Phenological and Biophysical Mediterranean Orchard Assessment Using Ground-Based Methods and Sentinel 2 Data
Abstract
:1. Introduction
- A ceptometer is a tool often used to estimate crop PAI. It measures the solar radiation above and below canopy and calculates the canopy PAI based on the ratio between the two [61];
- Hemispherical photography uses photographs of the canopy acquired with a hemispherical (fish-eye) lens [62,63]. The can-eye software.495 developed by Weiss et al. (2008) [63] (https://www6.paca.inrae.fr/can-eye/ accessed on 2 August 2024) facilitates the computation of the main biophysical variables;
- The Viticanopy application proposed by De Bei et al. (2016) [64] is an easy-to-use method based on photographs collected from a smartphone.
2. Data and Methodology
2.1. Study Areas
2.1.1. Description of Orchards Monitored in the Ouvèze–Ventoux Watershed
2.1.2. Description of Orchards Monitored in the La Crau Area
2.2. Sentinel 2 Data and Image Processing
2.3. Ground Measurements for Monitoring of Orchard Development
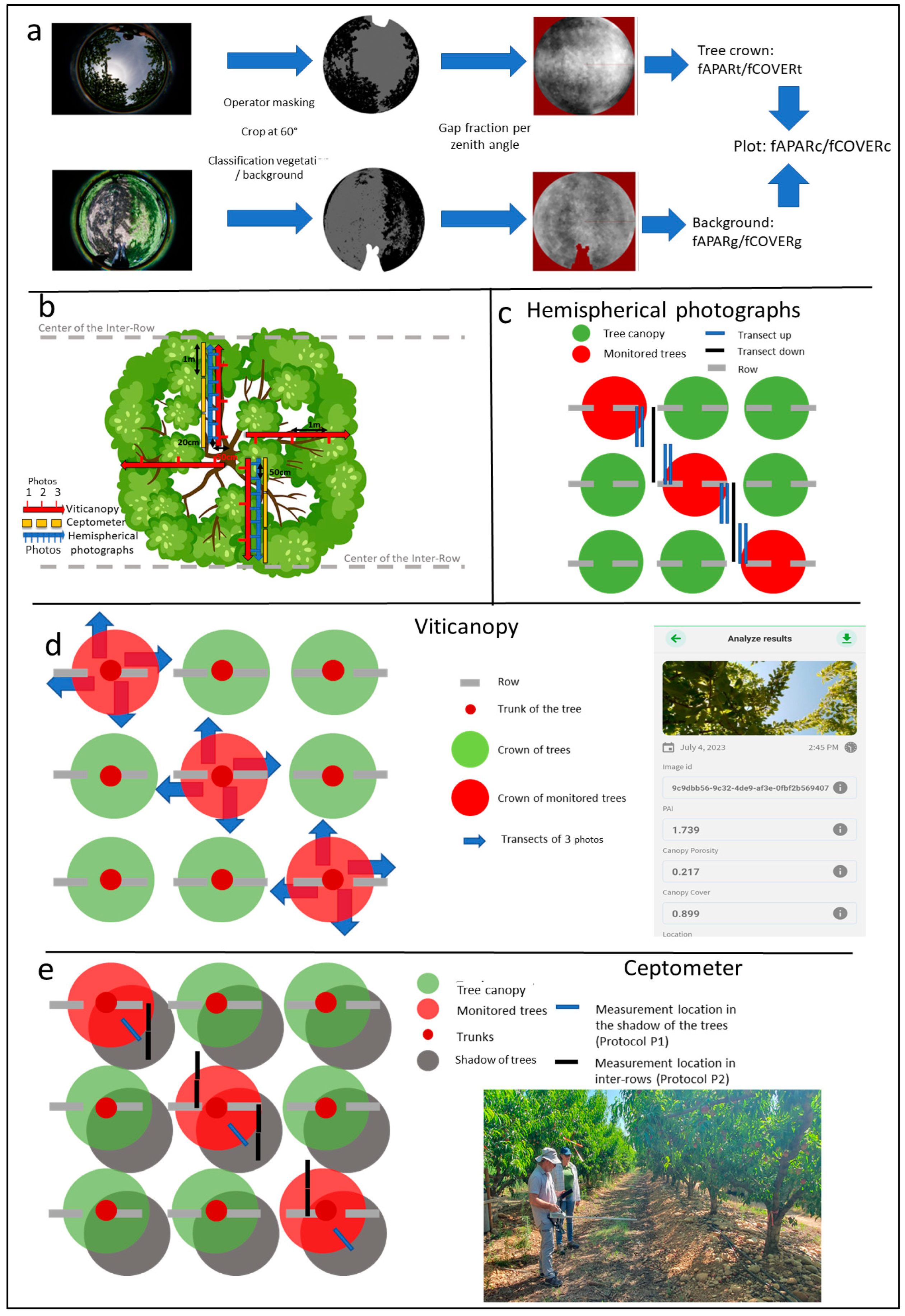
2.3.1. Hemispherical Photographs: Acquisition and Processing
2.3.2. Use of the Viticanopy Application to Estimate PAI and Gap Fraction
2.3.3. Ceptometer Measurements to Estimate PAI
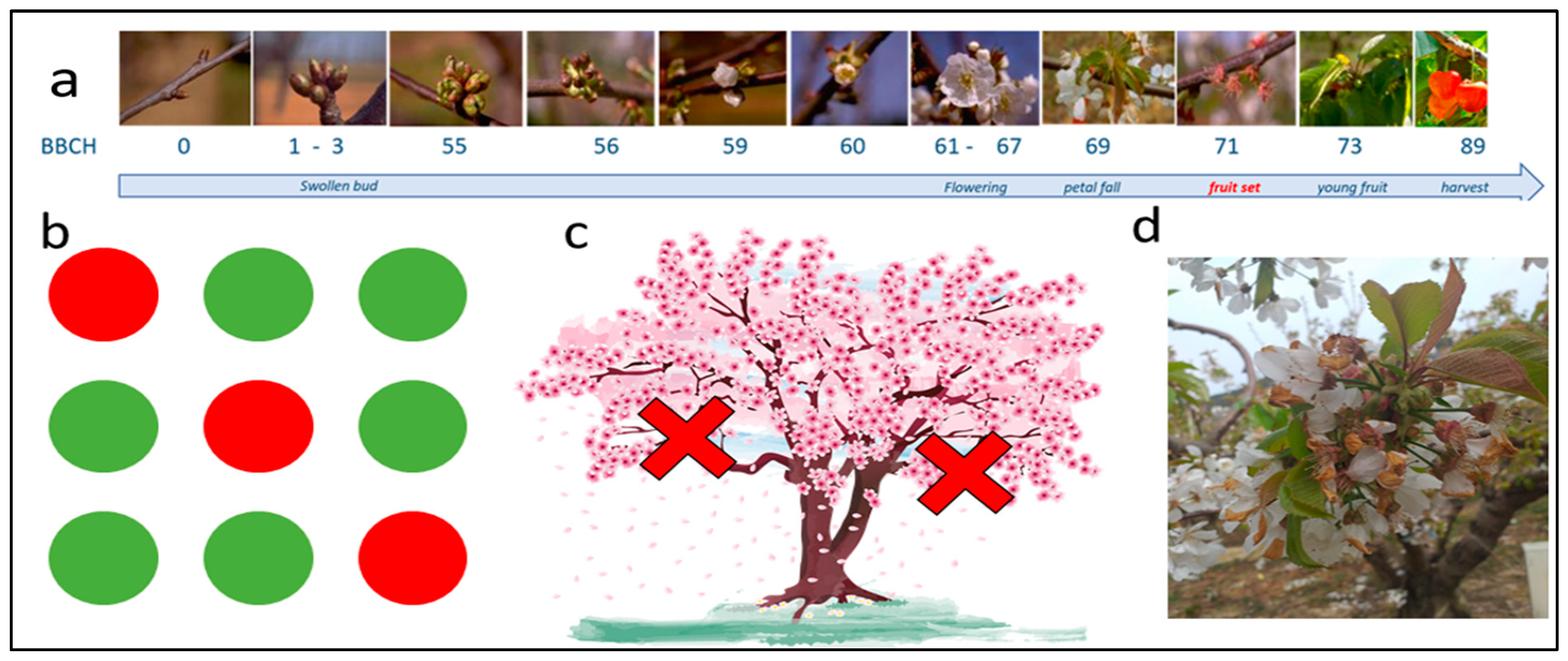
2.4. Phenology Monitoring
3. Results
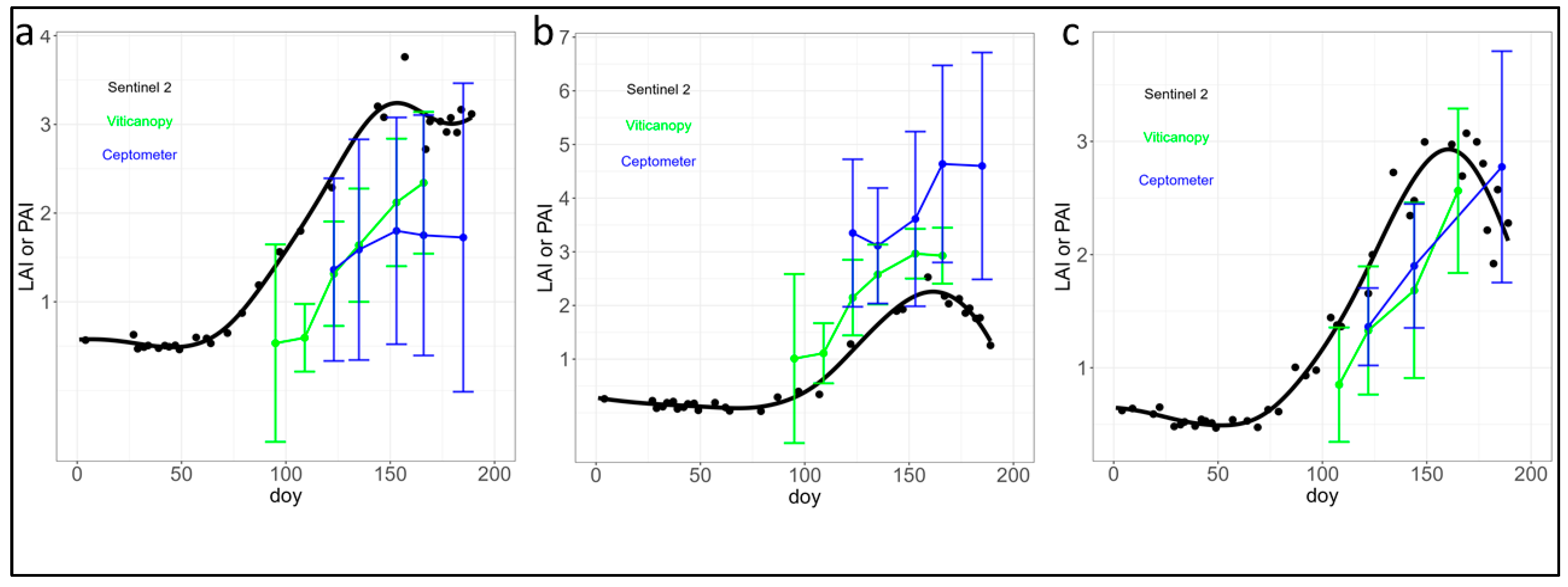
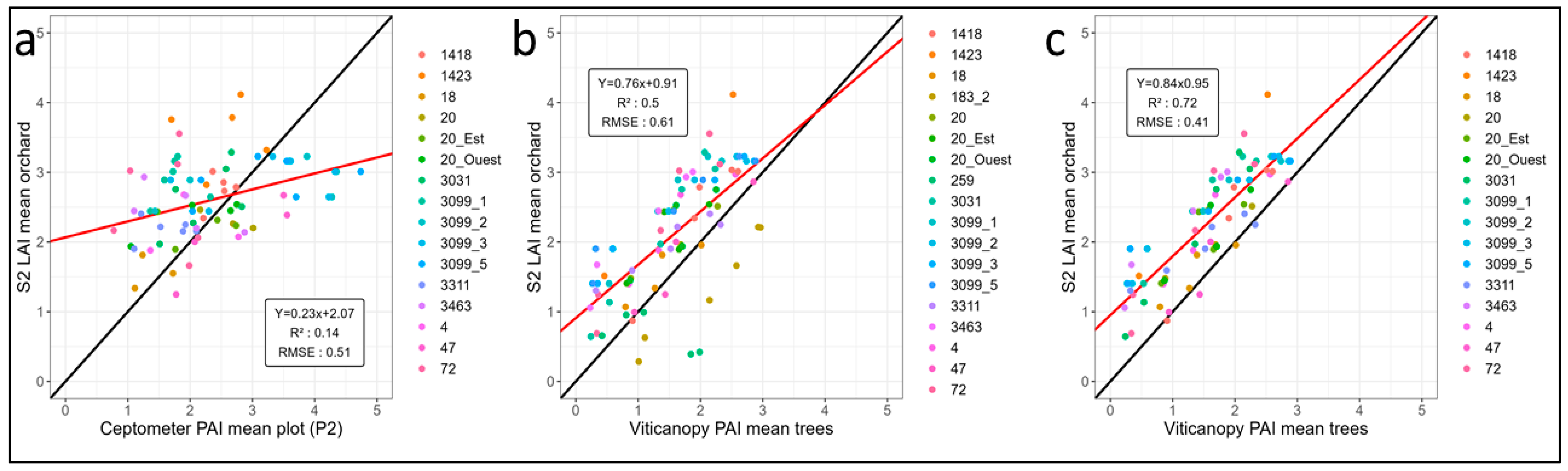
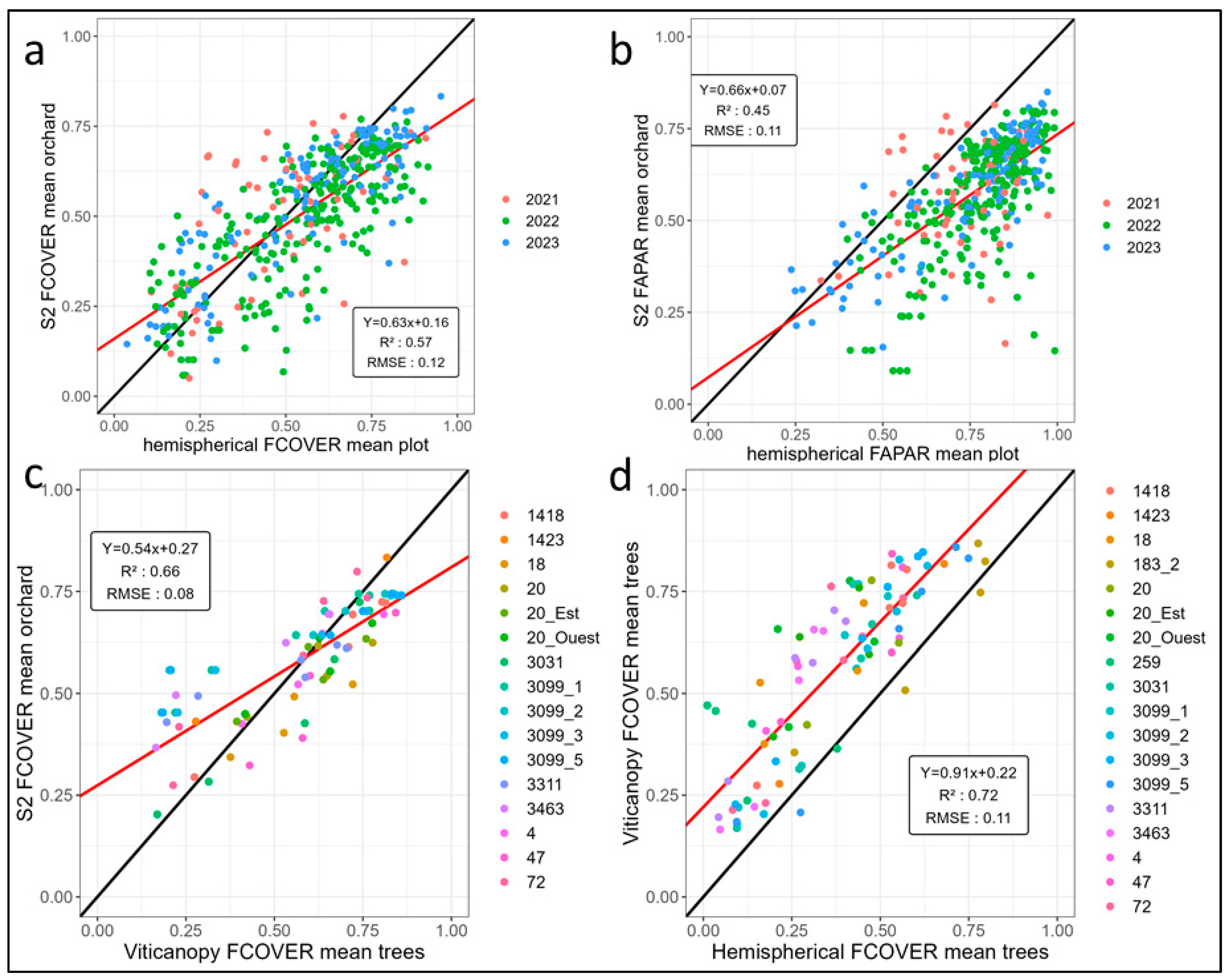
3.1. Analysis of Ground Measurements Compared to Estimations from Sentinel 2
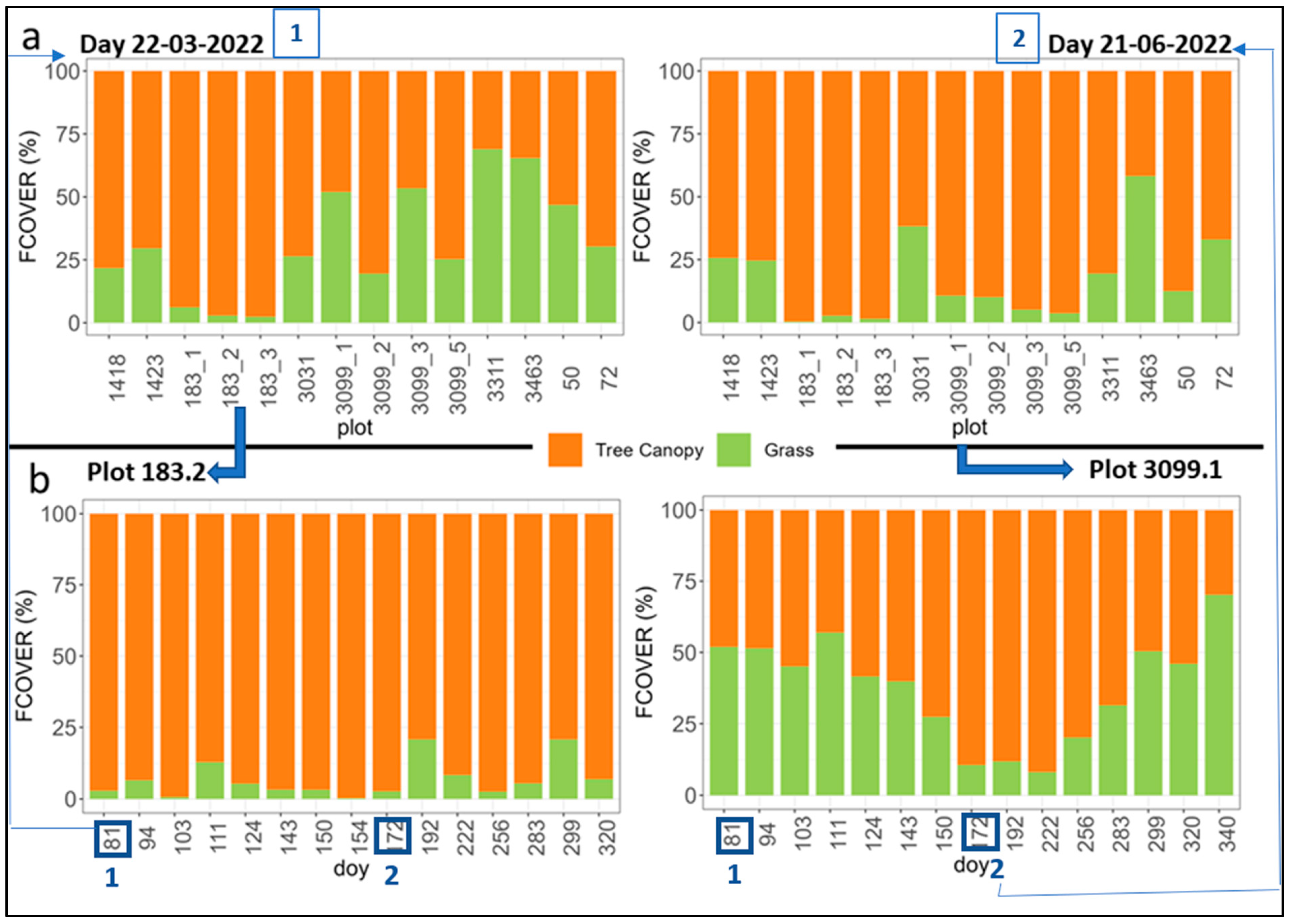
3.2. Analysis of Inter-Row Impact on the Biophysical Variables Assessed from Hemispherical Photographs
3.3. Validation of the Aggregative Model
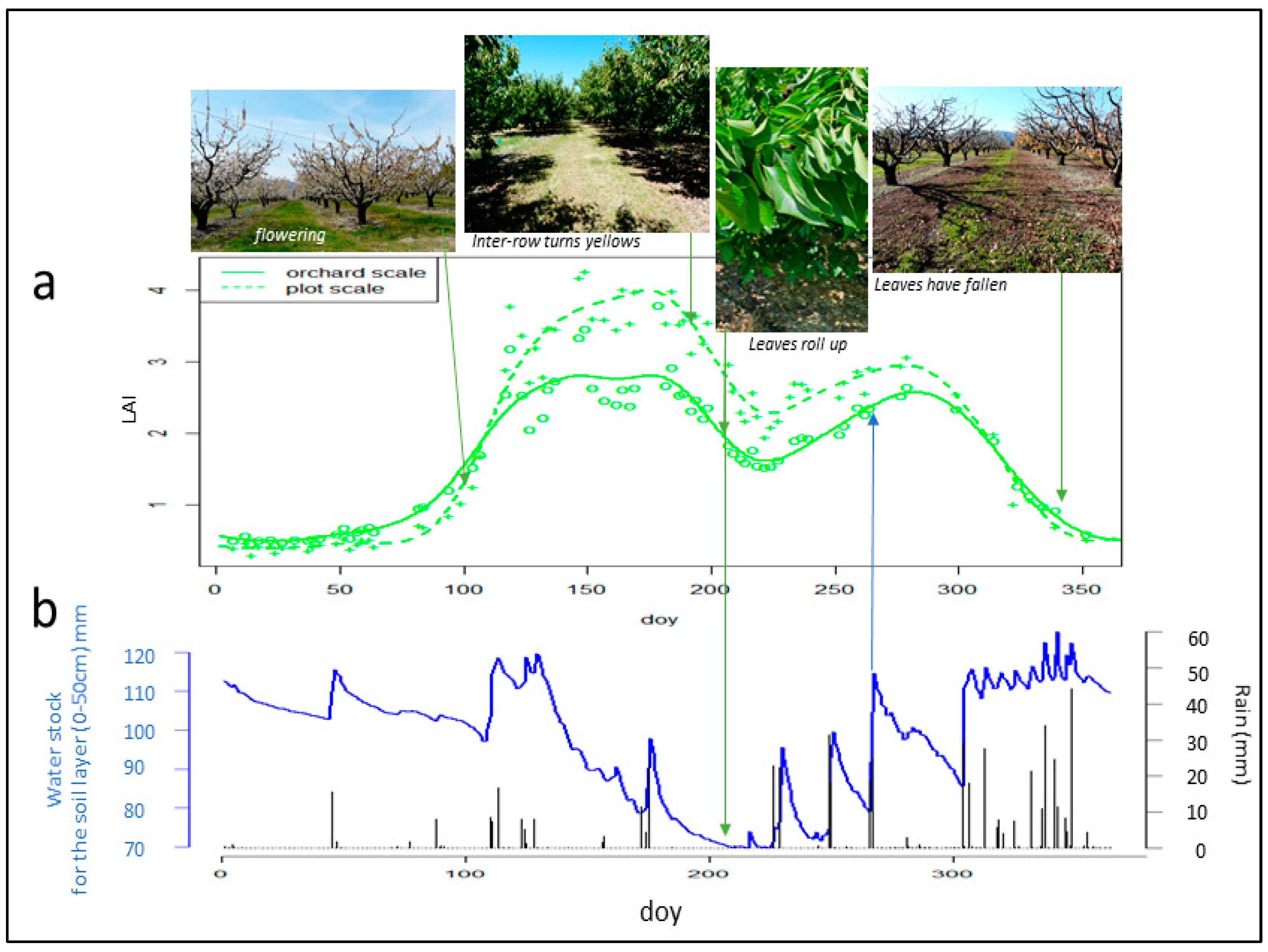
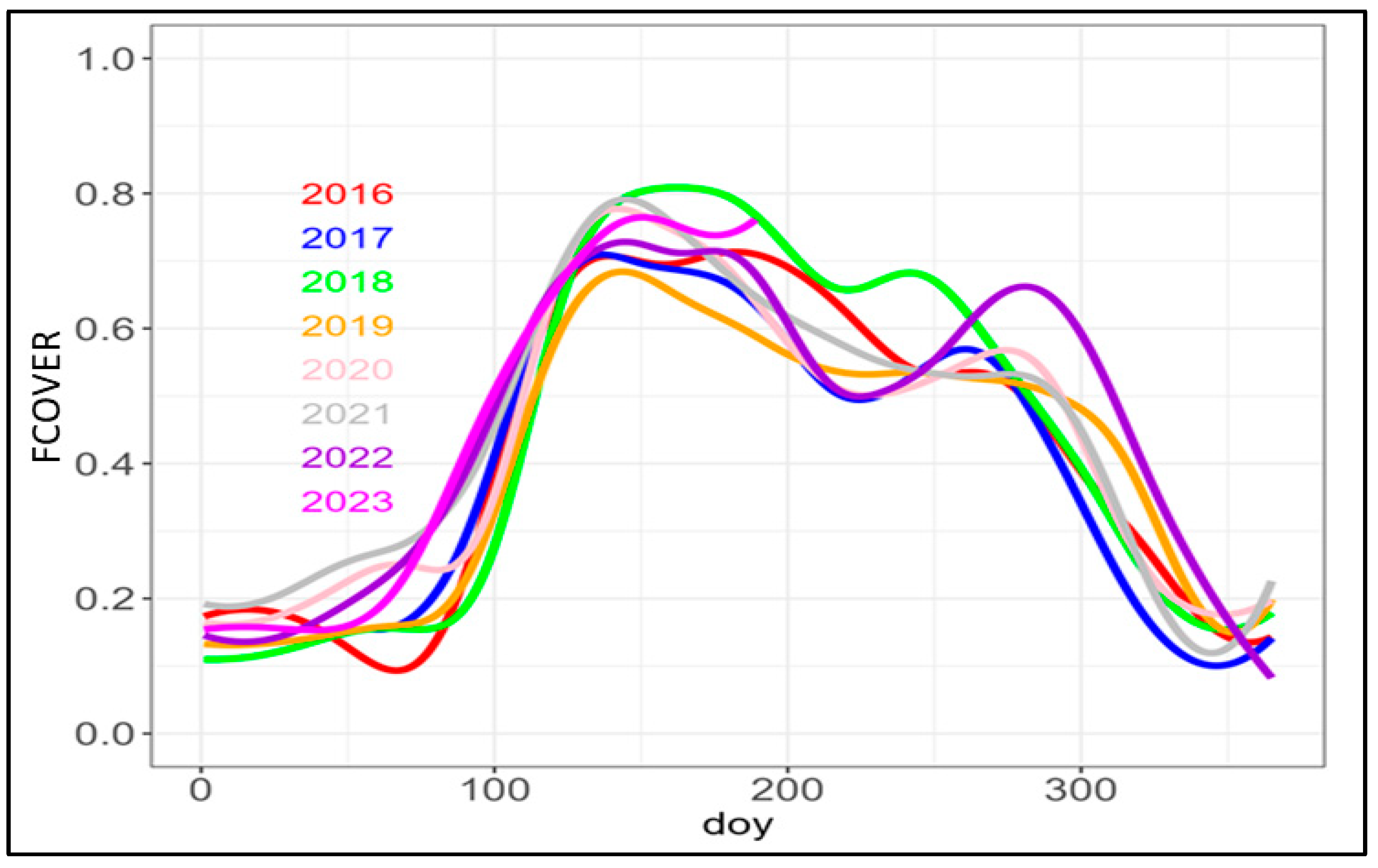
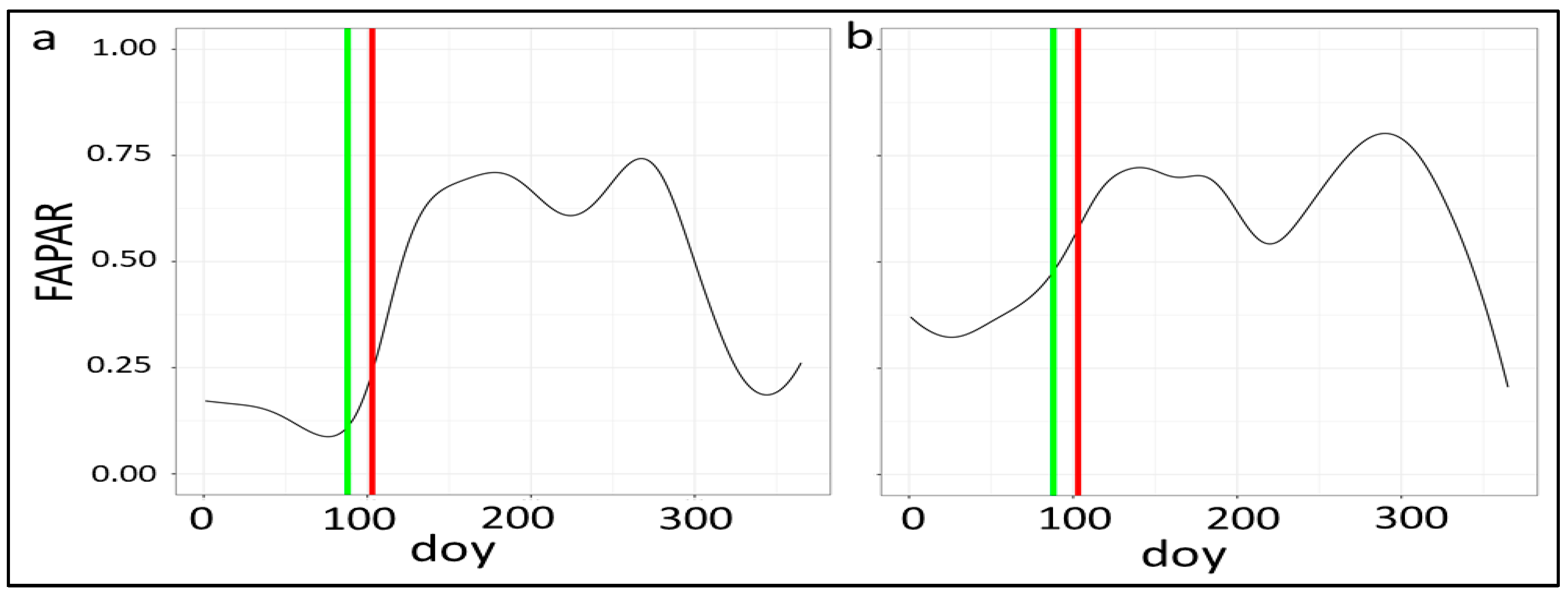
3.4. Analysis of Leaf Development and Identification of Key Phenological Stages from Sentinel 2 Biophysical Variables
4. Discussion
4.1. Ground Measurement Protocol to Estimate Biophysical Variables and Comparison with Sentinel 2-Based Estimations
4.2. Sentinel 2 Potential for Orchard Monitoring and the Detection of Key Phenological Stages
4.3. Operational Applicability of Methods, Limitations, and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cherif, S.; Doblas-Miranda, E.; Lionello, P.; Borrego, C.; Giorgi, F.; Rilov, G.; Iglesias, A.; Jebari, S.; Mahmoudi, E.; Moriondo, M.; et al. Drivers of Change. In Climate and Environmental Change in the Mediterranean Basin–Current Situation and Risks for the Future; First Mediterranean Assessment Report; Union for the Mediterranean, Plan Bleu, UNEP/MAP: Marseille, France, 2020; pp. 59–180. [Google Scholar] [CrossRef]
- MedECC. Climate and Environmental Change in the Mediterranean Basin–Current Situation and Risks for the Future; First Mediterranean Assessment Report; Union for the Mediterranean, Plan Bleu, UNEP/MAP: Marseille, France, 2020; ISBN 978-2-9577416-0-1. [Google Scholar] [CrossRef]
- Nagai, S.; Saitoh, T.M.; Yoshitake, S. Cultural Ecosystem Services Provided by Flowering of Cherry Trees under Climate Change: A Case Study of the Relationship between the Periods of Flowering and Festivals. Int. J. Biometeorol. 2019, 63, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Benmoussa, H.; Ben Mimoun, M.; Ghrab, M.; Luedeling, E. Climate Change Threatens Central Tunisian Nut Orchards. Int. J. Biometeorol. 2018, 62, 2245–2255. [Google Scholar] [CrossRef] [PubMed]
- Campoy, J.A.; Ruiz, D.; Egea, J. Dormancy in Temperate Fruit Trees in a Global Warming Context: A Review. Sci. Hortic. 2011, 130, 357–372. [Google Scholar] [CrossRef]
- El Yaacoubi, A.; Malagi, G.; Oukabli, A.; Hafidi, M.; Legave, J.-M. Global Warming Impact on Floral Phenology of Fruit Trees Species in Mediterranean Region. Sci. Hortic. 2014, 180, 243–253. [Google Scholar] [CrossRef]
- Primack, R.B.; Higuchi, H.; Miller-Rushing, A.J. The Impact of Climate Change on Cherry Trees and Other Species in Japan. Biol. Conserv. 2009, 142, 1943–1949. [Google Scholar] [CrossRef]
- Mihailescu, E.; Bruno Soares, M. The Influence of Climate on Agricultural Decisions for Three European Crops: A Systematic Review. Front. Sustain. Food Syst. 2020, 4, 64. [Google Scholar] [CrossRef]
- Fernández, J.E. Plant-Based Sensing to Monitor Water Stress: Applicability to Commercial Orchards. Agric. Water Manag. 2014, 142, 99–109. [Google Scholar] [CrossRef]
- Bujdosó, G.; Hrotko, K. Cherry Production. In Cherries: Botany, Production and Uses; CABI: Wallingford, UK, 2017; pp. 1–13. ISBN 978-1-78064-837-8. [Google Scholar]
- Blanco, V.; Blaya-Ros, P.J.; Castillo, C.; Soto-Vallés, F.; Torres-Sánchez, R.; Domingo, R. Potential of UAS-Based Remote Sensing for Estimating Tree Water Status and Yield in Sweet Cherry Trees. Remote Sens. 2020, 12, 2359. [Google Scholar] [CrossRef]
- Fadón, E.; Herrero, M.; Rodrigo, J. Flower Development in Sweet Cherry Framed in the BBCH Scale. Sci. Hortic. 2015, 192, 141–147. [Google Scholar] [CrossRef]
- Gobin, A.; Sallah, A.-H.M.; Curnel, Y.; Delvoye, C.; Weiss, M.; Wellens, J.; Piccard, I.; Planchon, V.; Tychon, B.; Goffart, J.-P.; et al. Crop Phenology Modelling Using Proximal and Satellite Sensor Data. Remote Sens. 2023, 15, 2090. [Google Scholar] [CrossRef]
- Guimarães, N.; Sousa, J.J.; Pádua, L.; Bento, A.; Couto, P. Remote Sensing Applications in Almond Orchards: A Comprehensive Systematic Review of Current Insights, Research Gaps, and Future Prospects. Appl. Sci. 2024, 14, 1749. [Google Scholar] [CrossRef]
- Shin, N.; Saitoh, T.M.; Takeuchi, Y.; Miura, T.; Aiba, M.; Kurokawa, H.; Onoda, Y.; Ichii, K.; Nasahara, K.N.; Suzuki, R.; et al. Review: Monitoring of Land Cover Changes and Plant Phenology by Remote-Sensing in East Asia. Ecol. Res. 2023, 38, 111–133. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, M.; Zeng, H.; Tian, F.; Potgieter, A.B.; Qin, X.; Yan, N.; Chang, S.; Zhao, Y.; Dong, Q.; et al. Challenges and Opportunities in Remote Sensing-Based Crop Monitoring: A Review. Natl. Sci. Rev. 2023, 10, nwac290. [Google Scholar] [CrossRef]
- Courault, D.; Hossard, L.; Demarez, V.; Dechatre, H.; Irfan, K.; Baghdadi, N.; Flamain, F.; Ruget, F. STICS Crop Model and Sentinel-2 Images for Monitoring Rice Growth and Yield in the Camargue Region. Agron. Sustain. Dev. 2021, 41, 49. [Google Scholar] [CrossRef]
- Rivas, H.; Delbart, N.; Ottlé, C.; Maignan, F.; Vaudour, E. Disaggregated PROBA-V Data Allows Monitoring Individual Crop Phenology at a Higher Observation Frequency than Sentinel-2. Int. J. Appl. Earth Obs. Geoinf. 2021, 104, 102569. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, Y.; Jia, S.; Shao, H.; Liu, M.; Tao, S.; Dai, X. Impacts of Mining on Vegetation Phenology and Sensitivity Assessment of Spectral Vegetation Indices to Mining Activities in Arid/Semi-Arid Areas. J. Environ. Manag. 2024, 356, 120678. [Google Scholar] [CrossRef]
- Boschetti, M.; Stroppiana, D.; Brivio, P.A.; Bocchi, S. Multi-Year Monitoring of Rice Crop Phenology through Time Series Analysis of MODIS Images. Int. J. Remote Sens. 2009, 30, 4643–4662. [Google Scholar] [CrossRef]
- Duchemin, B.; Hadria, R.; Er-Raki, S.; Boulet, G.; Maisongrande, P.; Chehbouni, A.; Escadafal, R.; Ezzahar, J.; Hoedjes, J.; Kharrou, H.; et al. Monitoring Wheat Phenology and Irrigation in Central Morocco: On the Use of Relationships between Evapotranspiration, Crops Coefficients, Leaf Area Index and Remotely-Sensed Vegetation Indices. Agric. Water Manag. 2006, 97, 1–27. [Google Scholar] [CrossRef]
- Nasrallah, A.; Baghdadi, N.; El Hajj, M.; Darwish, T.; Belhouchette, H.; Faour, G.; Darwich, S.; Mhawej, M. Sentinel-1 Data for Winter Wheat Phenology Monitoring and Mapping. Remote Sens. 2019, 11, 2228. [Google Scholar] [CrossRef]
- Kowalski, K.; Senf, C.; Hostert, P.; Pflugmacher, D. Characterizing Spring Phenology of Temperate Broadleaf Forests Using Landsat and Sentinel-2 Time Series. Int. J. Appl. Earth Obs. Geoinf. 2020, 92, 102172. [Google Scholar] [CrossRef]
- Quesada-Ruiz, L.C.; Caparros-Santiago, J.A.; Garcia-Perez, M.A.; Rodriguez-Galiano, V. Characterising the Spring and Autumn Land Surface Phenology of Macaronesian Species Using Sentinel-2 Data: The Case of Canary Island. In Proceedings of the Remote Sensing for Agriculture, Ecosystems, and Hydrology XXIII, Online, 13–17 September 2021; Neale, C.M., Maltese, A., Eds.; SPIE: Bellingham, WA, USA, 2021; p. 7. [Google Scholar]
- Grabska-Szwagrzyk, E.; Tymińska-Czabańska, L. Sentinel-2 Time Series: A Promising Tool in Monitoring Temperate Species Spring Phenology. For. Int. J. For. Res. 2024, 97, 267–281. [Google Scholar] [CrossRef]
- Misra, G.; Cawkwell, F.; Wingler, A. Status of Phenological Research Using Sentinel-2 Data: A Review. Remote Sens. 2020, 12, 2760. [Google Scholar] [CrossRef]
- Sawant, S.A.; Chakraborty, M.; Suradhaniwar, S.; Adinarayana, J.; Durbha, S.S. Time Series Analysis of Remote Sensing Observations for Citrus Crop Growth Stage and Evapotranspiration Estimation. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2016, XLI-B8, 1037–1042. [Google Scholar] [CrossRef]
- Abubakar, M.A.; Chanzy, A.; Flamain, F.; Courault, D. Characterisation of Grapevine Canopy Leaf Area and Inter-Row Management Using Sentinel-2 Time Series. OENO One 2023, 57. [Google Scholar] [CrossRef]
- Rao, P.; Zhou, W.; Bhattarai, N.; Srivastava, A.K.; Singh, B.; Poonia, S.; Lobell, D.B.; Jain, M. Using Sentinel-1, Sentinel-2, and Planet Imagery to Map Crop Type of Smallholder Farms. Remote Sens. 2021, 13, 1870. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Hornero, A.; Beck, P.S.A.; Kattenborn, T.; Kempeneers, P.; Hernández-Clemente, R. Chlorophyll Content Estimation in an Open-Canopy Conifer Forest with Sentinel-2A and Hyperspectral Imagery in the Context of Forest Decline. Remote Sens. Environ. 2019, 223, 320–335. [Google Scholar] [CrossRef]
- Fang, H.; Baret, F.; Plummer, S.; Schaepman-Strub, G. An Overview of Global Leaf Area Index (LAI): Methods, Products, Validation, and Applications. Rev. Geophys. 2019, 57, 739–799. [Google Scholar] [CrossRef]
- Monteith, J.L. Principles of Environmental Physics. Agric. Meteorol. 1973, 13, 429–430. [Google Scholar] [CrossRef]
- Weiss, M.; Jacob, F.; Duveiller, G. Remote Sensing for Agricultural Applications: A Meta-Review. Remote Sens. Environ. 2020, 236, 111402. [Google Scholar] [CrossRef]
- Weiss, M.; Baret, F.; Jay, S. S2ToolBox Level 2 Products LAI, FAPAR, FCOVER 2.0. 2020. Available online: https://step.esa.int/docs/extra/ATBD_S2ToolBox_V2.1.pdf (accessed on 2 August 2024).
- López-Lozano, R.; Baret, F.; García de Cortázar-Atauri, I.; Bertrand, N.; Casterad, M.A. Optimal Geometric Configuration and Algorithms for LAI Indirect Estimates under Row Canopies: The Case of Vineyards. Agric. For. Meteorol. 2009, 149, 1307–1316. [Google Scholar] [CrossRef]
- Gower, S.T.; Kucharik, C.J.; Norman, J.M. Direct and Indirect Estimation of Leaf Area Index, fAPAR, and Net Primary Production of Terrestrial Ecosystems. Remote Sens. Environ. 1999, 70, 29–51. [Google Scholar] [CrossRef]
- Prince, S.D. Satellite Remote Sensing of Primary Production: Comparison of Results for Sahelian Grasslands 1981–1988. Int. J. Remote Sens. 1991, 12, 1301–1311. [Google Scholar] [CrossRef]
- Shobairi, S.R.; Roudbari, S.H.; Ayombekov, Q.; Sadeghi, H.; Beirami, B.; Pirbasti, M. Tracking the Impact of Climate Factors on Vegetation Dynamics across the Alashan Plateau Semi Desert Ecoregion. Comput. Ecol. Softw. 2024, 14, 77. [Google Scholar]
- Belda, S.; Pipia, L.; Verrelst, J. Trends in Satellite Time Series Processing for Vegetation Phenology Monitoring. In Multitemporal Earth Observation Image Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2024; pp. 151–183. ISBN 978-1-394-30665-7. [Google Scholar]
- Han, D.; Cai, H.; Zhang, L.; Wen, Y. Multi-Sensor High Spatial Resolution Leaf Area Index Estimation by Combining Surface Reflectance with Vegetation Indices for Highly Heterogeneous Regions: A Case Study of the Chishui River Basin in Southwest China. Ecol. Inform. 2024, 80, 102489. [Google Scholar] [CrossRef]
- Camacho, F.; Martínez-Sánchez, E.; Brown, L.A.; Morris, H.; Morrone, R.; Williams, O.; Dash, J.; Origo, N.; Sánchez-Zapero, J.; Boccia, V. Validation and Conformity Testing of Sentinel-3 Green Instantaneous FAPAR and Canopy Chlorophyll Content Products. Remote Sens. 2024, 16, 2698. [Google Scholar] [CrossRef]
- López-Lozano, R.; Duveiller, G.; Seguini, L.; Meroni, M.; García-Condado, S.; Hooker, J.; Leo, O.; Baruth, B. Towards Regional Grain Yield Forecasting with 1km-Resolution EO Biophysical Products: Strengths and Limitations at Pan-European Level. Agric. For. Meteorol. 2015, 206, 12–32. [Google Scholar] [CrossRef]
- Ines, A.V.M.; Das, N.N.; Hansen, J.W.; Njoku, E.G. Assimilation of Remotely Sensed Soil Moisture and Vegetation with a Crop Simulation Model for Maize Yield Prediction. Remote Sens. Environ. 2013, 138, 149–164. [Google Scholar] [CrossRef]
- Li, L.; Mu, X.; Jiang, H.; Chianucci, F.; Hu, R.; Song, W.; Qi, J.; Liu, S.; Zhou, J.; Chen, L.; et al. Review of Ground and Aerial Methods for Vegetation Cover Fraction (fCover) and Related Quantities Estimation: Definitions, Advances, Challenges, and Future Perspectives. ISPRS J. Photogramm. Remote Sens. 2023, 199, 133–156. [Google Scholar] [CrossRef]
- Valdés-Gómez, H.; Celette, F.; de Cortázar-Atauri, I.G.; Jara-Rojas, F.; Ortega-Farías, S.; Gary, C. Modelling Soil Water Content and Grapevine Growth and Development with the Stics Crop-Soil Model under Two Different Water Management Strategies. OENO One 2009, 43, 13–28. [Google Scholar] [CrossRef]
- Weiss, M.; Baret, F.; Leroy, M.; Hautecoeur, O.; Bacour, C.; Prévot, L.; Bruguier, N. Validation d’une Méthode Basée Sur l’utilisation de Réseaux de Neurones Pour l’estimation de Variables Biophysiques Des Couverts Végétaux à Partir de Données de Télédétection. Agronomie 2002, 22, 547–553. [Google Scholar] [CrossRef]
- Kalaitzidis, C.; Heinzel, V.; Zianis, D. A Review of Multispectral Vegetation Indices for Biomass Estimation. In Imagin[e,g] Europe; IOS Press: Amsterdam, The Netherlands, 2010; pp. 201–208. [Google Scholar] [CrossRef]
- Odi-Lara, M.; Campos, I.; Neale, C.M.U.; Ortega-Farías, S.; Poblete-Echeverría, C.; Balbontín, C.; Calera, A. Estimating Evapotranspiration of an Apple Orchard Using a Remote Sensing-Based Soil Water Balance. Remote Sens. 2016, 8, 253. [Google Scholar] [CrossRef]
- Pôças, I.; Paço, T.A.; Paredes, P.; Cunha, M.; Pereira, L.S. Estimation of Actual Crop Coefficients Using Remotely Sensed Vegetation Indices and Soil Water Balance Modelled Data. Remote Sens. 2015, 7, 2373–2400. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, Z.; Yu, R.; Tian, Q.; Zhu, X. Extraction of Information about Individual Trees from High-Spatial-Resolution UAV-Acquired Images of an Orchard. Remote Sens. 2020, 12, 133. [Google Scholar] [CrossRef]
- Özdarici-OK, A.; Ok, A. Using Remote Sensing to Identify Individual Tree Species in Orchards: A Review. Sci. Hortic. 2023, 321, 112333. [Google Scholar] [CrossRef]
- Park, S.; Nolan, A.; Ryu, D.; Fuentes, S.; Hernandez, E.; Chung, H.; O’Connell, M. Estimation of Crop Water Stress in a Nectarine Orchard Using High-Resolution Imagery from Unmanned Aerial Vehicle (UAV). In Proceedings of the 21st International Congress on Modelling and Simulation, Gold Coast, QLD, Australia, 29 November–4 December 2015; Volume 29. [Google Scholar]
- Houborg, R.; McCabe, M. Application of a Regularized Model Inversion System (REGFLEC) to Multi-Temporal RapidEye Imagery for Retrieving Vegetation Characteristics. In Proceedings of the SPIE Remote Sensing Conference, Toulouse, France, 21–24 September 2015. [Google Scholar]
- Bréda, N.J.J. Ground-based Measurements of Leaf Area Index: A Review of Methods, Instruments and Current Controversies. J. Exp. Bot. 2003, 54, 2403–2417. [Google Scholar] [CrossRef]
- Jonckheere, I.; Fleck, S.; Nackaerts, K.; Muys, B.; Coppin, P.; Weiss, M.; Baret, F. Review of Methods for in Situ Leaf Area Index Determination: Part I. Theories, Sensors and Hemispherical Photography. Agric. For. Meteorol. 2004, 121, 19–35. [Google Scholar] [CrossRef]
- Weiss, M.; Baret, F.; Smith, G.J.; Jonckheere, I.; Coppin, P. Review of Methods for in Situ Leaf Area Index (LAI) Determination: Part II. Estimation of LAI, Errors and Sampling. Agric. For. Meteorol. 2004, 121, 37–53. [Google Scholar] [CrossRef]
- Demarez, V.; Duthoit, S.; Baret, F.; Weiss, M.; Dedieu, G. Estimation of Leaf Area and Clumping Indexes of Crops with Hemispherical Photographs. Agric. For. Meteorol. 2008, 148, 644–655. [Google Scholar] [CrossRef]
- Andrieu, B.; Allirand, J.M.; Jaggard, K. Ground Cover and Leaf Area Index of Maize and Sugar Beet Crops. Agronomie 1997, 17, 315–321. [Google Scholar] [CrossRef]
- Jackson, J.E. The Biology of Apples and Pears; Cambridge University Press: New York, NY, USA, 2003; ISBN 978-1-139-43705-9. [Google Scholar]
- Steiner, M.; Magyar, L.; Gueviki, M.; Hrotkó, K. Optimization of Light Interception in Intensive Sweet Cherry Orchard. Horticulture 2015, LIX. Available online: https://horticulturejournal.usamv.ro/pdf/2015/art17.pdf (accessed on 2 August 2024).
- Pokovai, K.; Fodor, N. Adjusting Ceptometer Data to Improve Leaf Area Index Measurements. Agronomy 2019, 9, 866. [Google Scholar] [CrossRef]
- Rich, P.M. Characterizing Plant Canopies with Hemispherical Photographs. Remote Sens. Rev. 1990, 5, 13–29. [Google Scholar] [CrossRef]
- Weiss, M.; Baret, F.; de Solan, B.; Demarez, V. CAN-EYE, logiciel de traitement d’images pour l’estimation de l’indice foliaire. Cah. Tech. L’inra 2008, 159. Available online: https://hal.science/hal-01496819/document (accessed on 2 August 2024).
- De Bei, R.; Fuentes, S.; Gilliham, M.; Tyerman, S.; Edwards, E.; Bianchini, N.; Smith, J.; Collins, C. VitiCanopy: A Free Computer App to Estimate Canopy Vigor and Porosity for Grapevine. Sensors 2016, 16, 585. [Google Scholar] [CrossRef] [PubMed]
- Makhloufi, A.; Kallel, A.; Chaker, R.; Gastellu-Etchegorry, J.-P. Retrieval of Olive Tree Biophysical Properties from Sentinel-2 Time Series Based on Physical Modelling and Machine Learning Technique. Int. J. Remote Sens. 2021, 42, 8542–8571. [Google Scholar] [CrossRef]
- Orlando, F.; Movedi, E.; Paleari, L.; Gilardelli, C.; Foi, M.; Dell’Oro, M.; Confalonieri, R. Estimating Leaf Area Index in Tree Species Using the PocketLAI Smart App. Appl. Veg. Sci. 2015, 18, 716–723. [Google Scholar] [CrossRef]
- Poblete-Echeverría, C.; Fuentes, S.; Ortega-Farias, S.; Gonzalez-Talice, J.; Yuri, J.A. Digital Cover Photography for Estimating Leaf Area Index (LAI) in Apple Trees Using a Variable Light Extinction Coefficient. Sensors 2015, 15, 2860–2872. [Google Scholar] [CrossRef]
- Majasalmi, T.; Rautiainen, M.; Stenberg, P.; Lukeš, P. An Assessment of Ground Reference Methods for Estimating LAI of Boreal Forests. For. Ecol. Manag. 2013, 292, 10–18. [Google Scholar] [CrossRef]
- Rouault, P.; Courault, D.; Flamain, F.; Pouget, G.; Doussan, C.; Lopez-Lozano, R.; McCabe, M.; Debolini, M. High-Resolution Satellite Imagery to Assess Orchard Characteristics Impacting Water Use. Agric. Water Manag. 2024, 295, 108763. [Google Scholar] [CrossRef]
- Paltineanu, C.; Chitu, E. Climate Change Impact on Phenological Stages of Sweet and Sour Cherry Trees in a Continental Climate Environment. Sci. Hortic. 2020, 261, 109011. [Google Scholar] [CrossRef]
- Vilà, M.; Sardans, J. Plant Competition in Mediterranean-type Vegetation. J. Veg. Sci. 1999, 10, 281–294. [Google Scholar] [CrossRef]
- Abubakar, M.A.; Chanzy, A.; Flamain, F.; Pouget, G.; Courault, D. Delineation of Orchard, Vineyard, and Olive Trees Based on Phenology Metrics Derived from Time Series of Sentinel-2. Remote Sens. 2023, 15, 2420. [Google Scholar] [CrossRef]
- Mohammed, G. Modélisation Biogéochimique du Système “Irrigation-Sol-Plante-Nappe”: Application à la Durabilité du Système de Culture du foin de Crau. Doctoral Thesis, Université d’Avignon, Avignon, France, 2017; p. 285. [Google Scholar]
- Trolard, F.; Bourrié, G.; Baillieux, A.; Buis, S.; Chanzy, A.; Clastre, P.; Closet, J.-F.; Courault, D.; Dangeard, M.-L.; Di Virgilio, N.; et al. The PRECOS Framework: Measuring the Impacts of the Global Changes on Soils, Water, Agriculture on Territories to Better Anticipate the Future. J. Environ. Manag. 2016, 181, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Olioso, A.; Lecerf, R.; Baillieux, A.; Chanzy, A.; Ruget, F.; Banton, O.; Lecharpentier, P.; Trolard, F.; Cognard-Plancq, A.-L. Modelling of Drainage and Hay Production over the Crau Aquifer for Analysing Impact of Global Change on Aquifer Recharge. Procedia Environ. Sci. 2013, 19, 691–700. [Google Scholar] [CrossRef]
- Djamai, N.; Fernandes, R.; Weiss, M.; McNairn, H.; Goïta, K. Validation of the Sentinel Simplified Level 2 Product Prototype Processor (SL2P) for Mapping Cropland Biophysical Variables Using Sentinel-2/MSI and Landsat-8/OLI Data. Remote Sens. Environ. 2019, 225, 416–430. [Google Scholar] [CrossRef]
- Weiss, M.; Baret, F. Evaluation of Canopy Biophysical Variable Retrieval Performances from the Accumulation of Large Swath Satellite Data. Remote Sens. Environ. 1999, 70, 293–306. [Google Scholar] [CrossRef]
- Claverie, M.; Vermote, E.F.; Weiss, M.; Baret, F.; Hagolle, O.; Demarez, V. Validation of Coarse Spatial Resolution LAI and FAPAR Time Series over Cropland in Southwest France. Remote Sens. Environ. 2013, 139, 216–230. [Google Scholar] [CrossRef]
- Delloye, C.; Weiss, M.; Defourny, P. Retrieval of the Canopy Chlorophyll Content from Sentinel-2 Spectral Bands to Estimate Nitrogen Uptake in Intensive Winter Wheat Cropping Systems. Remote Sens. Environ. 2018, 216, 245–261. [Google Scholar] [CrossRef]
- Zérah, Y.; Valero, S.; Inglada, J. Physics-constrained deep learning for biophysical parameter retrieval from Sentinel-2 images: Inversion of the PROSAIL model. Remote Sens. Environ. 2024, 312, 114309. [Google Scholar] [CrossRef]
- Eilers, P.H.C. A Perfect Smoother. Anal. Chem. 2003, 75, 3631–3636. [Google Scholar] [CrossRef]
- Pichon, L.; Taylor, J.; Tisseyre, B. Using Smartphone Leaf Area Index Data Acquired in a Collaborative Context within Vineyards in Southern France. OENO One 2020, 54, 123–130. [Google Scholar] [CrossRef]
- Xue, J.; Fan, Y.; Su, B.; Fuentes, S. Assessment of Canopy Vigor Information from Kiwifruit Plants Based on a Digital Surface Model from Unmanned Aerial Vehicle Imagery. Int. J. Agric. Biol. Eng. 2019, 12, 165–171. [Google Scholar] [CrossRef]
- Fuentes, S.; Chacon, G.; Torrico, D.D.; Zarate, A.; Gonzalez Viejo, C. Spatial Variability of Aroma Profiles of Cocoa Trees Obtained through Computer Vision and Machine Learning Modelling: A Cover Photography and High Spatial Remote Sensing Application. Sensors 2019, 19, 3054. [Google Scholar] [CrossRef] [PubMed]
- Tongson, E.J.; Fuentes, S.; Carrasco-Benavides, M.; Mora, M. Canopy Architecture Assessment of Cherry Trees by Cover Photography Based on Variable Light Extinction Coefficient Modelled Using Artificial Neural Networks. Acta Hortic. 2019, 1235, 183–188. [Google Scholar] [CrossRef]
- Ilniyaz, O.; Kurban, A.; Du, Q. Leaf Area Index Estimation of Pergola-Trained Vineyards in Arid Regions Based on UAV RGB and Multispectral Data Using Machine Learning Methods. Remote Sens. 2022, 14, 415. [Google Scholar] [CrossRef]
- Champion, G.T.; Froud-Williams, R.J.; Holland, J.M. Interactions between Wheat (Triticum aestivum L.) Cultivar, Row Spacing and Density and the Effect on Weed Suppression and Crop Yield. Ann. Appl. Biol. 1998, 133, 443–453. [Google Scholar] [CrossRef]
- Francone, C.; Pagani, V.; Foi, M.; Cappelli, G.; Confalonieri, R. Comparison of Leaf Area Index Estimates by Ceptometer and PocketLAI Smart App in Canopies with Different Structures. Field Crops Res. 2014, 155, 38–41. [Google Scholar] [CrossRef]
- Keane, R.E.; Reinhardt, E.D.; Scott, J.; Gray, K.; Reardon, J. Estimating Forest Canopy Bulk Density Using Six Indirect Methods. Can. J. For. Res. 2005, 35, 724–739. [Google Scholar] [CrossRef]
- Lopes, D.; Nunes, L.; Walford, N.; Aranha, J.; Sette, C.J.; Viana, H.; Hernandez, C. A Simplified Methodology for the Correction of Leaf Area Index (LAI) Measurements Obtained by Ceptometer with Reference to Pinus Portuguese Forests. iFor.-Biogeosci. For. 2014, 7, 186. [Google Scholar] [CrossRef]
- Wünsche, J.N.; Lakso, A.N.; Robinson, T.L. Comparison of Four Methods for Estimating Total Light Interception by Apple Trees of Varying Forms. HortScience 1995, 30, 272–276. [Google Scholar] [CrossRef]
- López-Lozano, R.; Casterad, M. Comparison of Different Protocols for Indirect Measurement of Leaf Area Index with Ceptometers in Vertically Trained Vineyards. Aust. J. Grape Wine Res. 2013, 19, 116–122. [Google Scholar] [CrossRef]
- Daymond, A.J.; Hadley, P.; Machado, R.C.R.; Ng, E. Canopy characteristics of contrasting clones of cacao (Theobroma cacao). Exp. Agric. 2002, 38, 359–367. [Google Scholar] [CrossRef]
- Louarn, G. Analyse et Modélisation de l’organogenèse et de l’architecture Du Rameau de Vigne (Vitis vinifera L.). Ph.D. Thesis, 2005. Available online: https://www.researchgate.net/publication/358146863_Analyse_et_modelisation_de_l%27organogenese_et_de_l%27architecture_du_rameau_de_vigne_Vitis_vinifera_L (accessed on 2 August 2024).
- Gates, D.M. Transpiration and Leaf Temperature. Annu. Rev. Plant. Physiol. 1968, 19, 211–238. [Google Scholar] [CrossRef]
- Jackson, J.E.; Palmer, J.W. Interception of Light by Model Hedgerow Orchards in Relation to Latitude, Time of Year and Hedgerow Configuration and Orientation. J. Appl. Ecol. 1972, 9, 341. [Google Scholar] [CrossRef]
- Wagenmakers, P.S.; Callesen, O. Light Distribution in Apple Orchard Systems in Relation to Production and Fruit Quality. J. Hortic. Sci. 1995, 70, 935–948. [Google Scholar] [CrossRef]
- Meier, U.; Graf, H.; Hack, H.; Heß, M.; Kennel, W.; Klose, R.; Mappes, D.; Seipp, D.; Stauß, R.; Steif, J.; et al. Phänologische Entwicklungsstadien Des Kernobstes (Malus Domestica Borkh. und Pyrus communis L.), Des Steinobstes (Prunus-Arten), Der Johannisbeere (Ribes-Arten) und Der Erdbeere (Fragaria × Ananassa Duch.). Heft 7 1994, 46, 141–153. [Google Scholar]
- Grasselly, D.; Perron, G.; Navarro, E. Western Flower Thrips in Peach Orchards in France. In Thrips Biology and Management; Parker, B.L., Skinner, M., Lewis, T., Eds.; NATO ASI Series; Springer: Boston, MA, USA, 1995; pp. 389–392. ISBN 978-1-4899-1409-5. [Google Scholar]
- Sawamura, Y.; Suesada, Y.; Sugiura, T.; Yaegaki, H. Chilling Requirements and Blooming Dates of Leading Peach Cultivars and a Promising Early Maturing Peach Selection, Momo Tsukuba 127. Hortic. J. 2017, 86, 426–436. [Google Scholar] [CrossRef]
- Oukabli, A.; Laghezali, M. Évaluation phénologique et pomologique d’une collection variétale de cerisiers en conditions de moyenne altitude au Maroc. Fruits 2000, 55, 83–92. [Google Scholar]
- Rejeb, H.; Albouchi, A. Déroulement d’un Cycle Végétatif de Jeunes Plants de Cerisiers (Hybrides Prunus cerasus × Prunus avium). Prémisses D’amélior. Vigueur 2011, 26, 167–184. [Google Scholar]
- Yan, G.; Hu, R.; Luo, J.; Weiss, M.; Jiang, H.; Mu, X.; Xie, D.; Zhang, W. Review of Indirect Optical Measurements of Leaf Area Index: Recent Advances, Challenges, and Perspectives. Agric. For. Meteorol. 2019, 265, 390–411. [Google Scholar] [CrossRef]
- Liu, C.; Kang, S.; Li, F.; Li, S.; Du, T. Canopy Leaf Area Index for Apple Tree Using Hemispherical Photography in Arid Region. Sci. Hortic. 2013, 164, 610–615. [Google Scholar] [CrossRef]
- Bsaibes, A.; Courault, D.; Baret, F.; Weiss, M.; Olioso, A.; Jacob, F.; Hagolle, O.; Marloie, O.; Bertrand, N.; Desfond, V.; et al. Albedo and LAI Estimates from FORMOSAT-2 Data for Crop Monitoring. Remote Sens. Environ. 2009, 113, 716–729. [Google Scholar] [CrossRef]
- Lai, Y.; Mu, X.; Li, W.; Zou, J.; Bian, Y.; Zhou, K.; Hu, R.; Li, L.; Xie, D.; Yan, G. Correcting for the Clumping Effect in Leaf Area Index Calculations Using One-Dimensional Fractal Dimension. Remote Sens. Environ. 2022, 281, 113259. [Google Scholar] [CrossRef]
- Dian, Y.; Liu, X.; Hu, L.; Zhang, J.-Z.; Hu, C.; Liu, Y.; Zhang, J.; Zhang, W.; Hu, Q.; Zhang, Y.; et al. Characteristics of Photosynthesis and Vertical Canopy Architecture of Citrus Trees under Two Labor-Saving Cultivation Modes Using UAV-Based LiDAR Data in Citrus Orchards. Hortic. Res. 2023, 10, uhad018. [Google Scholar] [CrossRef]
- Elfarkh, J.; Johansen, K.; El Hajj, M.M.; Almashharawi, S.K.; McCabe, M.F. Evapotranspiration, Gross Primary Productivity and Water Use Efficiency over a High-Density Olive Orchard Using Ground and Satellite Based Data. Agric. Water Manag. 2023, 287, 108423. [Google Scholar] [CrossRef]
- Padilla-Díaz, C.M.; Rodriguez-Dominguez, C.M.; Hernandez-Santana, V.; Perez-Martin, A.; Fernandes, R.D.M.; Montero, A.; García, J.M.; Fernández, J.E. Water Status, Gas Exchange and Crop Performance in a Super High Density Olive Orchard under Deficit Irrigation Scheduled from Leaf Turgor Measurements. Agric. Water Manag. 2018, 202, 241–252. [Google Scholar] [CrossRef]
- Carmona-García, J.M.; Arredondo-Bustillos, A.; Salas-Salazar, N.A.; Parra-Quezada, R.A.; Rodríguez-Roque, M.J.; Flores-Córdova, M.A.; Ojeda-Barrios, D.L.; Soto-Caballero, M.C. Monitoring Flowering Phenology of Apple Trees Using Remote Sensing Techniques. Nova Geod. 2024, 4, 196. [Google Scholar] [CrossRef]
- Tubau Comas, A.; Valente, J.; Kooistra, L. Automatic apple tree blossom estimation from uav rgb imagery. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2019, XLII-2-W13, 631–635. [Google Scholar] [CrossRef]
- Horton, R.; Cano, E.; Bulanon, D.; Fallahi, E. Peach Flower Monitoring Using Aerial Multispectral Imaging. J. Imaging 2017, 3, 2. [Google Scholar] [CrossRef]
- Chen, B.; Jin, Y.; Brown, P. An Enhanced Bloom Index for Quantifying Floral Phenology Using Multi-Scale Remote Sensing Observations. ISPRS J. Photogramm. Remote Sens. 2019, 156, 108–120. [Google Scholar] [CrossRef]
- Torgbor, B.A.; Rahman, M.M.; Robson, A.; Brinkhoff, J.; Khan, A. Assessing the Potential of Sentinel-2 Derived Vegetation Indices to Retrieve Phenological Stages of Mango in Ghana. Horticulturae 2022, 8, 11. [Google Scholar] [CrossRef]
- Veloso, A.; Mermoz, S.; Bouvet, A.; Le Toan, T.; Planells, M.; Dejoux, J.-F.; Ceschia, E. Understanding the Temporal Behavior of Crops Using Sentinel-1 and Sentinel-2-like Data for Agricultural Applications. Remote Sens. Environ. 2017, 199, 415–426. [Google Scholar] [CrossRef]
- Giuliani, R.; Flore, J.A. Potential Use of Infra-Red Thermometry for the Detection of Water Stress in Apple Trees. Acta Hortic. 2000, 537, 383–392. [Google Scholar] [CrossRef]
- Isbérie, C.; Regnard, J.L.; Colonges, T.; Virlet, N.; Roux, B.; Labbé, S.; Jolivot, A.; Marti, R.; Bégué, A.; Akakpo, K.; et al. Some Contributions of Remote Sensing for Orchard Irrigation Scheduling Resulting from the TELERIEG Research Program in the South-West of France. Acta Hortic. 2014, 1038, 255. [Google Scholar] [CrossRef]
- McCabe, M.; Miralles, D.; Holmes, T.; Fisher, J. Advances in the Remote Sensing of Terrestrial Evaporation. Available online: https://www.mdpi.com/2072-4292/11/9/1138 (accessed on 4 July 2023).
- Roujean, J.-L.; Bhattacharya, B.; Gamet, P.; Pandya, M.R.; Boulet, G.; Olioso, A.; Singh, S.K.; Shukla, M.V.; Mishra, M.; Briottet, X.; et al. TRISHNA: An Indo-French Space Mission to Study the Thermography of the Earth at Fine Spatio-Temporal Resolution. In Proceedings of the 2021 IEEE International India Geoscience and Remote Sensing Symposium (InGARSS), Ahmedabad, India, 6–10 December 2021. [Google Scholar]
- Brisson, N.; Bussière, F.; Ozier-Lafontaine, H.; Tournebize, R.; Sinoquet, H. Adaptation of the Crop Model STICS to Intercropping. Theoretical Basis and Parameterisation. Agronomie 2004, 24, 409–421. [Google Scholar] [CrossRef]
- Garcia de Cortazar Atauri, I. Adaptation Du Modèle STICS à La Vigne (Vitis vinifera L.): Utilisation Dans Le Cadre d’une Étude d’impact Du Changement Climatique à l’échelle de La France. Doctoral Thesis, École nationale supérieure agronomique (Montpellier), Montpellier, France, 2006. [Google Scholar]
- Demestihas, C.; Plénet, D.; Génard, M.; Garcia de Cortazar-Atauri, I.; Launay, M.; Ripoche, D.; Beaudoin, N.; Simon, S.; Charreyron, M.; Raynal, C.; et al. Analyzing Ecosystem Services in Apple Orchards Using the STICS Model. Eur. J. Agron. 2018, 94, 108–119. [Google Scholar] [CrossRef]
- Courault, D.; Hadria, R.; Ruget, F.; Olioso, A.; Duchemin, B.; Hagolle, O.; Dedieu, G. Combined Use of FORMOSAT-2 Images with a Crop Model for Biomass and Water Monitoring of Permanent Grassland in Mediterranean Region. Hydrol. Earth Syst. Sci. 2010, 14, 1731–1744. [Google Scholar] [CrossRef]
- Sun, G.; Chen, S.; Zhang, S.; Chen, S.; Liu, J.; He, Q.; Hu, T.; Zhang, F. Responses of Leaf Nitrogen Status and Leaf Area Index to Water and Nitrogen Application and Their Relationship with Apple Orchard Productivity. Agric. Water Manag. 2024, 296, 108810. [Google Scholar] [CrossRef]
- Garcia, L.; Celette, F.; Gary, C.; Ripoche, A.; Valdés-Gómez, H.; Metay, A. Management of Service Crops for the Provision of Ecosystem Services in Vineyards: A Review. Agric. Ecosyst. Environ. 2018, 251, 158–170. [Google Scholar] [CrossRef]
- Yang, L.; Mao, Y.-F.; Hu, Y.-L.; Wang, Y.-Y.; Zhang, L.-L.; Yin, Y.-J.; Pang, H.-L.; Su, X.-F.; Liu, Y.-P.; Shen, X. Effects of orchard grass on soil fertility and apple tree nutrition. J. Plant Nutr. Fertil. 2020, 26, 325–337. [Google Scholar] [CrossRef]
- Heikal, N.H.; Rady, M.H.; Merdan, B.A.; El-Abbassi, T.S.; El-Genaidy, M.A.; Azazy, A.M.; Yones, M.S.; Essa, E.E. Early Detection of Bactrocera Zonata Infestation in Peach Fruit Using Remote Sensing Technique and Application of Nematodes for Its Control. Kuwait J. Sci. 2024, 51, 100191. [Google Scholar] [CrossRef]





| Id | Crop | Cultivar | Monitoring Years | Number of Plots | Area (ha) | Planting Pattern (m) (Inter-Tree × Inter-Row) | Irrigation Type | Grassy |
|---|---|---|---|---|---|---|---|---|
| 50 | Cherry | 1 Summit + 1 Belge + 1 Burlat | 2021 to 2022 | 1 | 0.72 | 6 × 7 | Drip | Yes |
| 56 | Cherry | 2021 | 1 | 0.84 | 8 × 7 | Drip | Yes | |
| 72 | Cherry | 2 Belge + 1 Summit | 2021 to 2023 | 1 | 0.89 | 7 × 7 | Micro-sprinkler | Yes |
| 183.1 183.2 183.3 | Cherry | 2 Summit + 1 Sweetheart 2 Belge + 1 Summit 2 Belge+ 1 Summit | 2021 to 2023 | 1 (2021) 3 (2022) 1 (2023) | 1.09 | 5 × 5 | Drip | No |
| 259 | Apricot | 2023 | 1 | 0.48 | 4 × 4 | Non-irrigated | No | |
| 1378 | Cherry | 2021 | 1 | 0.54 | 7 × 7 | Non-irrigated | Yes | |
| 1409 | Cherry | 2 Belge + 1 Noir de Meched | 2021 | 1 | 0.42 | 6 × 7 | Drip | Yes |
| 1418 | Cherry | 2 Folfer + 1 Earlise | 2021 to 2023 | 1 | 0.22 | 6 × 7 | Micro-sprinkler | Yes |
| 1423 | Cherry | 3 Belge | 2021 to 2023 | 1 | 0.62 | 6 × 7 | Micro-sprinkler | Yes |
| 3031 | Cherry | 3 Summer Charm | 2021 to 2023 | 1 | 0.43 | 6 × 7 | Micro-sprinkler | Yes |
| 3099.1 3099.2 3099.3 3099.5 | Cherry | 3 Prime Giant 2 Belge + 1 Summit 2 Belge + 1 Folfer 2 Belge + Summit | 2021 to 2023 | 1 (2021) 4 (2022/2023) | 4.57 | 5.5 × 7 | Drip | Yes |
| 3150 | Cherry | 2 Belge + 1 Summit | 2021 | 1 | 3.09 | 5 × 5 | Drip | Yes |
| 3311 | Cherry | Belge + Summit | 2021 to 2023 | 1 | 0.74 | 7 × 7 | Drip | Yes |
| 3463 | Cherry | 2 Van + Burlat | 2021 to 2023 | 1 | 0.33 | 6 × 7 | Micro-sprinkler | Yes |
| Id | Crop | Cultivar | Precocity | Water Restrictions | Monitoring Years | Planting Pattern (m) (Inter-Tree × Inter-Row) |
|---|---|---|---|---|---|---|
| 4 | Nectarine | Big Fire | Early | No | 2022 to 2023 | 5.5 × 3.5 |
| 18 | Apricot | (Under numbers) | Late | No | 2022 to 2023 | 5.5 × 3.5 |
| 20 | Nectarine | Queen Glory | Season | No | 2022 to 2023 | 5.5 × 3.5 |
| 20a | Nectarine | Queen Glory | Season | Yes (−30%) | 2023 | 5.5 × 3.5 |
| 20b | Nectarine | Queen Glory | Season | No | 2023 | 5.5 × 3.5 |
| 47 | Nectarine | Nectasweet | Early | No | 2022 to 2023 | 5.5 × 3.5 |
| Plot | BBCH 67 2021 | BBCH 67 2022 | BBCH 67 2023 | Plot | BBCH 69 2021 | BBCH 69 2022 | BBCH 69 2023 |
|---|---|---|---|---|---|---|---|
| 50 | 109 (19/04) | 111 (21/04) | 50 | 125 (05/05) | 124 (04/05) | ||
| 72 | 111 (21/04) | 109 (19/04) | 72 | 124 (04/05) | 123 (03/05) | ||
| 183_1 | 103 (13/04) | 183_1 | 124 (04/05) | ||||
| 183_2 | 103 (13/04) | 104 (14/04) | 183_2 | 124 (04/05) | 123 (03/05) | ||
| 183_3 | 103 (13/04) | 183_3 | 124 (04/05) | ||||
| 1401 | 109 (19/04) | 111 (21/04) | 109 (19/04) | 1401 | 125 (05/05) | 124 (04/05) | 123 (03/05) |
| 1418 | 99 (09/04) | 94 (04/04) | 104 (14/04) | 1418 | 125 (05/05) | 111 (21/04) | 109 (19/04) |
| 1423 | 111 (21/04) | 109 (19/04) | 1423 | 124 (04/05) | 123 (03/05) | ||
| 1424 | 99 (09/04) | 1424 | 125 (05/05) | ||||
| 3031 | 103 (13/04) | 104 (14/04) | 3031 | 124 (04/05) | 123 (03/05) | ||
| 3099_1 | 99 (09/04) | 103 (13/04) | 104 (14/04) | 3099_1 | 125 (05/05) | 124 (04/05) | 123 (03/05) |
| 3099_2 | 111 (21/04) | 111 (21/04) | 3099_2 | 124 (04/05) | |||
| 3099_3 | 103 (13/04) | 111 (21/04) | 3099_3 | 124 (04/05) | 123 (03/05) | ||
| 3099_5 | 111 (21/04) | 111 (21/04) | 3099_5 | 124 (04/05) | 123 (03/05) | ||
| 3311 | 111 (21/04) | 109 (19/04) | 3311 | 124 (04/05) | 123 (03/05) | ||
| 3347 | 92 (02/04) | 103 (13/04) | 95 (05/04) | 3347 | 125 (05/05) | 111 (21/04) | 109 (19/04) |
| 3463 | 111 (21/04) | 109 (19/04) | 3463 | 124 (04/05) | 123 (03/05) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouault, P.; Courault, D.; Pouget, G.; Flamain, F.; Diop, P.-K.; Desfonds, V.; Doussan, C.; Chanzy, A.; Debolini, M.; McCabe, M.; et al. Phenological and Biophysical Mediterranean Orchard Assessment Using Ground-Based Methods and Sentinel 2 Data. Remote Sens. 2024, 16, 3393. https://doi.org/10.3390/rs16183393
Rouault P, Courault D, Pouget G, Flamain F, Diop P-K, Desfonds V, Doussan C, Chanzy A, Debolini M, McCabe M, et al. Phenological and Biophysical Mediterranean Orchard Assessment Using Ground-Based Methods and Sentinel 2 Data. Remote Sensing. 2024; 16(18):3393. https://doi.org/10.3390/rs16183393
Chicago/Turabian StyleRouault, Pierre, Dominique Courault, Guillaume Pouget, Fabrice Flamain, Papa-Khaly Diop, Véronique Desfonds, Claude Doussan, André Chanzy, Marta Debolini, Matthew McCabe, and et al. 2024. "Phenological and Biophysical Mediterranean Orchard Assessment Using Ground-Based Methods and Sentinel 2 Data" Remote Sensing 16, no. 18: 3393. https://doi.org/10.3390/rs16183393
APA StyleRouault, P., Courault, D., Pouget, G., Flamain, F., Diop, P.-K., Desfonds, V., Doussan, C., Chanzy, A., Debolini, M., McCabe, M., & Lopez-Lozano, R. (2024). Phenological and Biophysical Mediterranean Orchard Assessment Using Ground-Based Methods and Sentinel 2 Data. Remote Sensing, 16(18), 3393. https://doi.org/10.3390/rs16183393







