; Aiba, S.; et al. Long-term carbon sink in Borneo’s forests halted by drought and vulnerable to edge effects. Nat. Commun. 2017, 8, 1966. [Google Scholar] [CrossRef]
- Schmidt, M.; Jochheim, H.; Kersebaum, K.C.; Lischeid, G.; Nendel, C. Gradients of microclimate, carbon and nitrogen in transition zones of fragmented landscapes—A review. Agric. For. Meteorol. 2017, 232, 659–671. [Google Scholar] [CrossRef]
- Ding, L.; Lu, J.; Xu, G.; Wu, J. Effects of ecological protection and development on landscape pattern in the Thousand-Island Lake region, Zhejiang Province. Biodivers. Sci. 2004, 12, 473. [Google Scholar]
- Ren, G.; Young, S.S.; Wang, L.; Wang, W.; Long, Y.; Wu, R.; Li, J.; Zhu, J.; Yu, D.W. Effectiveness of China’s national forest protection program and nature reserves. Conserv. Biol. 2015, 29, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; He, Y.H. Species classification using Unmanned Aerial Vehicle (UAV)-acquired high spatial resolution imagery in a heterogeneous grassland. ISPRS J. Photogramm. Remote Sens. 2017, 128, 73–85. [Google Scholar] [CrossRef]
- Xiao, X.M.; Hollinger, D.; Aber, J.; Goltz, M.; Davidson, E.A.; Zhang, Q.Y.; Moore, B. Satellite-based modeling of gross primary production in an evergreen needleleaf forest. Remote Sens. Environ. 2004, 89, 519–534. [Google Scholar] [CrossRef]
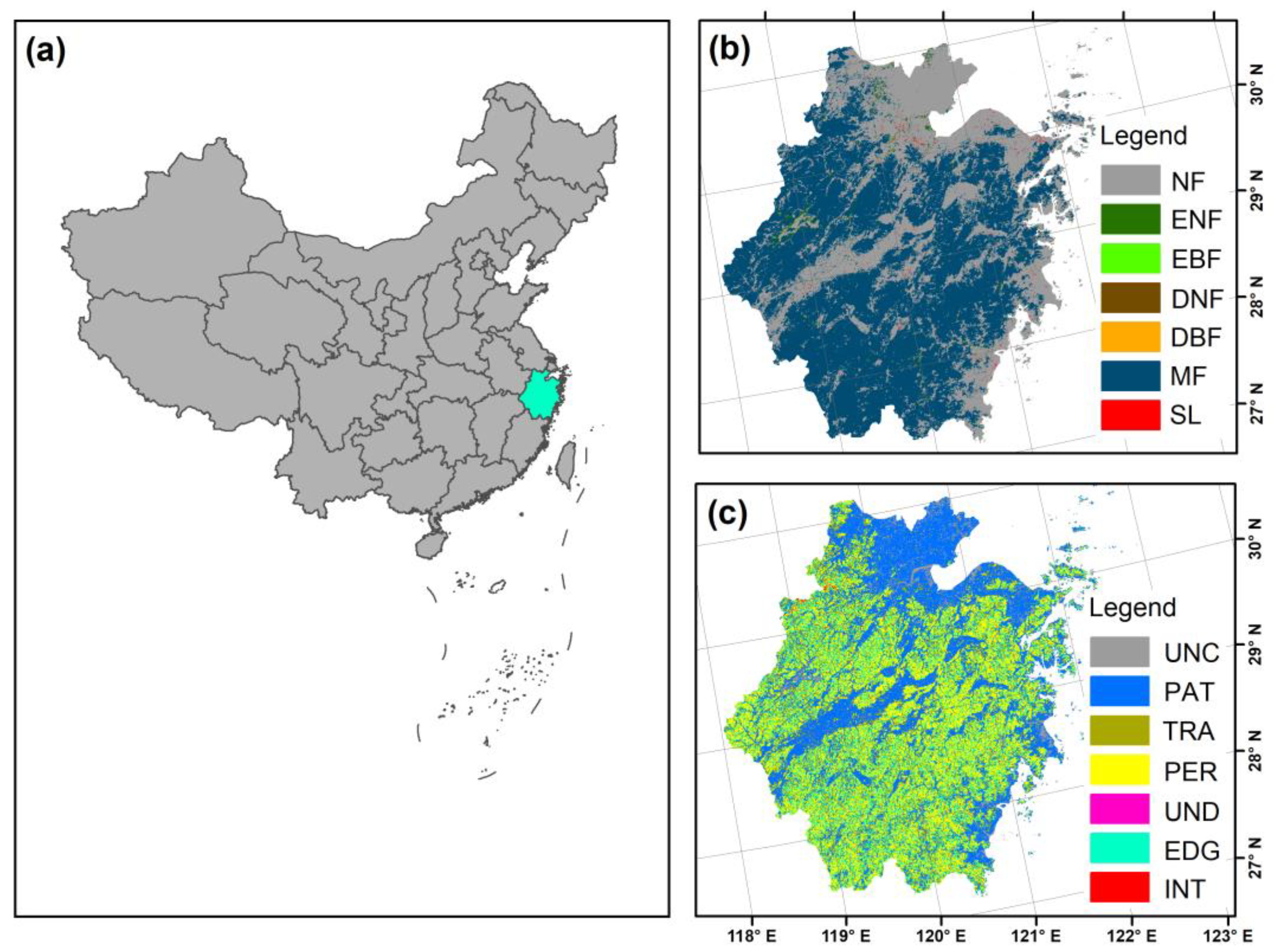
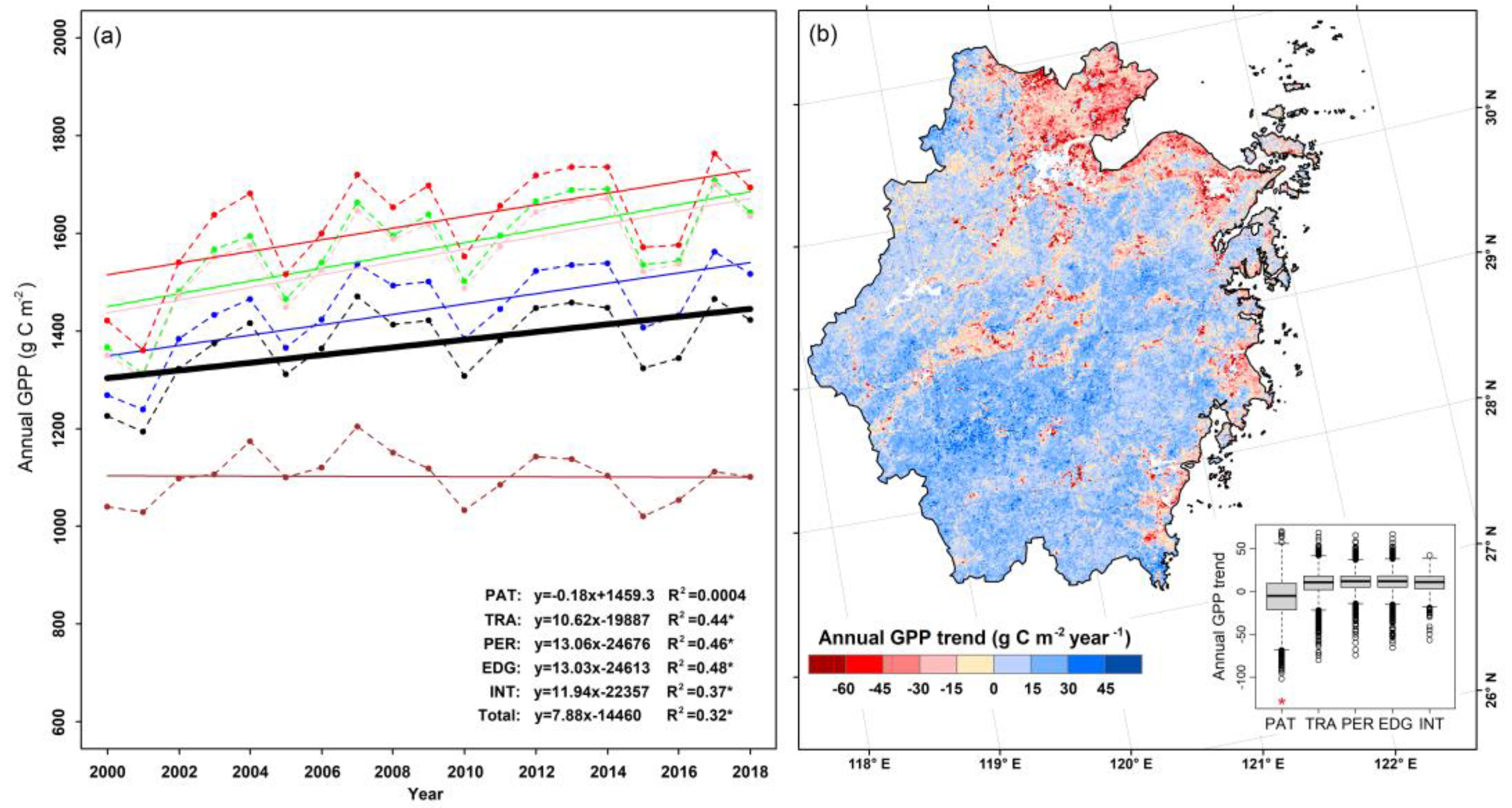

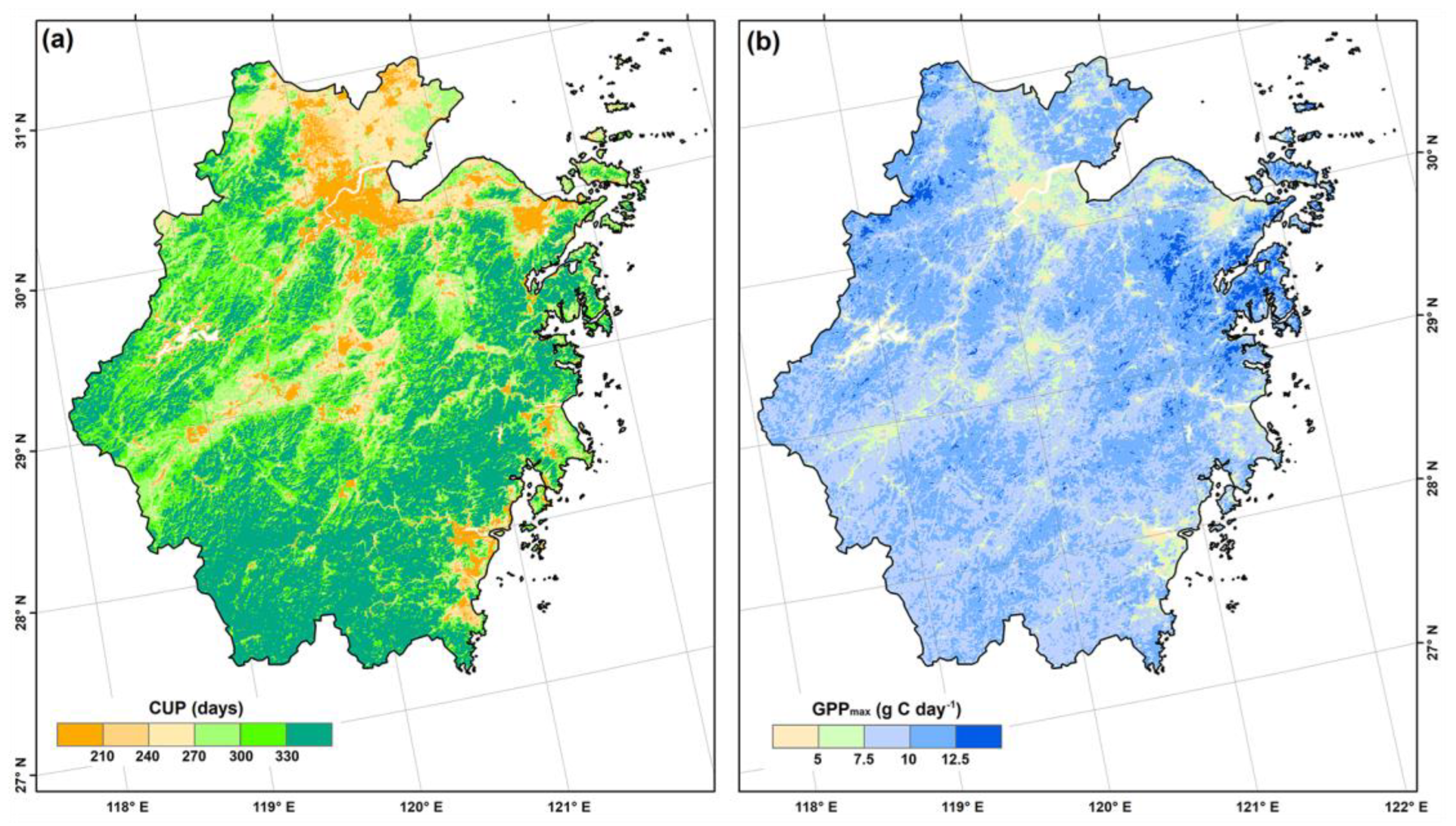
| Fragmentation Categories | Classification Criteria | Area (104 ha) | Area Percentage (%) |
|---|---|---|---|
| PAT | Pf < 0.4 | 263.0 | 33.7 |
| TRA | 0.4 < Pf < 0.6 | 129.5 | 16.6 |
| PER | 0.6 <= Pf < 1 and Pf > Pff | 226.1 | 28.9 |
| UND | 0.6 <= Pf < 1 and Pf = Pff | 0.7 | 0.1 |
| EDG | 0.6 <= Pf < 1 and Pf < Pff | 153.0 | 19.6 |
| INT | Pff = 1 | 9.0 | 1.2 |
| Fragmentation Categories | Mean Annual GPP (g C m−2) | Mean Normalized Annual GPP (g C m−2) | ||
|---|---|---|---|---|
| 2000 | 2018 | 2000 | 2018 | |
| PAT | 1040.4 ± 319.1 a | 1101.6 ± 449.4 a | 11,611.3 ± 17,514.1 d | 11,337.4 ± 16,718.4 d |
| TRA | 1268.7 ± 256.4 b | 1516.8 ± 351.1 b | 2979.0 ± 4071.6 c | 3505.2 ± 4629.4 c |
| PER | 1366.5 ± 193.1 bc | 1643.4 ± 231.5 bc | 1870.6 ± 1276.2 b | 2246.4 ± 1487.0 b |
| EDG | 1349.8 ± 202.4 bc | 1635.2 ± 246.3 bc | 2199.1 ± 2681.0 b | 2656.5 ± 3158.8 b |
| INT | 1421.4 ± 197.6 c | 1693.9 ± 213.9 c | 1657.0 ± 1222.9 a | 1972.6 ± 1478.9 a |
| Fragmentation Categories | CUP (days) | GPPmax (g C m−2 day−1) | CUP × GPPmax |
|---|---|---|---|
| PAT | 256.1 ± 73.4 a | 8.7 ± 2.2 a | 2339.4 ± 949.7 a |
| TRA | 310.7 ± 49.7 b | 9.6 ± 1.6 b | 3041.2 ± 737.1 b |
| PER | 329.8 ± 28.5 b | 10.2 ± 1.3 bc | 3369.1 ± 548.5 c |
| EDG | 327.8 ± 30.3 b | 10.1 ± 1.3 bc | 3320.6 ± 551.2 bc |
| INT | 332.9 ± 28.1 b | 10.7 ± 1.5 c | 3547.7 ± 524.7 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, J.; Cheng, Y.; Hong, P.; Ma, J.; Yao, L.; Jiang, B.; Xu, X.; Wu, C. Impact of Fragmentation on Carbon Uptake in Subtropical Forest Landscapes in Zhejiang Province, China. Remote Sens. 2024, 16, 2393. https://doi.org/10.3390/rs16132393
Jiao J, Cheng Y, Hong P, Ma J, Yao L, Jiang B, Xu X, Wu C. Impact of Fragmentation on Carbon Uptake in Subtropical Forest Landscapes in Zhejiang Province, China. Remote Sensing. 2024; 16(13):2393. https://doi.org/10.3390/rs16132393
Chicago/Turabian StyleJiao, Jiejie, Yan Cheng, Pinghua Hong, Jun Ma, Liangjin Yao, Bo Jiang, Xia Xu, and Chuping Wu. 2024. "Impact of Fragmentation on Carbon Uptake in Subtropical Forest Landscapes in Zhejiang Province, China" Remote Sensing 16, no. 13: 2393. https://doi.org/10.3390/rs16132393
APA StyleJiao, J., Cheng, Y., Hong, P., Ma, J., Yao, L., Jiang, B., Xu, X., & Wu, C. (2024). Impact of Fragmentation on Carbon Uptake in Subtropical Forest Landscapes in Zhejiang Province, China. Remote Sensing, 16(13), 2393. https://doi.org/10.3390/rs16132393










