New Hyperspectral Geometry Ratio Index for Monitoring Rice Blast Disease from Leaf Scale to Canopy Scale
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Design
2.2. Data Acquisition
2.2.1. Measurement of Leaf and Canopy Reflectance Spectra
2.2.2. Estimation of the Proportion of Leaf Spots and Canopy Disease Index
2.2.3. Hyperspectral Vegetation Index Commonly Used in Vegetation Disease Identification
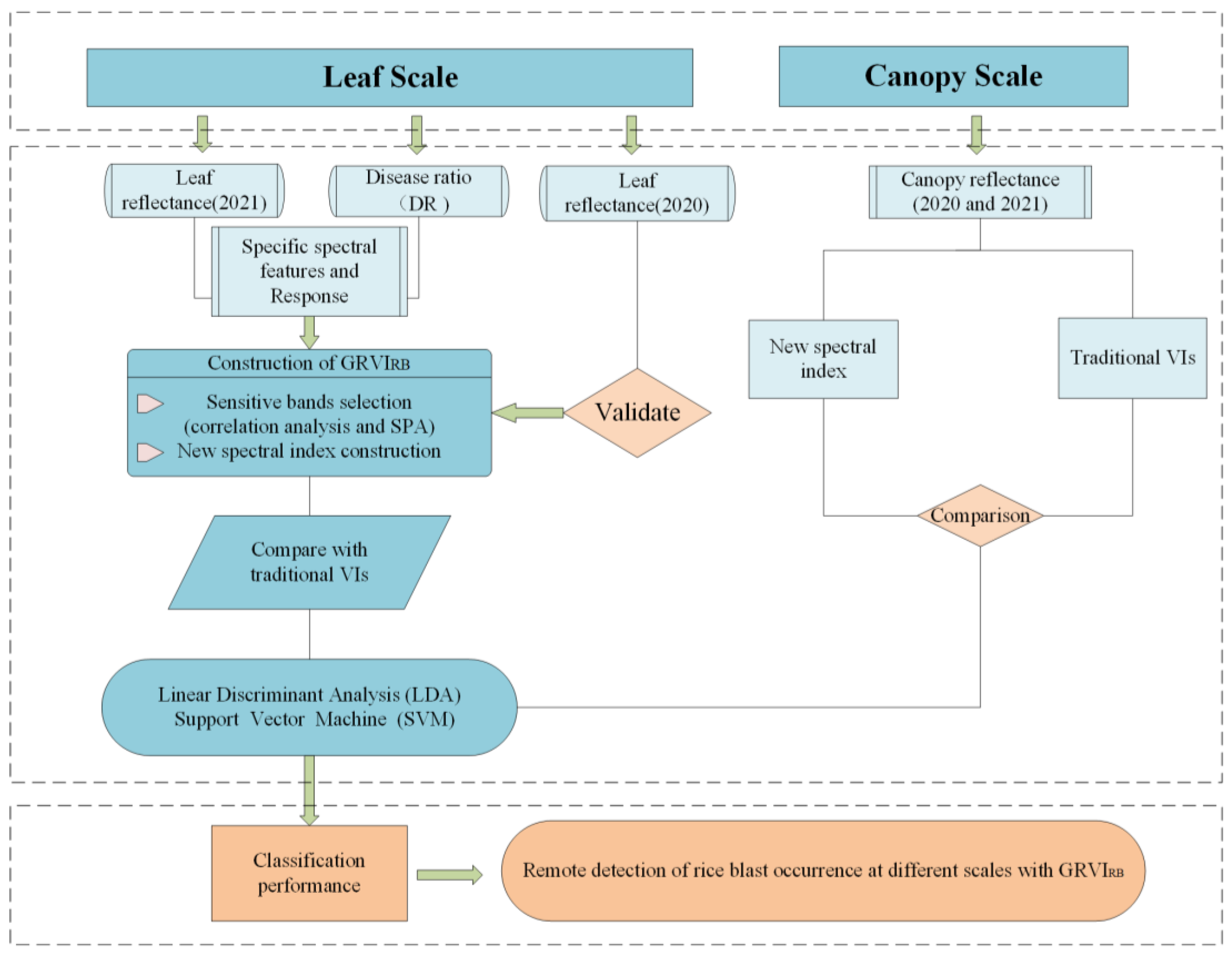
2.3. Methodology
2.3.1. Selection of Spectral Features of Rice Blast
2.3.2. Linear Discriminant Analysis
2.3.3. Support Vector Machine
2.3.4. Precision Evaluation
3. Results
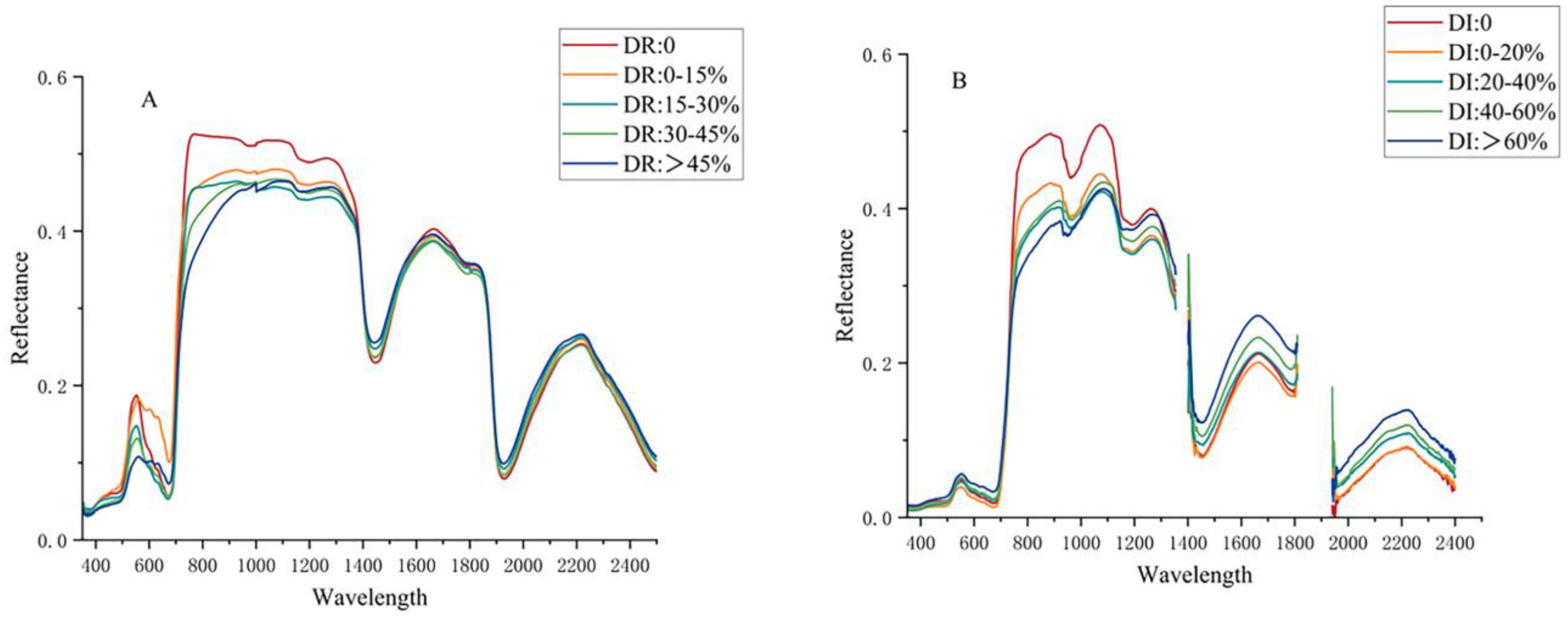
3.1. Responses of Reflectance at Both Leaf and Canopy Scales to Rice Blast Infection
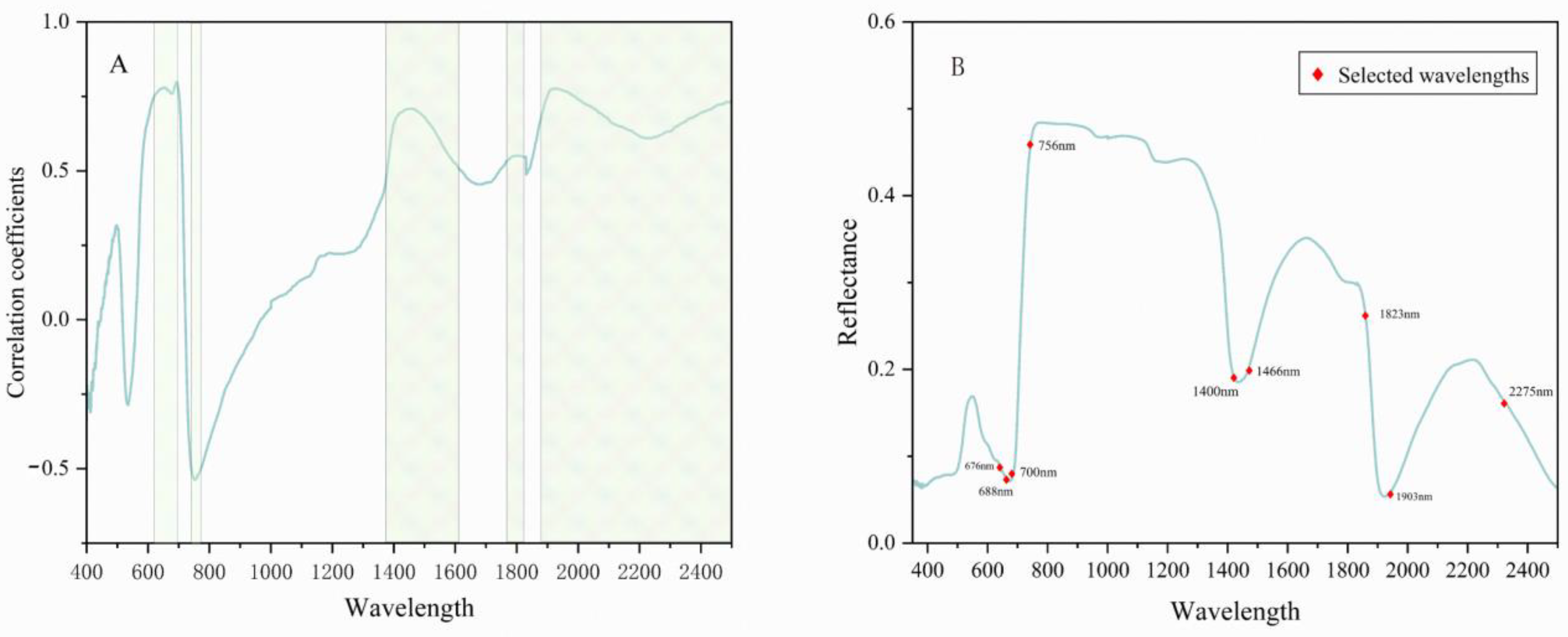
3.2. Spectral Feature Selection
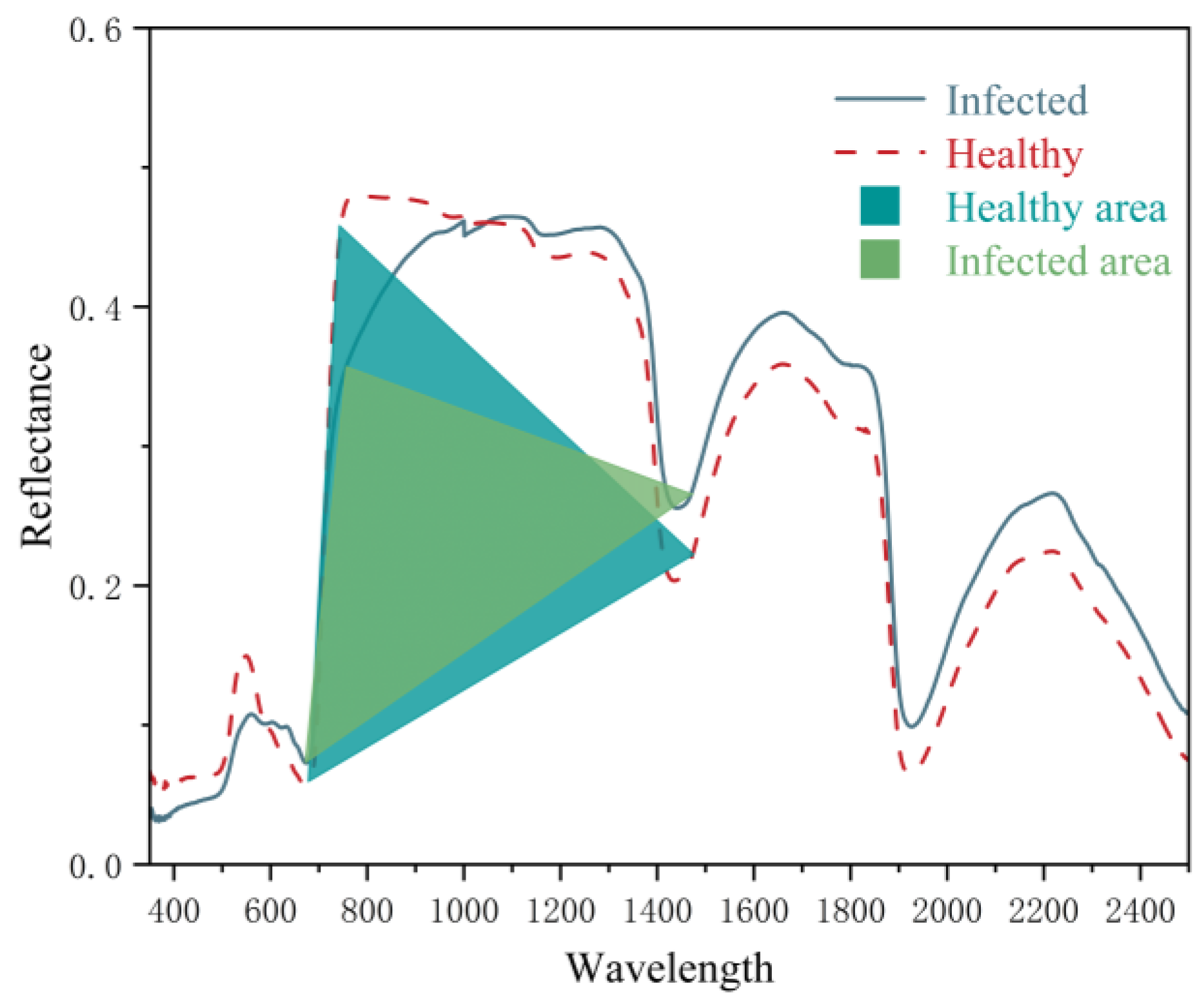
3.3. Formatting of Mathematical Components
3.4. Classification of Infected and Healthy Leaves Using GRVIRB
3.4.1. Comparative Analysis of GRVIRB and Traditional VIs at the Leaf Scale
3.4.2. Comparative Analysis of GRVIRB and Traditional VIs at the Canopy Scale
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, T.R.; Rai, A.K.; Gupta, S.K.; Vijayan, J.; Devanna, B.N.; Ray, S. Rice Blast Management Through Host-Plant Resistance: Retrospect and Prospects. Agric. Res. 2012, 1, 37–52. [Google Scholar] [CrossRef]
- Deng, Y.; Zhai, K.; Xie, Z.; Yang, D.; Zhu, X.; Liu, J.; Wang, X.; Qin, P.; Yang, Y.; Zhang, G.; et al. Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science 2017, 355, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Wang, Z.; Xue, B.; Li, D.; Zheng, H.; Yao, X.; Zhu, Y.; Cao, W.; Cheng, T. A disease-specific spectral index tracks Magnaporthe oryzae infection in paddy rice from ground to space. Remote Sens. Environ. 2023, 285, 113384. [Google Scholar] [CrossRef]
- Dean, R.A.; Talbot, N.J.; Ebbole, D.J.; Farman, M.L.; Mitchell, T.K.; Orbach, M.J.; Thon, M.; Kulkarni, R.; Xu, J.R.; Pan, H.; et al. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature 2005, 434, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.A.; Talbot, N.J. Under pressure: Investigating the biology of plant infection by Magnaporthe oryzae. Nat. Rev. Microbiol. 2009, 7, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, H.; Li, M.; Wang, Z.; Zhou, J.; Wang, S.; Lu, G.; Fu, F. Early diagnosis of blast fungus, Magnaporthe oryzae, in rice plant by using an ultra-sensitive electrically magnetic-controllable electrochemical biosensor. Anal. Chim. Acta 2014, 850, 85–91. [Google Scholar] [CrossRef]
- Zhao, D.; Cao, Y.; Li, J.; Cao, Q.; Li, J.; Guo, F.; Feng, S.; Xu, T. Early Detection of Rice Leaf Blast Disease Using Unmanned Aerial Vehicle Remote Sensing: A Novel Approach Integrating a New Spectral Vegetation Index and Machine Learning. Agronomy 2024, 14, 602. [Google Scholar] [CrossRef]
- Zheng, Q.; Huang, W.; Xia, Q.; Dong, Y.; Ye, H.; Jiang, H.; Chen, S.; Huang, S. Remote Sensing Monitoring of Rice Diseases and Pests from Different Data Sources: A Review. Agronomy 2023, 13, 1851. [Google Scholar] [CrossRef]
- Huang, W.; Lamb, D.W.; Niu, Z.; Zhang, Y.; Liu, L.; Wang, J. Identification of yellow rust in wheat using in-situ spectral reflectance measurements and airborne hyperspectral imaging. Precis. Agric. 2007, 8, 187–197. [Google Scholar] [CrossRef]
- Huang, W.; Guan, Q.; Luo, J.; Zhang, J.; Zhao, J.; Liang, D.; Huang, L.; Zhang, D. New Optimized Spectral Indices for Identifying and Monitoring Winter Wheat Diseases. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 2516–2524. [Google Scholar] [CrossRef]
- Franceschini, M.H.D.; Bartholomeus, H.; van Apeldoorn, D.F.; Suomalainen, J.; Kooistra, L. Feasibility of Unmanned Aerial Vehicle Optical Imagery for Early Detection and Severity Assessment of Late Blight in Potato. Remote Sens. 2019, 11, 224. [Google Scholar] [CrossRef]
- Zhang, J.; Pu, R.; Huang, W.; Yuan, L.; Luo, J.; Wang, J. Using in-situ hyperspectral data for detecting and discriminating yellow rust disease from nutrient stresses. Field Crops Res. 2012, 134, 165–174. [Google Scholar] [CrossRef]
- Liu, T.; Shi, T.; Zhang, H.; Wu, C. Detection of Rise Damage by Leaf Folder (Cnaphalocrocis medinalis) Using Unmanned Aerial Vehicle Based Hyperspectral Data. Sustainability 2020, 12, 9343. [Google Scholar] [CrossRef]
- Huang, J.; Liao, H.; Zhu, Y.; Sun, J.; Sun, Q.; Liu, X. Hyperspectral detection of rice damaged by rice leaf folder (Cnaphalocrocis medinalis). Comput. Electron. Agric. 2012, 82, 100–107. [Google Scholar] [CrossRef]
- Marang, I.J.; Filippi, P.; Weaver, T.B.; Evans, B.J.; Whelan, B.M.; Bishop, T.F.A.; Murad, M.O.F.; Al-Shammari, D.; Roth, G. Machine Learning Optimised Hyperspectral Remote Sensing Retrieves Cotton Nitrogen Status. Remote Sens. 2021, 13, 1428. [Google Scholar] [CrossRef]
- Anderegg, J.; Hund, A.; Karisto, P.; Mikaberidze, A. In-Field Detection and Quantification of Septoria Tritici Blotch in Diverse Wheat Germplasm Using Spectral–Temporal Features. Front. Plant Sci. 2019, 10, 1335. [Google Scholar] [CrossRef]
- Franceschini, M.D.; Bartholomeus, H.; van Apeldoorn, D.; Suomalainen, J.; Kooistra, L. Intercomparison of Unmanned Aerial Vehicle and Ground-Based Narrow Band Spectrometers Applied to Crop Trait Monitoring in Organic Potato Production. Sensors 2017, 17, 1428. [Google Scholar] [CrossRef]
- Zheng, Q.; Huang, W.; Cui, X.; Shi, Y.; Liu, L. New Spectral Index for Detecting Wheat Yellow Rust Using Sentinel-2 Multispectral Imagery. Sensors 2018, 18, 868. [Google Scholar] [CrossRef]
- Zheng, Q.; Huang, W.; Cui, X.; Shi, Y.; Liu, L. A Disease Index for Efficiently Detecting Wheat Fusarium Head Blight Using Sentinel-2 Multispectral Imagery. IEEE Access 2020, 8, 52181–52191. [Google Scholar] [CrossRef]
- Huo, L.; Persson, H.J.; Lindberg, E. Early detection of forest stress from European spruce bark beetle attack, and a new vegetation index: Normalized distance red & SWIR (NDRS). Remote Sens. Environ. 2021, 255, 112240. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel algorithms for remote estimation of vegetation fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef]
- Mandal, N.; Adak, S.; Das, D.K.; Sahoo, R.N.; Mukherjee, J.; Kumar, A.; Chinnusamy, V.; Das, B.; Mukhopadhyay, A.; Rajashekara, H.; et al. Spectral characterization and severity assessment of rice blast disease using univariate and multivariate models. Front. Plant Sci. 2023, 14, 1067189. [Google Scholar] [CrossRef]
- Liu, H.Q.; Huete, A. A feedback based modification of the NDVI to minimize canopy background and atmospheric noise. IEEE Trans. Geosci. Remote Sens. 1995, 33, 457–465. [Google Scholar] [CrossRef]
- Delalieux, S.; Somers, B.; Hereijgers, S.; Verstraeten, W.; Keulemans, W.; Coppin, P. A near-infrared narrow-waveband ratio to determine Leaf Area Index in orchards. Remote Sens. Environ. 2008, 112, 3762–3772. [Google Scholar] [CrossRef]
- Chen, J.M. Evaluation of Vegetation Indices and a Modified Simple Ratio for Boreal Applications. Can. J. Remote Sens. 1996, 22, 229–242. [Google Scholar] [CrossRef]
- Haboudane, D. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with Erts. NASA Spec. Publ. 1974, 351, 309. [Google Scholar]
- Chen, P.; Haboudane, D.; Tremblay, N.; Wang, J.; Vigneault, P.; Li, B. New spectral indicator assessing the efficiency of crop nitrogen treatment in corn and wheat. Remote Sens. Environ. 2010, 114, 1987–1997. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Ustin, S.L.; Whiting, M.L. Temporal and Spatial Relationships between Within-Field Yield Variability in Cotton and High-Spatial Hyperspectral Remote Sensing Imagery. Agron. J. 2005, 97, 641–653. [Google Scholar] [CrossRef]
- Gitelson, A.A. Remote estimation of canopy chlorophyll content in crops. Geophys. Res. Lett. 2005, 32, L08403. [Google Scholar] [CrossRef]
- Chappelle, E.W.; Kim, M.S.; McMurtrey, J.E. Ratio analysis of reflectance spectra (RARS): An algorithm for the remote estimation of the concentrations of chlorophyll A, chlorophyll B, and carotenoids in soybean leaves. Remote Sens. Environ. 1992, 39, 239–247. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y.U. Non-destructive optical detection of pigment changes during leaf senescence and fruit ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef]
- Bannari, A.; Khurshid, K.S.; Staenz, K.; Schwarz, J.W. A Comparison of Hyperspectral Chlorophyll Indices for Wheat Crop Chlorophyll Content Estimation Using Laboratory Reflectance Measurements. IEEE Trans. Geosci. Remote Sens. 2007, 45, 3063–3074. [Google Scholar] [CrossRef]
- Datt, B. A New Reflectance Index for Remote Sensing of Chlorophyll Content in Higher Plants: Tests using Eucalyptus Leaves. J. Plant Physiol. 1999, 154, 30–36. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Pushnik, J.C.; Dobrowski, S.; Ustin, S.L. Steady-state chlorophyll a fluorescence detection from canopy derivative reflectance and double-peak red-edge effects. Remote Sens. Environ. 2003, 84, 283–294. [Google Scholar] [CrossRef]
- Nagler, P.L.; Daughtry, C.S.T.; Goward, S.N. Plant Litter and Soil Reflectance. Remote Sens. Environ. 2000, 71, 207–215. [Google Scholar] [CrossRef]
- Araújo, M.C.U.; Saldanha, T.C.B.; Galvão, R.K.H.; Yoneyama, T.; Chame, H.C.; Visani, V. The successive projections algorithm for variable selection in spectroscopic multicomponent analysis. Chemom. Intell. Lab. Syst. 2001, 57, 65–73. [Google Scholar] [CrossRef]
- Fisher, R.A. The Use of Multiple Measurements in Taxonomic Problems. Ann. Eugen. 1936, 7, 179–188. [Google Scholar] [CrossRef]
- Schölkopf, B. Learning with Kernels: Support Vector Machines, Regularization, Optimization, and Beyond. IEEE Trans. Neural Netw. 2005, 16, 781. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Pu, R.; Gonzalez-Moreno, P.; Yuan, L.; Wu, K.; Huang, W. Monitoring plant diseases and pests through remote sensing technology: A review. Comput. Electron. Agric. 2019, 165, 104943. [Google Scholar] [CrossRef]
- Tian, L.; Xue, B.; Wang, Z.; Li, D.; Yao, X.; Cao, Q.; Zhu, Y.; Cao, W.; Cheng, T. Spectroscopic detection of rice leaf blast infection from asymptomatic to mild stages with integrated machine learning and feature selection. Remote Sens. Environ. 2021, 257, 112350. [Google Scholar] [CrossRef]
- Burchett, S.; Burchett, S. Plant Pathology; Garland Science, Taylor & Francis Group, Cop: New York, NY, USA, 2018. [Google Scholar]
- Cooke, B.M.; Jones, D.G.; Kaye, B. The Epidemiology of Plant Diseases; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Jacquemoud, S.; Baret, F. PROSPECT: A model of leaf optical properties spectra. Remote Sens. Environ. 1990, 34, 75–91. [Google Scholar] [CrossRef]
- Apan, A.; Held, A.; Phinn, S.; Markley, J. Detecting sugarcane orange rust disease using EO-1 Hyperion hyperspectral imagery. Int. J. Remote Sens. 2004, 25, 489–498. [Google Scholar] [CrossRef]
- McLellan, T.M.; Aber, J.D.; Martin, M.E.; Melillo, J.M.; Nadelhoffer, K.J. Determination of nitrogen, lignin, and cellulose content of decomposing leaf material by near infrared reflectance spectroscopy. Can. J. For. Res. 1991, 21, 1684–1688. [Google Scholar] [CrossRef]
- Wang, J.; Shi, T.; Liu, H.; Wu, G. Successive projections algorithm-based three-band vegetation index for foliar phosphorus estimation. Ecol. Indic. 2016, 67, 12–20. [Google Scholar] [CrossRef]
- Cao, Z.; Cheng, T.; Ma, X.; Tian, Y.; Zhu, Y.; Yao, X.; Chen, Q.; Liu, S.; Guo, Z.; Zhen, Q.; et al. A new three-band spectral index for mitigating the saturation in the estimation of leaf area index in wheat. Int. J. Remote Sens. 2017, 38, 3865–3885. [Google Scholar] [CrossRef]
- Pacheco-Labrador, J.; El-Madany, T.S.; van der Tol, C.; Martin, M.P.; Gonzalez-Cascon, R.; Perez-Priego, O.; Guan, J.; Moreno, G.; Carrara, A.; Reichstein, M.; et al. Senscope: Modeling mixed canopies combining green and brown senesced leaves. Evaluation in a Mediterranean Grassland. Remote Sens. Environ. 2021, 257, 112352. [Google Scholar] [CrossRef]
- Ustin, S.L. Remote sensing of canopy chemistry. Proc. Natl. Acad. Sci. USA 2013, 110, 804–805. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Verhoef, W.; Baret, F.; Bacour, C.; Zarco-Tejada, P.J.; Asner, G.P.; François, C.; Ustin, S.L. PROSPECT+SAIL models: A review of use for vegetation characterization. Remote Sens. Environ. 2009, 113, S56–S66. [Google Scholar] [CrossRef]
- Ollinger, S.V. Sources of variability in canopy reflectance and the convergent properties of plants. New Phytol. 2010, 189, 375–394. [Google Scholar] [CrossRef]
- Abdullah, H.; Skidmore, A.K.; Darvishzadeh, R.; Heurich, M. Sentinel-2 accurately maps green-attack stage of European spruce bark beetle (Ips typographus, L.) compared with Landsat-8. Remote Sens. Ecol. Conserv. 2018, 5, 87–106. [Google Scholar] [CrossRef]
- Féret, J.-B.; Gitelson, A.A.; Noble, S.D.; Jacquemoud, S. PROSPECT-D: Towards modeling leaf optical properties through a complete lifecycle. Remote Sens. Environ. 2017, 193, 204–215. [Google Scholar] [CrossRef]
- Mahlein, A.-K.; Alisaac, E.; Al Masri, A.; Behmann, J.; Dehne, H.-W.; Oerke, E.-C. Comparison and Combination of Thermal, Fluorescence, and Hyperspectral Imaging for Monitoring Fusarium Head Blight of Wheat on Spikelet Scale. Sensors 2019, 19, 2281. [Google Scholar] [CrossRef] [PubMed]

| Level | Year | Number of Samples | Sampling Date | Healthy Sample | Infected Sample |
|---|---|---|---|---|---|
| Leaf | 2020 | 148 | 9.11 | 51 | 97 |
| 2021 | 121 | 6.06 | 40 | 81 | |
| Canopy | 2020 | 78 | 9.11 | 31 | 47 |
| 2021 | 101 | 6.06 | 42 | 59 |
| Related | Index | Reference | |
|---|---|---|---|
| Disease stress | RIBInir | [3] | |
| RIBIred | [3] | ||
| RBI | [22] | ||
| Structural index | EVI | [23] | |
| LAIDI | [24] | ||
| MSR | [25] | ||
| MTVI1 | [26] | ||
| NDVI675/750 | [27] | ||
| NRI | [28] | ||
| Pigments | BGI | [29] | |
| Chlrededge | [30] | ||
| RARS | [31] | ||
| PSRI | [32] | ||
| PSSRa | [33] | ||
| LCI | [34] | ||
| Water | SRWI | [35] | |
| Biochemical parameters | CAI | [36] |
| Methods | GRVIRB | Healthy | Infected | UA (%) | OA (%) | Kappa | |
|---|---|---|---|---|---|---|---|
| Training dataset (2021) | SVM | Healthy | 40 | 0 | 100 | 98.35 | 0.97 |
| Infected | 2 | 79 | 97.53 | ||||
| PA (%) | 95.24 | 100 | |||||
| LDA | Healthy | 40 | 0 | 100 | 95.04 | 0.91 | |
| Infected | 6 | 75 | 92.59 | ||||
| PA (%) | 86.96 | 100 | |||||
| Validation dataset (2020) | SVM | Healthy | 27 | 24 | 52.94 | 80.41 | 0.7 |
| Infected | 5 | 92 | 94.85 | ||||
| PA (%) | 84.38 | 79.31 | |||||
| LDA | Healthy | 30 | 21 | 58.82 | 79.73 | 0.67 | |
| Infected | 9 | 88 | 90.72 | ||||
| PA (%) | 76.92 | 80.73 |
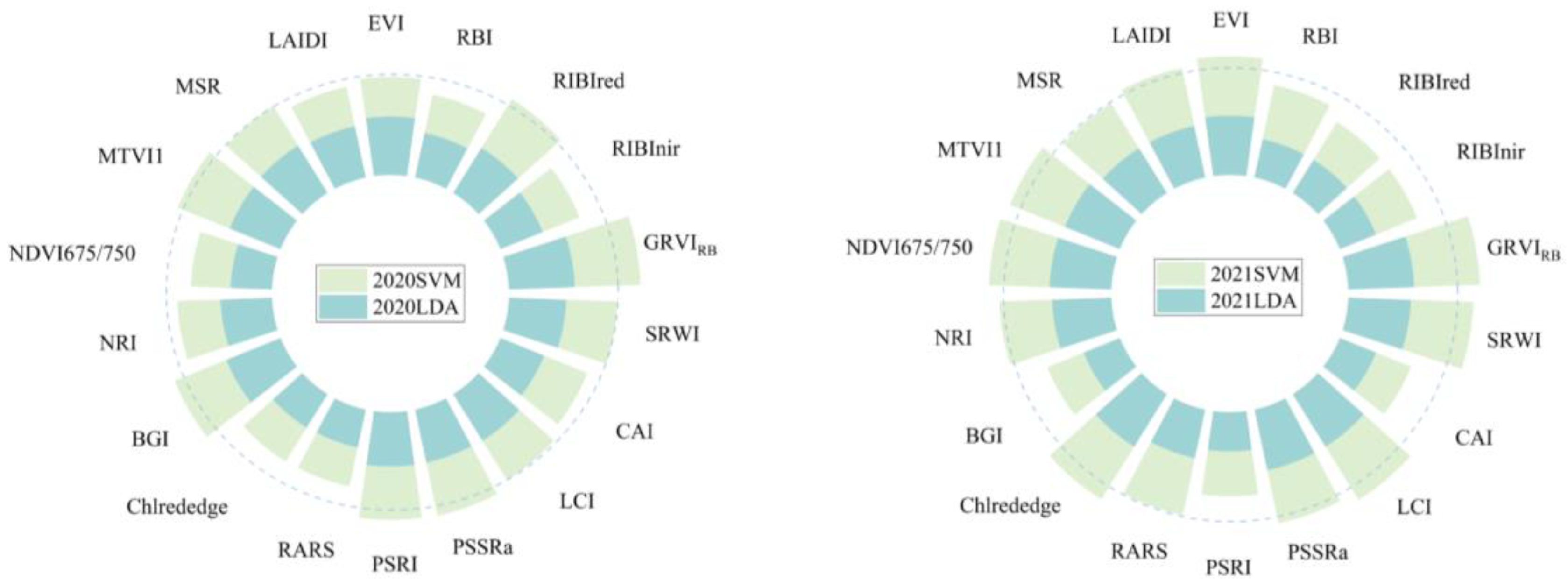
| Year | VIs | Overall Classification Accuracy (%) | |
|---|---|---|---|
| SVM | LDA | ||
| 2021 | GRVIRB | 98.35 | 95.04 |
| RIBIred | 97.52 | 93.39 | |
| MSR | 92.56 | 92.56 | |
| PSSRa | 92.56 | 92.56 | |
| RARS | 83.47 | 85.12 | |
| Chlrededge | 76.39 | 90.91 | |
| EVI | 73.55 | 94.04 | |
| MTVI1 | 71.07 | 94.87 | |
| LAIDI | 70.07 | 85.95 | |
| RIBInir | 69.94 | 92.56 | |
| NRI | 66.94 | 94.21 | |
| RBI | 66.94 | 89.26 | |
| NDVI675/750 | 66.34 | 90.91 | |
| PSRI | 66.94 | 78.51 | |
| SRWI | 66.94 | 94.21 | |
| BGI | 66.94 | 63.64 | |
| CAI | 66.29 | 65.29 | |
| LCI | 66.29 | 80.17 | |
| 2020 | GRVIRB | 80.41 | 79.73 |
| MTVI1 | 74.32 | 79.05 | |
| SRWI | 60.22 | 60.81 | |
| NRI | 65.54 | 61.49 | |
| EVI | 70.12 | 79.05 | |
| RIBIred | 65.54 | 66.22 | |
| RIBInir | 69.94 | 66.22 | |
| MSR | 72.97 | 74.32 | |
| PSSRa | 75.68 | 73.65 | |
| NDVI675/750 | 65.54 | 74.32 | |
| Chlrededge | 65.10 | 78.41 | |
| RBI | 66.72 | 78.32 | |
| RARS | 68.92 | 65.54 | |
| LAIDI | 65.54 | 78.08 | |
| LCI | 65.54 | 72.30 | |
| PSRI | 60.22 | 60.14 | |
| CAI | 68.92 | 68.54 | |
| BGI | 65.54 | 65.54 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Q.; Chen, Y.; Xia, Q.; Zhang, Y.; Li, D.; Jiang, H.; Wang, C.; Zhao, L.; Huang, W.; Dong, Y.; et al. New Hyperspectral Geometry Ratio Index for Monitoring Rice Blast Disease from Leaf Scale to Canopy Scale. Remote Sens. 2024, 16, 4681. https://doi.org/10.3390/rs16244681
Zheng Q, Chen Y, Xia Q, Zhang Y, Li D, Jiang H, Wang C, Zhao L, Huang W, Dong Y, et al. New Hyperspectral Geometry Ratio Index for Monitoring Rice Blast Disease from Leaf Scale to Canopy Scale. Remote Sensing. 2024; 16(24):4681. https://doi.org/10.3390/rs16244681
Chicago/Turabian StyleZheng, Qiong, Yihao Chen, Qing Xia, Yunfei Zhang, Dan Li, Hao Jiang, Chongyang Wang, Longlong Zhao, Wenjiang Huang, Yingying Dong, and et al. 2024. "New Hyperspectral Geometry Ratio Index for Monitoring Rice Blast Disease from Leaf Scale to Canopy Scale" Remote Sensing 16, no. 24: 4681. https://doi.org/10.3390/rs16244681
APA StyleZheng, Q., Chen, Y., Xia, Q., Zhang, Y., Li, D., Jiang, H., Wang, C., Zhao, L., Huang, W., Dong, Y., & Wang, C. (2024). New Hyperspectral Geometry Ratio Index for Monitoring Rice Blast Disease from Leaf Scale to Canopy Scale. Remote Sensing, 16(24), 4681. https://doi.org/10.3390/rs16244681











