Climate Sensitivity and Drought Legacy of Tree Growth in Plantation Forests in Northeast China Are Species- and Age-Dependent
Abstract
1. Introduction
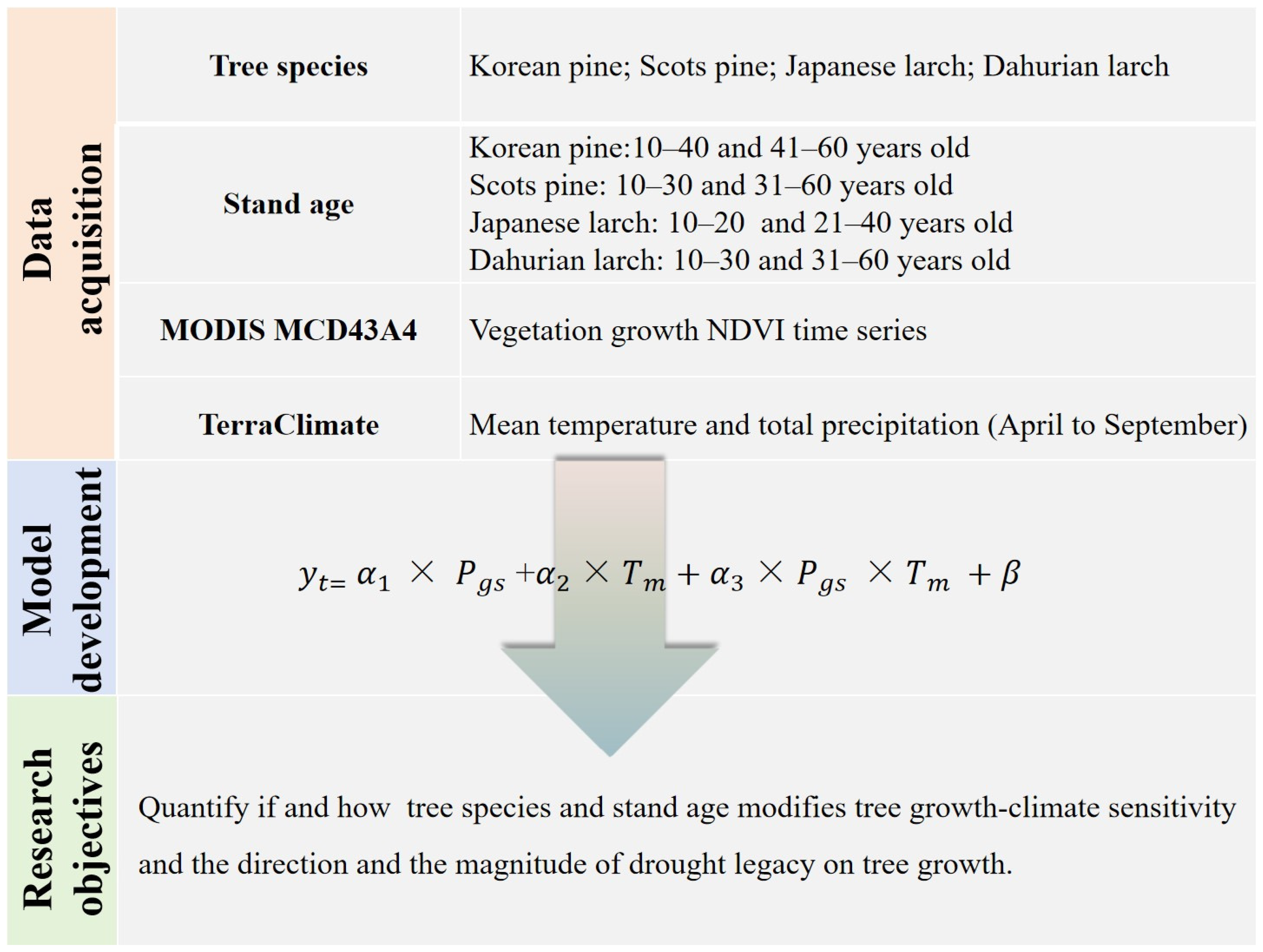
2. Materials and Methods
2.1. Study Area
2.2. MODIS-Derived NDVI Time-Series Data
2.3. Climate Data
2.4. Statistical Analysis
3. Results
3.1. Tree-Growth–Climate Sensitivity for Each Species and Age Group
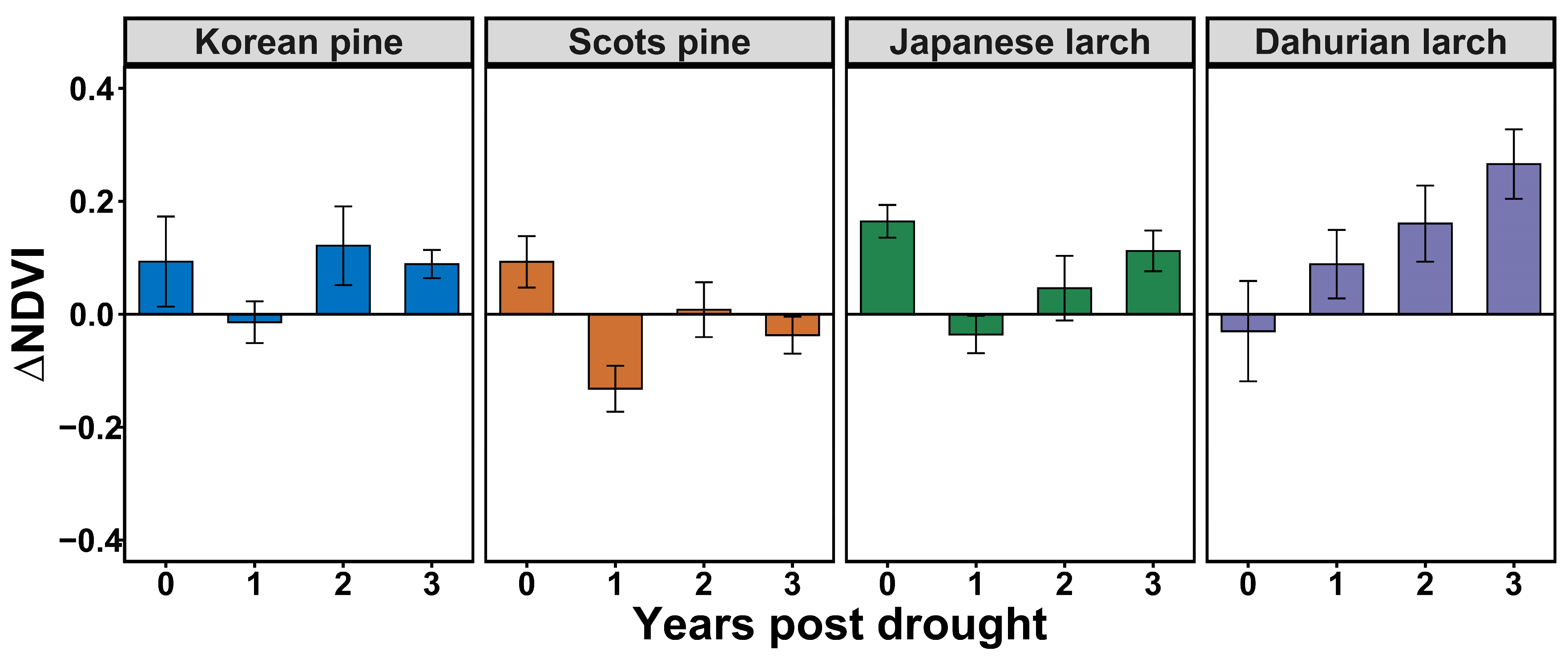
3.2. Drought Legacy Effects on NDVI Varied among Species
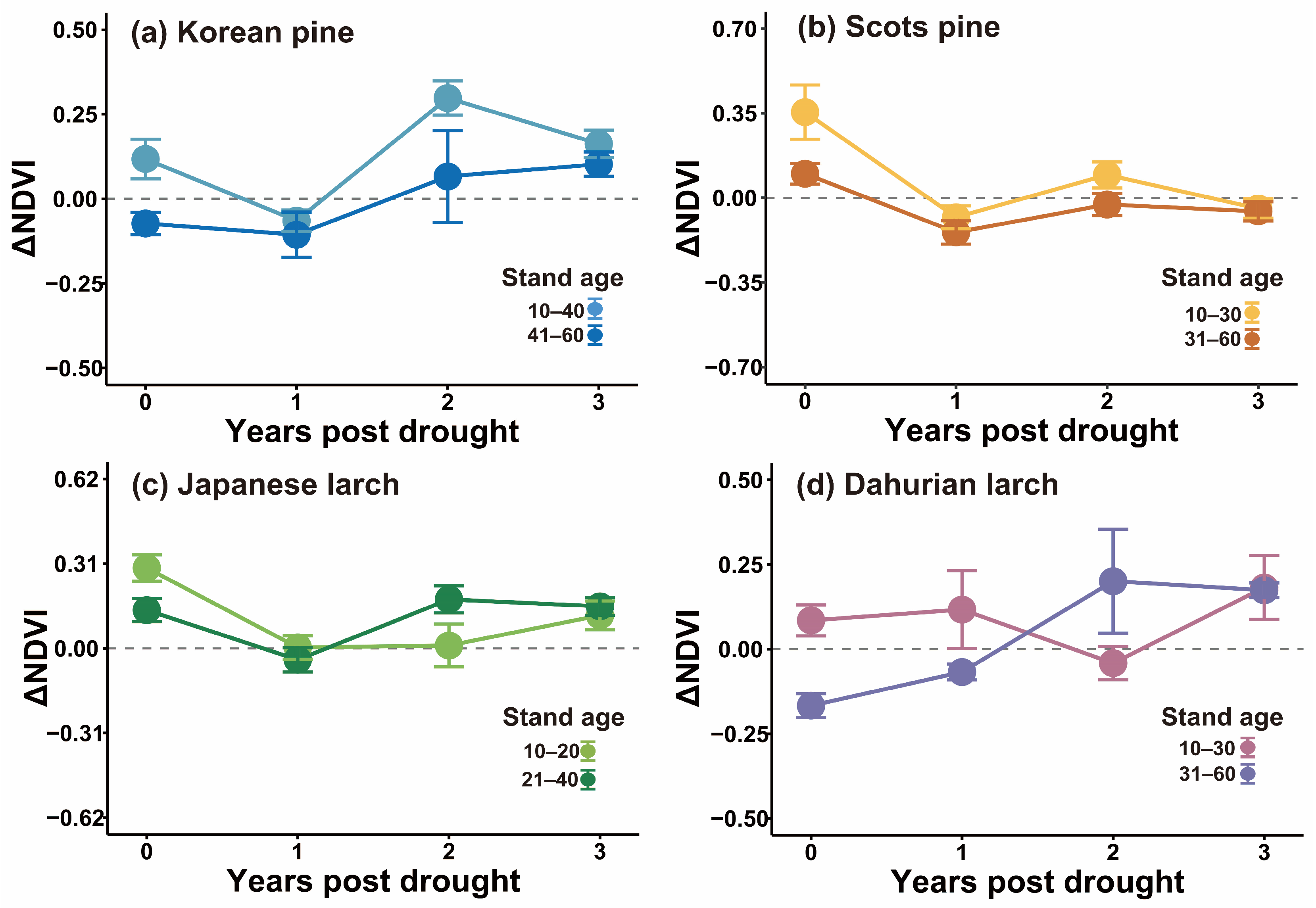
3.3. Stand Age Modified Drought Legacy
4. Discussion
4.1. Tree-Growth–Climate Sensitivity and Drought Legacy Varied among Species
4.2. Stand Age Modified Tree-Growth–Climate Sensitivity and Drought Legacy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anderegg, W.R.L.; Trugman, A.T.; Badgley, G.; Anderson, C.M.; Bartuska, A.; Ciais, P.; Cullenward, D.; Field, C.B.; Freeman, J.; Goetz, S.J.; et al. Climate-driven risks to the climate mitigation potential of forests. Science 2020, 368, eaaz7005. [Google Scholar] [CrossRef]
- Diffenbaugh, N.S.; Field, C.B. Changes in Ecologically Critical Terrestrial Climate Conditions. Science 2013, 341, 486–492. [Google Scholar] [CrossRef]
- Schwalm, C.R.; Anderegg, W.R.L.; Michalak, A.M.; Fisher, J.B.; Biondi, F.; Koch, G.; Litvak, M.; Ogle, K.; Shaw, J.D.; Wolf, A.; et al. Global patterns of drought recovery. Nature 2017, 548, 202–205. [Google Scholar] [CrossRef]
- Hartmann, H.; Bastos, A.; Das, A.J.; Esquivel-Muelbert, A.; Hammond, W.M.; Martinez-Vilalta, J.; McDowell, N.G.; Powers, J.S.; Pugh, T.A.M.; Ruthrof, K.X.; et al. Climate Change Risks to Global Forest Health: Emergence of Unexpected Events of Elevated Tree Mortality Worldwide. Annu. Rev. Plant Biol. 2022, 73, 673–702. [Google Scholar] [CrossRef]
- Clark, J.S.; Iverson, L.; Woodall, C.W.; Allen, C.D.; Bell, D.M.; Bragg, D.C.; D’Amato, A.W.; Davis, F.W.; Hersh, M.H.; Ibanez, I.; et al. The impacts of increasing drought on forest dynamics, structure, and biodiversity in the United States. Glob. Chang. Biol. 2016, 22, 2329–2352. [Google Scholar] [CrossRef]
- Brando, P.M.; Paolucci, L.; Ummenhofer, C.C.; Ordway, E.M.; Hartmann, H.; Cattau, M.E.; Rattis, L.; Medjibe, V.; Coe, M.T.; Balch, J. Droughts, Wildfires, and Forest Carbon Cycling: A Pantropical Synthesis. Annu. Rev. Earth Planet. Sci. 2019, 47, 555–581. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Kim, J.B.; Trugman, A.T.; Kim, Y.; Still, C.J. Linking tree physiological constraints with predictions of carbon and water fluxes at an old-growth coniferous forest. Ecosphere 2019, 10, e02692. [Google Scholar] [CrossRef]
- Ogaya, R.; Barbeta, A.; Basnou, C.; Penuelas, J. Satellite data as indicators of tree biomass growth and forest dieback in a Mediterranean holm oak forest. Ann. For. Sci. 2015, 72, 135–144. [Google Scholar] [CrossRef]
- Zhou, L.M.; Tian, Y.H.; Myneni, R.B.; Ciais, P.; Saatchi, S.; Liu, Y.Y.; Piao, S.L.; Chen, H.S.; Vermote, E.F.; Song, C.H.; et al. Widespread decline of Congo rainforest greenness in the past decade. Nature 2014, 509, 86–90. [Google Scholar] [CrossRef]
- Muller, L.M.; Bahn, M. Drought legacies and ecosystem responses to subsequent drought. Glob. Chang. Biol. 2022, 28, 5086–5103. [Google Scholar] [CrossRef]
- Huang, M.T.; Wang, X.H.; Keenan, T.F.; Piao, S.L. Drought timing influences the legacy of tree growth recovery. Glob. Chang. Biol. 2018, 24, 3546–3559. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Kerry, J.T.; Connolly, S.R.; Baird, A.H.; Eakin, C.M.; Heron, S.F.; Hoey, A.S.; Hoogenboom, M.O.; Jacobson, M.; Liu, G.; et al. Ecological memory modifies the cumulative impact of recurrent climate extremes. Nat. Clim. Chang. 2019, 9, 40–43. [Google Scholar] [CrossRef]
- Serra-Maluquer, X.; Mencuccini, M.; Martinez-Vilalta, J. Changes in tree resistance, recovery and resilience across three successive extreme droughts in the northeast Iberian Peninsula. Oecologia 2018, 187, 343–354. [Google Scholar] [CrossRef]
- Manrique-Alba, A.; Begueria, S.; Camarero, J.J. Long-term effects of forest management on post-drought growth resilience: An analytical framework. Sci. Total Environ. 2022, 810, 152374. [Google Scholar] [CrossRef] [PubMed]
- Canning, C.M.; Mood, B.J.; Bonsal, B.; Howat, B.; Laroque, C.P. Comparison of tree-growth drought legacies of three shelterbelt species in the Canadian Prairies. Agric. For. Meteorol. 2023, 330, 109317. [Google Scholar] [CrossRef]
- Lee, H.; Jeon, J.; Kang, M.; Cho, S.; Park, J.; Lee, M.; Lee, H.; Kim, D.; Kim, H.S. The resilience of the carbon cycles of temperate coniferous and broadleaved forests to drought. For. Ecol. Manag. 2021, 491, 119178. [Google Scholar] [CrossRef]
- Peltier, D.M.P.; Ogle, K. Tree growth sensitivity to climate is temporally variable. Ecol. Lett. 2020, 23, 1561–1572. [Google Scholar] [CrossRef]
- Colangelo, M.; Camarero, J.J.; Gazol, A.; Piovesan, G.; Borghetti, M.; Baliva, M.; Gentilesca, T.; Rita, A.; Schettino, A.; Ripullone, F. Mediterranean old-growth forests exhibit resistance to climate warming. Sci. Total Environ. 2021, 801, 149684. [Google Scholar] [CrossRef]
- Fang, K.Y.; Chen, D.; Gou, X.H.; D’Arrigo, R.; Davi, N. Influence of non-climatic factors on the relationships between tree growth and climate over the Chinese Loess Plateau. Glob. Planet. Chang. 2015, 132, 54–63. [Google Scholar] [CrossRef]
- Xu, L.L.; Meng, P.; Tong, X.J.; Zhang, J.S.; Li, J.; Wang, X.; Xie, H.; Liu, P.R. Productivity and water use efficiency of Pinus tabulaeformis responses to climate change in the temperate monsoon region. Agric. For. Meteorol. 2022, 327, 109188. [Google Scholar] [CrossRef]
- Wang, J.; Feng, L.; Palmer, P.I.; Liu, Y.; Fang, S.X.; Bosch, H.; O’Dell, C.W.; Tang, X.P.; Yang, D.X.; Liu, L.X.; et al. Large Chinese land carbon sink estimated from atmospheric carbon dioxide data. Nature 2020, 586, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhu, J.J.; Zhang, M.; Yan, Q.L.; Sun, O.J. Soil microbial biomass carbon and nitrogen in forest ecosystems of Northeast China: A comparison between natural secondary forest and larch plantation. J. Plant Ecol. 2010, 3, 175–182. [Google Scholar] [CrossRef]
- Faria, J.; Sanchez-Oliver, J.S.; Beja, P.; Moreira, F.; Catry, I.; Vasconcelos, S.; Pina, S.; Rotenberry, J.T.; Reino, L.; Santana, J. Contrasting effects of eucalyptus, pine and oak plantations on nest predation risk in Mediterranean grasslands. For. Ecol. Manag. 2022, 511, 120116. [Google Scholar] [CrossRef]
- Hua, F.Y.; Bruijnzeel, L.A.; Meli, P.; Martin, P.A.; Zhang, J.; Nakagawa, S.; Miao, X.R.; Wang, W.Y.; McEvoy, C.; Pena-Arancibia, J.L.; et al. The biodiversity and ecosystem service contributions and trade-offs of forest restoration approaches. Science 2022, 376, 839–844. [Google Scholar] [CrossRef]
- Rubio-Cuadrado, A.; Camarero, J.J.; Aspizua, R.; Sanchez-Gonzalez, M.; Gil, L.; Montes, F. Abiotic factors modulate post-drought growth resilience of Scots pine plantations and rear-edge Scots pine and oak forests. Dendrochronologia 2018, 51, 54–65. [Google Scholar] [CrossRef]
- Steckel, M.; del Rio, M.; Heym, M.; Aldea, J.; Bielak, K.; Brazaitis, G.; Cerny, J.; Coll, L.; Collet, C.; Ehbrecht, M.; et al. Species mixing reduces drought susceptibility of Scots pine (Pinus sylvestris L.) and oak (Quercus robur L., Quercus petraea (Matt.) Liebl.)—Site water supply and fertility modify the mixing effect. For. Ecol. Manag. 2020, 461, 117908. [Google Scholar] [CrossRef]
- Zhang, X.L.; Yu, P.; Wang, D.Z.; Xu, Z.Q. Density- and age- dependent influences of droughts and intrinsic water use efficiency on growth in temperate plantations. Agric. For. Meteorol. 2022, 325, 109134. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 31 October 2022).
- GB/T 38590-2020; Technical Regulations for Continuous Forest Inventory. State Administration for Market Regulation: Beijing, China; National Standardization Administration: Beijing, China, 2020; 56p.
- Tucker, C.J.; Vanpraet, C.L.; Sharman, M.J.; Van Ittersum, G. Satellite remote sensing of total herbaceous biomass production in the senegalese sahel: 1980–1984. Remote Sens. Environ. 1985, 17, 233–249. [Google Scholar] [CrossRef]
- Heumann, B.W.; Seaquist, J.W.; Eklundh, L.; Jönsson, P. AVHRR derived phenological change in the Sahel and Soudan, Africa, 1982–2005. Remote Sens. Environ. 2007, 108, 385–392. [Google Scholar] [CrossRef]
- Li, P.L.; Zhu, D.; Wang, Y.L.; Liu, D. Elevation dependence of drought legacy effects on vegetation greenness over the Tibetan Plateau. Agric. For. Meteorol. 2020, 295, 108190. [Google Scholar] [CrossRef]
- Dong, B.G.; Yu, Y.; Pereira, P. Non-growing season drought legacy effects on vegetation growth in southwestern China. Sci. Total Environ. 2022, 846, 157334. [Google Scholar] [CrossRef] [PubMed]
- Schowengerdt, R.A. Remote Sensing: Models and Methods for Image Processing; Academic Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Abatzoglou, J.T.; Dobrowski, S.Z.; Parks, S.A.; Hegewisch, K.C. Data Descriptor: TerraClimate, a high-resolution global dataset of monthly climate and climatic water balance from 1958–2015. Sci. Data 2018, 5, 170191. [Google Scholar] [CrossRef]
- Chu, H.S.; Venevsky, S.; Wu, C.; Wang, M.H. NDVI-based vegetation dynamics and its response to climate changes at Amur-Heilongjiang River Basin from 1982 to 2015. Sci. Total Environ. 2019, 650, 2051–2062. [Google Scholar] [CrossRef] [PubMed]
- Azen, R.; Budescu, D.V. The dominance analysis approach for comparing predictors in multiple regression. Psychol. Methods 2003, 8, 129–148. [Google Scholar] [CrossRef]
- Li, W.Q.; Jiang, Y.; Dong, M.Y.; Du, E.Z.; Wu, F.; Zhao, S.D.; Xu, H. Species-specific growth-climate responses of Dahurian larch (Larix gmelinii) and Mongolian pine (Pinus sylvestris var. mongolica) in the Greater Khingan Range, northeast China. Dendrochronologia 2021, 65, 125803. [Google Scholar] [CrossRef]
- Fu, Y.H.; Piao, S.; Delpierre, N.; Hao, F.; Hänninen, H.; Liu, Y.; Sun, W.; Janssens, I.A.; Campioli, M. Larger temperature response of autumn leaf senescence than spring leaf-out phenology. Glob. Chang. Biol. 2018, 24, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Jeong, S.; Park, C.E.; Xu, H.; Li, L.Z.X.; Wang, T.; Gentine, P.; Penuelas, J.; Piao, S.L. Biophysical impacts of northern vegetation changes on seasonal warming patterns. Nat. Commun. 2022, 13, 3925. [Google Scholar] [CrossRef]
- Gastwirth, J.L.; Gel, Y.R.; Miao, W.W. The Impact of Levene’s Test of Equality of Variances on Statistical Theory and Practice. Stat. Sci. 2009, 24, 343–360. [Google Scholar] [CrossRef]
- Bates, D.; Machler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Feichtinger, L.M.; Eilmann, B.; Buchmann, N.; Rigling, A. Growth adjustments of conifers to drought and to century-long irrigation. For. Ecol. Manag. 2014, 334, 96–105. [Google Scholar] [CrossRef]
- Lyu, S.N.; Wang, X.C.; Zhang, Y.D.; Li, Z.S. Different responses of Korean pine (Pinus koraiensis) and Mongolia oak (Quercus mongolica) growth to recent climate warming in northeast China. Dendrochronologia 2017, 45, 113–122. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Schwalm, C.; Biondi, F.; Camarero, J.J.; Koch, G.; Litvak, M.; Ogle, K.; Shaw, J.D.; Shevliakova, E.; Williams, A.P.; et al. Pervasive drought legacies in forest ecosystems and their implications for carbon cycle models. Science 2015, 349, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Kannenberg, S.A.; Schwalm, C.R.; Anderegg, W.R.L. Ghosts of the past: How drought legacy effects shape forest functioning and carbon cycling. Ecol. Lett. 2020, 23, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.K.; Gessler, A.; Bolte, A.; Bottero, A.; Buras, A.; Cailleret, M.; Camarero, J.J.; Haeni, M.; Heres, A.M.; Hevia, A.; et al. Growth and resilience responses of Scots pine to extreme droughts across Europe depend on predrought growth conditions. Glob. Chang. Biol. 2020, 26, 4521–4537. [Google Scholar] [CrossRef] [PubMed]
- Peltier, D.M.P.; Guo, J.; Nguyen, P.; Bangs, M.; Wilson, M.; Samuels-Crow, K.; Yocom, L.L.; Liu, Y.; Fell, M.K.; Shaw, J.D.; et al. Temperature memory and non-structural carbohydrates mediate legacies of a hot drought in trees across the southwestern USA. Tree Physiol. 2022, 42, 71–85. [Google Scholar] [CrossRef]
- Martin-Gomez, P.; Aguilera, M.; Peman, J.; Gil-Pelegrin, E.; Ferrio, J.P. Contrasting ecophysiological strategies related to drought: The case of a mixed stand of Scots pine (Pinus sylvestris) and a submediterranean oak (Quercus subpyrenaica). Tree Physiol. 2017, 37, 1478–1492. [Google Scholar] [CrossRef]
- Rowland, L.; da Costa, A.C.L.; Galbraith, D.R.; Oliveira, R.S.; Binks, O.J.; Oliveira, A.A.R.; Pullen, A.M.; Doughty, C.E.; Metcalfe, D.B.; Vasconcelos, S.S.; et al. Death from drought in tropical forests is triggered by hydraulics not carbon starvation. Nature 2015, 528, 119–122. [Google Scholar] [CrossRef]
- Klein, T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- Manzoni, S.; Vico, G.; Thompson, S.; Beyer, F.; Weih, M. Contrasting leaf phenological strategies optimize carbon gain under droughts of different duration. Adv. Water Resour. 2015, 84, 37–51. [Google Scholar] [CrossRef]
- Sanguesa-Barreda, G.; Gazol, A.; Camarero, J.J. Drops in needle production are early-warning signals of drought-triggered dieback in Scots pine. Trees-Struct. Funct. 2023, 37, 1137–1151. [Google Scholar] [CrossRef]
- He, W.Q.; Liu, H.Y.; Qi, Y.; Liu, F.; Zhu, X.R. Patterns in nonstructural carbohydrate contents at the tree organ level in response to drought duration. Glob. Chang. Biol. 2020, 26, 3627–3638. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.N.; Gessler, A.; Saurer, M.; Hagedorn, F.; Gao, D.C.; Wang, X.Y.; Schaub, M.; Li, M.H.; Shen, W.J.; Schonbeck, L. Root carbon and nutrient homeostasis determines downy oak sapling survival and recovery from drought. Tree Physiol. 2021, 41, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Salguero, R.; Navarro-Cerrillo, R.M.; Camarero, J.J.; Fernandez-Cancio, A. Selective drought-induced decline of pine species in southeastern Spain. Clim. Chang. 2012, 113, 767–785. [Google Scholar] [CrossRef]
- Gessler, A.; Bottero, A.; Marshall, J.; Arend, M. The way back: Recovery of trees from drought and its implication for acclimation. New Phytol. 2020, 228, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Schwalm, C.R.; Samuels-Crow, K.E.; Ogle, K. Ecological memory of daily carbon exchange across the globe and its importance in drylands. Ecol. Lett. 2019, 22, 1806–1816. [Google Scholar] [CrossRef]
- Primicia, I.; Camarero, J.J.; Janda, P.; Cada, V.; Morrissey, R.C.; Trotsiuk, V.; Bace, R.; Teodosiu, M.; Svoboda, M. Age, competition, disturbance and elevation effects on tree and stand growth response of primary Picea abies forest to climate. For. Ecol. Manag. 2015, 354, 77–86. [Google Scholar] [CrossRef]
- Au, T.F.; Maxwell, J.T.; Robeson, S.M.; Li, J.B.; Siani, S.M.O.; Novick, K.A.; Dannenberg, M.P.; Phillips, R.P.; Li, T.; Chen, Z.J.; et al. Younger trees in the upper canopy are more sensitive but also more resilient to drought. Nat. Clim. Chang. 2022, 12, 1168–1174. [Google Scholar] [CrossRef]
- Scholz, F.G.; Phillips, N.G.; Bucci, S.J.; Meinzer, F.C.; Goldstein, G. Hydraulic Capacitance: Biophysics and Functional Significance of Internal Water Sources in Relation to Tree Size. In Size- and Age-Related Changes in Tree Structure and Function; Meinzer, F.C., Lachenbruch, B., Dawson, T.E., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 341–361. [Google Scholar] [CrossRef]
- Feraandez-Martinez, M.; Garbulsky, M.; Penuelas, J.; Peguero, G.; Espelta, J.M. Temporal trends in the enhanced vegetation index and spring weather predict seed production in Mediterranean oaks. Plant Ecol. 2015, 216, 1061–1072. [Google Scholar] [CrossRef]
- Ouyang, L.; Wu, J.; Zhao, P.; Zhu, L.W.; Ni, G.Y. Stand age rather than soil moisture gradient mainly regulates the compromise between plant growth and water use of Eucalyptus urophylla in hilly South China. Land Degrad. Dev. 2021, 32, 2423–2436. [Google Scholar] [CrossRef]
- Olson, M.E.; Soriano, D.; Rosell, J.A.; Anfodillo, T.; Donoghue, M.J.; Edwards, E.J.; Leon-Gomez, C.; Dawson, T.; Martinez, J.J.C.; Castorena, M.; et al. Plant height and hydraulic vulnerability to drought and cold. Proc. Natl. Acad. Sci. USA 2018, 115, 7551–7556. [Google Scholar] [CrossRef] [PubMed]
- Maclauchlan, L. Quantification of Dryocoetes confusus-caused mortality in subalpine fir forests of southern British Columbia. For. Ecol. Manag. 2016, 359, 210–220. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J.; Sanchez-Salguero, R.; Vicente-Serrano, S.M.; Serra-Maluquer, X.; Gutierrez, E.; de Luis, M.; Sanguesa-Barreda, G.; Novak, K.; Rozas, V.; et al. Drought legacies are short, prevail in dry conifer forests and depend on growth variability. J. Ecol. 2020, 108, 2473–2484. [Google Scholar] [CrossRef]
- Jiang, P.; Liu, H.Y.; Piao, S.L.; Ciais, P.; Wu, X.C.; Yin, Y.; Wang, H.Y. Enhanced growth after extreme wetness compensates for post-drought carbon loss in dry forests. Nat. Commun. 2019, 10, 195. [Google Scholar] [CrossRef]
- Zhou, Y. The Research of Two Species of Sawfly Damaged on the Coniferous Plantations Influence Carbon Sinks. Master’s Thesis, Northeast Forestry University, Harbin, China, 2014. [Google Scholar]
- Wang, C. Study on the Relationship between Pine Needles of Pinus koraiensis Plantation and Soil Nutrients under Different Terrain Conditions. Master’s Thesis, Northeast Forestry University, Harbin, China, 2014. [Google Scholar]
- Xu, T.; Zhu, J.; Yu, L.; Wang, R.; Zhang, J. Physical and Chemical Properties of Stemflow in Different Forest Types of a Secondary Forest Ecosystem in Montane Regions of Eastern Liaoning Province, China. Acta Ecol. Sin. 2013, 33, 3415–3424. [Google Scholar]
- Wang, A. Spatial Distribution Pattern and Interspecific Relationship of Dominant Species in Typical Forest Community in Mao’er Mountain. Shandong For. Sci. Technol. 2020, 50, 1–9+16. [Google Scholar]
- Xie, J.; Yan, Q.; Zhang, T. Temporal Effects of Thinning on the Composition and Growth of Regenerated Woody Plants in Larix Kaempferi Plantations. Chin. J. Appl. Ecol. 2020, 31, 2481–2490. [Google Scholar]
- Wu, M. Growth and Climatic Response of Pinus sylvestris var. mongolica in Sandy Land of Zhang-Gu-Tai, Liaoning Province. Master Thesis, Beijing Forestry University, Harbin, China, 2019. [Google Scholar]
- Chen, M. Water Uptake of Pinus sylvestris var. mongolica Plantation in Horqin Sandy Land. Master’s Thesis, Beijing Forestry University, Harbin, China, 2020. [Google Scholar]
- Lu, X. Study on the Radial Growth and Spatial Climate Response of Pinus sylvestris var. mongolica in Plantations in Jilin Province. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2018. [Google Scholar]
- Liu, B.; Wang, S.; Dao, R. Effect of Afforestation in Sandy Land on Soil Bulk Density and Particle-Size Composition in Duolun Count. Inn. Mong. For. Sci. Technol. 2013, 39, 11–14. [Google Scholar]
- Tang, S.; Gao, B.; Yu, B.; Wang, X.; Yin, S.; Shan, S.; Han, X.; Cao, L. Temperature Changes and Main Gas Release Characteristics of Larix Gmelinii Plantations During Smoldering in Daxing’anling Mountain Region of Heilongjiang Province, Northeastern China. J. Beijing For. Univ. 2022, 44, 1–7. [Google Scholar]
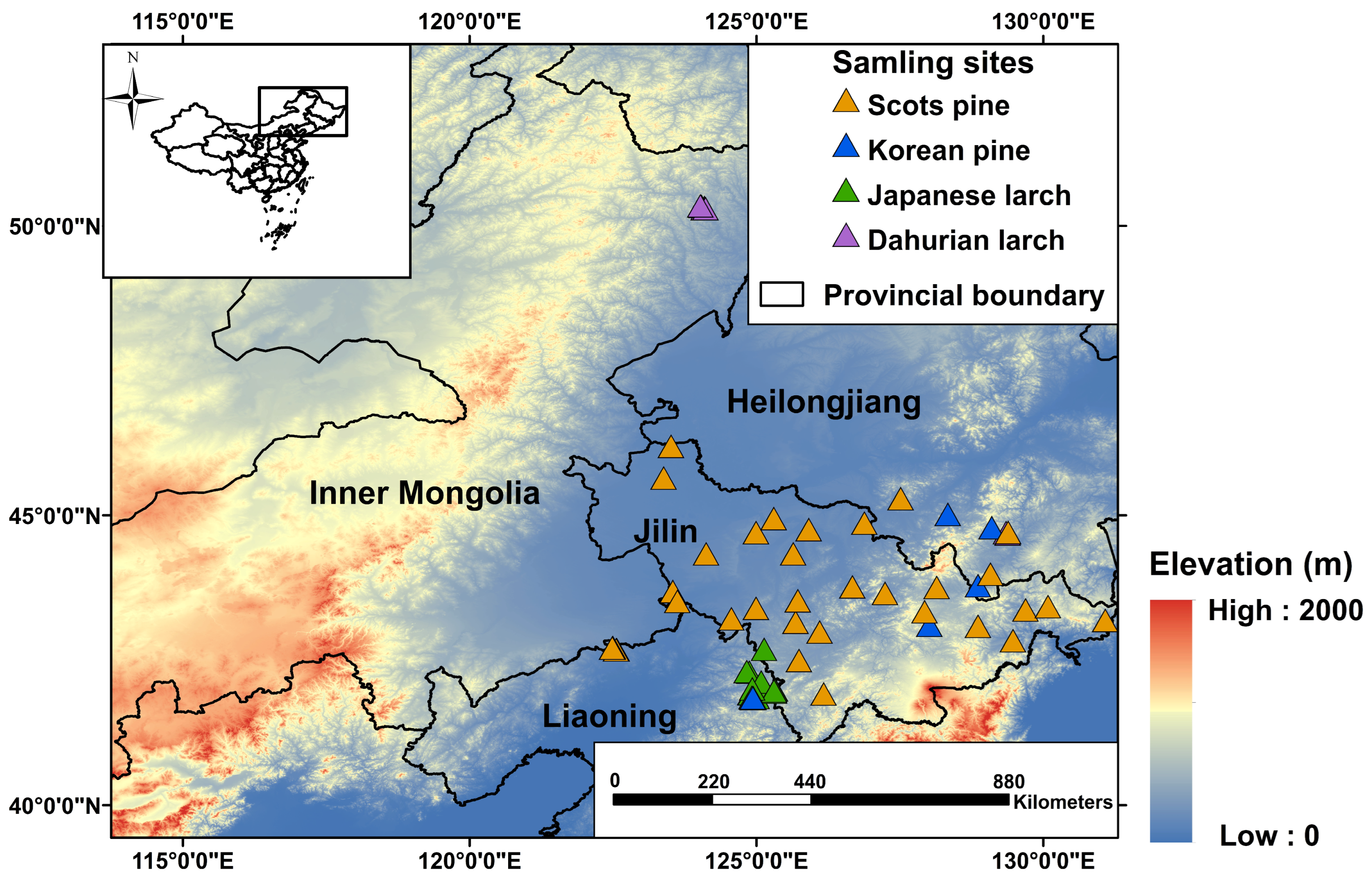


| Species | No. of Sites | Stand Age (Years) | Elevation (m) | Annual Mean Precipitation (mm) | Annual Mean Temperature (°C) |
|---|---|---|---|---|---|
| Korean pine (Pinus koraiensis) | 11 | 41 ± 4.2 | 543 | 714 | 4.57 |
| Scots pine (Pinus sylvestris) | 42 | 35 ± 11.7 | 338 | 554 | 6.04 |
| Japanese larch (Larix kaempferi) | 18 | 26 ± 7.7 | 500 | 821 | 5.70 |
| Dahurian larch (Larix gmelinii) | 11 | 32 ± 9.0 | 517 | 619 | 2.36 |
| Group | Species | F Value | R2 | p |
|---|---|---|---|---|
| By species | Korean pine | 8.23 | 0.12 | <0.001 |
| Scots pine | 15.62 | 0.07 | <0.001 | |
| Japanese larch | 4.89 | 0.05 | <0.05 | |
| Dahurian larch | 3.02 | 0.07 | <0.05 | |
| By age group | Young Korean pine | 3.12 | 0.09 | <0.05 |
| Old Korean pine | 4.63 | 0.16 | <0.05 | |
| Young Scots pine | 8.22 | 0.18 | <0.001 | |
| Old Scots pine | 10.25 | 0.06 | <0.001 | |
| Young Japanese larch | 3.24 | 0.07 | <0.05 | |
| Old Japanese larch | 2.94 | 0.06 | <0.05 | |
| Young Dahurian larch | 6.84 | 0.21 | <0.001 | |
| Old Dahurian larch | 6.29 | 0.17 | <0.001 |
| Species | Variable | General Dominance Statistics | Standardized | Ranking |
|---|---|---|---|---|
| Korean pine | Precipitation | 0.04 | 0.52 | 1 |
| Temperature | 0.03 | 0.41 | 2 | |
| Precipitation × temperature | 0.01 | 0.08 | 3 | |
| Scots pine | Precipitation | 0.02 | 0.50 | 1 |
| Temperature | 0.01 | 0.20 | 3 | |
| Precipitation × temperature | 0.01 | 0.31 | 2 | |
| Japanese larch | Precipitation | 0.03 | 0.29 | 2 |
| Temperature | 0.05 | 0.48 | 1 | |
| Precipitation × temperature | 0.03 | 0.24 | 3 | |
| Dahurian larch | Precipitation | 0.16 | 0.17 | 3 |
| Temperature | 0.53 | 0.57 | 1 | |
| Precipitation × temperature | 0.24 | 0.26 | 2 |
| Species | Explanatory Variables | ndf | ndf | F Value | p |
|---|---|---|---|---|---|
| Korean pine | Time | 3 | 36 | 7.3 | <0.05 |
| Stand age | 1 | 36 | 8.82 | <0.05 | |
| Time × stand age | 3 | 36 | 1.14 | 0.34 | |
| Scots pine | Time | 3 | 164 | 8.9 | <0.05 |
| Stand age | 1 | 164 | 5.21 | <0.05 | |
| Time × stand age | 3 | 164 | 1.12 | 0.34 | |
| Japanese larch | Time | 3 | 64 | 7.63 | <0.05 |
| Stand age | 1 | 64 | 0.03 | 0.87 | |
| Time × stand age | 3 | 64 | 3.63 | <0.05 | |
| Dahurian larch | Time | 3 | 36 | 9.97 | <0.05 |
| Stand age | 1 | 36 | 5.4 | <0.05 | |
| Time × stand age | 3 | 36 | 6.88 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Sun, Q.; Zou, H.; Marschner, P. Climate Sensitivity and Drought Legacy of Tree Growth in Plantation Forests in Northeast China Are Species- and Age-Dependent. Remote Sens. 2024, 16, 281. https://doi.org/10.3390/rs16020281
Li T, Sun Q, Zou H, Marschner P. Climate Sensitivity and Drought Legacy of Tree Growth in Plantation Forests in Northeast China Are Species- and Age-Dependent. Remote Sensing. 2024; 16(2):281. https://doi.org/10.3390/rs16020281
Chicago/Turabian StyleLi, Ting, Qiaoqi Sun, Hongfei Zou, and Petra Marschner. 2024. "Climate Sensitivity and Drought Legacy of Tree Growth in Plantation Forests in Northeast China Are Species- and Age-Dependent" Remote Sensing 16, no. 2: 281. https://doi.org/10.3390/rs16020281
APA StyleLi, T., Sun, Q., Zou, H., & Marschner, P. (2024). Climate Sensitivity and Drought Legacy of Tree Growth in Plantation Forests in Northeast China Are Species- and Age-Dependent. Remote Sensing, 16(2), 281. https://doi.org/10.3390/rs16020281




