Selenium Analysis and Speciation in Dietary Supplements Based on Next-Generation Selenium Ingredients
Abstract
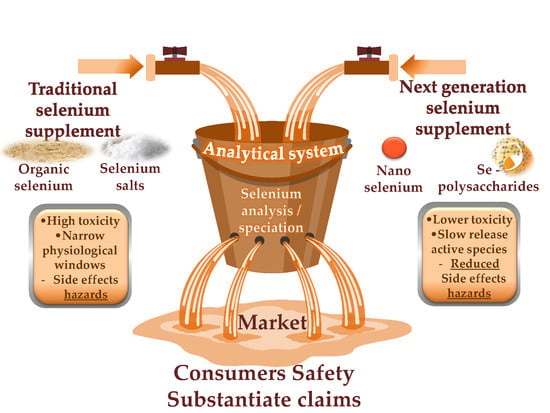
:1. Introduction
2. Conventional and Next-Generation Selenium Dietary Supplements
3. Analytical Methods for Selenium Detection and Speciation
3.1. Total Selenium
3.2. Inorganic Selenium
3.3. Organic Selenium
3.4. Selenium Next-Generation Ingredients
- In a single analysis using quadrupole instruments, which is the most common type of an ICP-MS instrument, it can measure only one isotope or maximum two isotopes;
- The nanoparticle size detection limit (LOD), expressed as the equivalent spherical diameter, ranges from 10 nm to 20 nm for monoisotopic nanoparticles.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1D SDS-PAGE | one-dimensional sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| A549 | Adenocarcinomic human alveolar basal epithelial cells |
| AAS | atomic absorption spectroscopy |
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) |
| AF4 | asymmetrical flow field-flow fractionation |
| AFM | atomic force microscopy |
| AFS | atomic fluorescence spectroscopy |
| AKI | acute kidney injuring |
| BALB/c | albino mice used in research |
| BGC823 | gastric cancer cell line |
| BMHT | betaine homocysteine methyltransferase |
| BSA | bovine serum albumin |
| CBIMMT | 4-(4′-chlorobenzylideneimino)-3-methyl-5-mercapto-1,2,4-triazole |
| Cys | cysteine |
| DAD | diode array detector |
| DLS | dynamic light scattering |
| DNA | deoxyribonucleic acid |
| DNMTs | DNA methyltransferases |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DS | dietary supplements |
| EELS | electron energy-loss spectroscopy |
| EFSA | European Food Safety Authority |
| ESI-MS | electrospray ionization mass spectrometry |
| ET-AAS | electrothermal atomic absorption spectroscopy |
| EXAFS | Extended X-ray absorption fine structure |
| FAAS | flame atomic absorption spectroscopy |
| FFF | (a)symmetrical flow field-flow fractionation |
| FRAP | ferric ion reducing antioxidant power |
| FRAPS | fluorescence recovery after photobleaching |
| FTIR | Fourier-transform infrared spectroscopy |
| GC | gas chromatography |
| GF-AAS | graphite furnace atomic absorption spectroscopy |
| GPX | glutathione peroxidase |
| H22 | murine hepatoma cell line |
| HA | humic acid |
| HCCLM9 | hepatocellular carcinoma cell line |
| HDACc | histone deacetylase |
| HEC | hydroxyethylcellulose |
| HeLa | cell line derived from cervical cancer cells |
| Hep G2 | human hepatocyte carcinoma cell |
| HG | hydride generation |
| HILIC | hydrophilic ion interaction chromatography |
| HPLC–HG-AAS | high-performance liquid chromatography hydride generation atomic absorption spectrometry |
| HR-CS | high-resolution continuous source |
| ICP-MS | inductively coupled plasma mass spectrometry |
| ICP-OES | inductively coupled plasma optical emission spectrometry |
| ICP-TOF-MS | inductively coupled plasma time-of-flight mass spectrometry |
| ICR | Institute of Cancer Research |
| IEF | isoelectric focusing electrophoresis |
| INAA | instrumental neutron activation analysis |
| iSE | inorganic selenium |
| LA-ICP-MS | laser ablation inductively coupled plasma mass spectrometry |
| LC–MS | liquid chromatography–mass spectrometry |
| LOD | limit of detection |
| MALS | multiple-angle light scattering |
| Met | methione |
| SeMet | selenomethionine |
| MeSeCys | methylselenocysteine |
| MeSeCys | methylselenocysteine |
| MCF-7 | human breast cancer cell line from Michigan Cancer Foundation |
| MDCK | Madin-Darby Canine Kidney cells |
| MP-AES | microwave plasma atomic emission spectrometry |
| MS | mass spectrometry |
| MSeA | methylseleninic acid |
| NOAEL | no observed adverse effect level |
| NPs | (selenium) nanoparticles |
| OTC | over-the-counter |
| PAGE | polyacrylamide gel electrophoresis |
| PC3 | prostate cancer cell line |
| photo-CVG | photochemical vapor generation |
| RDA | recommended daily allowance |
| SD | Sprague/Dawley (rat strain) |
| SDS | sodium dodecyl sulfate |
| SeCys | selenocysteine |
| SeCys2 | selenocystine |
| SEC | size-exclusion chromatography |
| SeCys | selenocysteine |
| SeHLan | selenohomolanthionine |
| SEM | scanning electron microscopy |
| SeMet | selenomethionine |
| SeNPs | selenium nanoparticles |
| SH-SY5Y | bone marrow neuroblast cell line |
| siRNA | small interfering ribonucleic acid |
| SPs | selenized polysaccharides |
| SP-ICP-MS | single-particle inductively coupled plasma mass spectrometry |
| TEM | transmission electron microscopy |
| TrxR | thioredoxin reductase |
| TXRF | total reflection X-ray fluorescence spectroscopy |
| UL | tolerable upper limit |
| US | Unites States |
| U.S. EPA | United States Environmental Protection Agency |
| UV-PVG | ultraviolet photochemical vapor generation system |
| XANES | X-ray absorption near-edge structure |
| XFA | X-ray fluorescence analysis |
| XRD | X-ray diffraction |
References
- Oldfield, J.E. The two faces of selenium. J. Nutr. 1987, 117, 2002–2008. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.H.; Benvenga, S. Selenium: An element for life. Endocrine 2015, 48, 756–775. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, E.; Vinceti, M. Selenium and human health: Witnessing a copernican revolution? J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2015, 33, 328–368. [Google Scholar] [CrossRef] [PubMed]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [PubMed]
- Rocourt, C.; Cheng, W.-H. Selenium supranutrition: Are the potential benefits of chemoprevention outweighed by the promotion of diabetes and insulin resistance? Nutrients 2013, 5, 1349–1365. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Filippini, T.; Del Giovane, C.; Dennert, G.; Zwahlen, M.; Brinkman, M.; Zeegers, M.P.A.; Horneber, M.; D’Amico, R.; Crespi, C.M. Selenium for preventing cancer. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Speckmann, B.; Sies, H. Toward understanding success and failures in the use of selenium for cancer prevention. Antioxid. Redox Signal. 2013, 19, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Brozmanová, J.; Mániková, D.; Vlčková, V.; Chovanec, M. Selenium: A double-edged sword for defense and offence in cancer. Arch. Toxicol. 2010, 84, 919–938. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L.; Hughes, D.J. The missing link? The potential role of selenium in the development of liver cancer and significance for the general population. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Rimm, E.B.; Siscovick, D.S.; Spiegelman, D.; Manson, J.E.; Morris, J.S.; Hu, F.B.; Mozaffarian, D. Toenail selenium and incidence of type 2 diabetes in U.S. Men and women. Diabetes Care 2012, 35, 1544–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayman, M.P.; Stranges, S. Epidemiology of selenium and type 2 diabetes: Can we make sense of it? Free Radic. Biol. Med. 2013, 65, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Solovyev, N.D. Importance of selenium and selenoprotein for brain function: From antioxidant protection to neuronal signalling. J. Inorg. Biochem. 2015, 153, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.R.; Roberts, B.R.; Bush, A.I.; Hare, D.J. Selenium, selenoproteins and neurodegenerative diseases. Metallomics 2015, 7, 1213–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillai, R.; Uyehara-Lock, J.H.; Bellinger, F.P. Selenium and selenoprotein function in brain disorders. IUBMB Life 2014, 66, 229–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solovyev, N.; Drobyshev, E.; Bjørklund, G.; Dubrovskii, Y.; Lysiuk, R.; Rayman, M.P. Selenium, selenoprotein p, and alzheimer’s disease: Is there a link? Free Radic. Biol. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Aaseth, J.; Alexander, J.; Bjørklund, G.; Hestad, K.; Dusek, P.; Roos, P.M.; Alehagen, U. Treatment strategies in alzheimer’s disease: A review with focus on selenium supplementation. BioMetals 2016, 29, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Mandrioli, J.; Borella, P.; Michalke, B.; Tsatsakis, A.; Finkelstein, Y. Selenium neurotoxicity in humans: Bridging laboratory and epidemiologic studies. Toxicol. Lett. 2014, 230, 295–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinceti, M.; Chiari, A.; Eichmüller, M.; Rothman, K.J.; Filippini, T.; Malagoli, C.; Weuve, J.; Tondelli, M.; Zamboni, G.; Nichelli, P.F.; et al. A selenium species in cerebrospinal fluid predicts conversion to alzheimer’s dementia in persons with mild cognitive impairment. Alzheimers Res. Ther. 2017, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Stoffaneller, R.; Morse, N.L. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.D. Selenium and iodine intakes and status in New Zealand and Australia. Br. J. Nutr. 2004, 91, 661–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Guan, X.; Yang, M.; Zeng, L.; Liu, C. Roles and potential mechanisms of selenium in countering thyrotoxicity of dehp. Sci. Total Environ. 2018, 619–620, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Arnaudguilhem, C.; Bierla, K.; Ouerdane, L.; Preud’homme, H.; Yiannikouris, A.; Lobinski, R. Selenium metabolomics in yeast using complementary reversed-phase/hydrophilic ion interaction (hilic) liquid chromatography–electrospray hybrid quadrupole trap/orbitrap mass spectrometry. Anal. Chim. Acta 2012, 757, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Fagan, S.; Owens, R.; Ward, P.; Connolly, C.; Doyle, S.; Murphy, R. Biochemical comparison of commercial selenium yeast preparations. Biol. Trace Elem. Res. 2015, 166, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Levander, O.A.; Burk, R.F. Update of human dietary standards for selenium. In Selenium: Its Molecular Biology and Role in Human Health; Hatfield, D.L., Berry, M.J., Gladyshev, V.N., Eds.; Springer: Boston, MA, USA, 2006; pp. 399–410. ISBN 978–0-387-33827-9. [Google Scholar]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H. Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Institute of Medicine, Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; The National Academies Press: Washington, DC, USA, 2000; p. 529.
- Rayman, M.P.; Winther, K.H.; Pastor-Barriuso, R.; Cold, F.; Thvilum, M.; Stranges, S.; Guallar, E.; Cold, S. Effect of long-term selenium supplementation on mortality: Results from a multiple-dose, randomised controlled trial. Free Radic. Biol. Med. 2018, 127, 46–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin e on risk of prostate cancer and other cancers: The selenium and vitamin e cancer prevention trial (select). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Speckmann, B.; Grune, T. Epigenetic effects of selenium and their implications for health. Epigenetics 2015, 10, 179–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, J.; Hu, X.; Ryan Smith, M.; Go, Y.-M.; Jones, D.P. Selenium at the redox interface of the genome, metabolome and exposome. Free Radic. Biol. Med. 2018, 127, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Benstoem, C.; Goetzenich, A.; Kraemer, S.; Borosch, S.; Manzanares, W.; Hardy, G.; Stoppe, C. Selenium and its supplementation in cardiovascular disease—What do we know? Nutrients 2015, 7, 3094–3118. [Google Scholar] [CrossRef] [PubMed]
- Febiyanto, N.; Yamazaki, C.; Kameo, S.; Sari, D.K.; Puspitasari, I.M.; Sunjaya, D.K.; Herawati, D.M.D.; Nugraha, G.I.; Fukuda, T.; Koyama, H. Effects of selenium supplementation on the diabetic condition depend on the baseline selenium status in kkay mice. Biol. Trace Elem. Res. 2018, 181, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Kristal, A.R.; Darke, A.K.; Morris, J.S.; Tangen, C.M.; Goodman, P.J.; Thompson, I.M.; Meyskens, F.L.; Goodman, G.E.; Minasian, L.M.; Parnes, H.L. Baseline selenium status and effects of selenium and vitamin e supplementation on prostate cancer risk. J. Natl. Cancer Inst. 2014, 106, 456. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Vanderlelie, J.J.; Perkins, A.V.; Redman, C.W.; Ahmadi, K.R.; Rayman, M.P. Genetic polymorphisms that affect selenium status and response to selenium supplementation in United Kingdom pregnant women. Am. J. Clin. Nutr. 2015, 103, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skalickova, S.; Milosavljevic, V.; Cihalova, K.; Horky, P.; Richtera, L.; Adam, V. Selenium nanoparticles as a nutritional supplement. Nutrition 2017, 33, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, C.; Tian, C.; Ren, Z.; Song, X.; Wang, X.; Xu, N.; Jing, H.; Li, S.; Liu, W.; et al. Characterization, antioxidation, anti-inflammation and renoprotection effects of selenized mycelia polysaccharides from oudemansiella radicata. Carbohydr. Polym. 2018, 181, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lamana, J.; Abad-Alvaro, I.; Bierla, K.; Laborda, F.; Szpunar, J.; Lobinski, R. Detection and characterization of biogenic selenium nanoparticles in selenium-rich yeast by single particle icpms. J. Anal. At. Spectrom. 2018, 33, 452–460. [Google Scholar] [CrossRef]
- Bhatia, P.; Aureli, F.; D’Amato, M.; Prakash, R.; Cameotra, S.S.; Nagaraja, T.P.; Cubadda, F. Selenium bioaccessibility and speciation in biofortified Pleurotus mushrooms grown on selenium-rich agricultural residues. Food chem. 2013, 140, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jing, H.; Zhang, J.; Che, G.; Zhou, M.; Gao, Z.; Li, S.; Ren, Z.; Hao, L.; Liu, Y.; et al. Optimization of mycelia selenium polysaccharide extraction from agrocybe cylindracea sl-02 and assessment of their antioxidant and anti-ageing activities. PLoS ONE 2016, 11, e0160799. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-l.; Yang, T.-b.; Wei, J.; Lei, G.-h.; Zeng, C. Association between serum selenium level and type 2 diabetes mellitus: A non-linear dose–response meta-analysis of observational studies. Nutr. J. 2016, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Méplan, C. Selenium and chronic diseases: A nutritional genomics perspective. Nutrients 2015, 7, 3621–3651. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Wang, C.; Yu, W.; Fan, W.; Wang, S.; Shen, N.; Wu, P.; Li, X.; Wang, F. Selenium exposure and cancer risk: An updated meta-analysis and meta-regression. Sci. Rep. 2016, 6, 19213. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Liu, C.; Guo, J.; Song, Y. Selenium status and cardiovascular diseases: Meta-analysis of prospective observational studies and randomized controlled trials. Eur. J. Clin. Nutr. 2015, 70, 162. [Google Scholar] [CrossRef] [PubMed]
- Helzlsouer, K.; Jacobs, R.; Morris, S. Acute selenium intoxication in the United-States. Fed. Proc. 1985, 44, 1670. [Google Scholar]
- Clark, R.F.; Strukle, E.; Williams, S.R.; Manoguerra, A.S. Selenium poisoning from a nutritional supplement. JAMA 1996, 275, 1087–1088. [Google Scholar] [CrossRef] [PubMed]
- MacFarquhar, J.K.; Broussard, D.L.; Melstrom, P.; Hutchinson, R.; Wolkin, A.; Martin, C.; Burk, R.F.; Dunn, J.R.; Green, A.L.; Hammond, R.; et al. Acute selenium toxicity associated with a dietary supplement. Arch. Intern. Med. 2010, 170, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.; Crane, S. Selenium toxicity from a misformulated dietary supplement, adverse health effects, and the temporal response in the nail biologic monitor. Nutrients 2013, 5, 1024–1057. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.R.; Jarrett, J.M.; Tevis, D.S.; Franklin, M.; Mullinix, N.J.; Wallon, K.L.; Derrick Quarles, C.; Caldwell, K.L.; Jones, R.L. Analysis of whole human blood for Pb, Cd, Hg, Se, and Mn by ICP-DRC-MS for biomonitoring and acute exposures. Talanta 2017, 162, 114–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalke, B.; Willkommen, D.; Drobyshev, E.; Solovyev, N. The importance of speciation analysis in neurodegeneration research. Trends Anal. Chem. 2017. [Google Scholar] [CrossRef]
- Schmidt, L.; Figueroa, J.A.L.; Dalla Vecchia, P.; Duarte, F.A.; Mello, P.A.; Caruso, J.A.; Flores, E.M.M. Bioavailability of hg and se from seafood after culinary treatments. Microchem. J. 2018, 139, 363–371. [Google Scholar] [CrossRef]
- Lazard, M.; Dauplais, M.; Blanquet, S.; Plateau, P. Recent advances in the mechanism of selenoamino acids toxicity in eukaryotic cells. Biomol. Concepts 2017, 8, 93–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plateau, P.; Saveanu, C.; Lestini, R.; Dauplais, M.; Decourty, L.; Jacquier, A.; Blanquet, S.; Lazard, M. Exposure to selenomethionine causes selenocysteine misincorporation and protein aggregation in saccharomyces cerevisiae. Sci. Rep. 2017, 7, 44761. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Yeon Chun, J.; Nadiminty, N.; Trump, D.L.; Ip, C.; Dong, Y.; Gao, A.C. Monomethylated selenium inhibits growth of lncap human prostate cancer xenograft accompanied by a decrease in the expression of androgen receptor and prostate-specific antigen (PSA). Prostate 2006, 66, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Abdulah, R.; Miyazaki, K.; Nakazawa, M.; Koyama, H. Chemical forms of selenium for cancer prevention. J. Trace Elem. Med. Biol. 2005, 19, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, M.; Konieczka, P. Evaluation of candidate reference material obtained from selenium-enriched sprouts for the purpose of selenium speciation analysis. LWT-Food Sci. Technol. 2016, 70, 286–295. [Google Scholar] [CrossRef]
- Ellis, D.R.; Salt, D.E. Plants, selenium and human health. Curr. Opin. Plant Biol. 2003, 6, 273–279. [Google Scholar] [CrossRef]
- Misra, S.; Boylan, M.; Selvam, A.; Spallholz, J.E.; Björnstedt, M. Redox-active selenium compounds—From toxicity and cell death to cancer treatment. Nutrients 2015, 7, 3536–3556. [Google Scholar] [CrossRef] [PubMed]
- Pyrzyńska, K. Speciation analysis of some organic selenium compounds. A review. Analyst 1996, 121, 77R–83R. [Google Scholar] [CrossRef]
- Uden, P.C.; Boakye, H.T.; Kahakachchi, C.; Hafezi, R.; Nolibos, P.; Block, E.; Johnson, S.; Tyson, J.F. Element selective characterization of stability and reactivity of selenium species in selenized yeast. J. Anal. At. Spectrom. 2004, 19, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Amoako, P.O.; Uden, P.C.; Tyson, J.F. Speciation of selenium dietary supplements; formation of S-(methylseleno)cysteine and other selenium compounds. Anal. Chim. Acta 2009, 652, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N. Nutritional selenium supplements: Product types, quality, and safety. J. Am. Coll. Nutr. 2001, 20, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, F.; Charrondiere, U.R.; Dusemund, B.; Galtier, P.; Gilbert, J.; Gott, D.M.; Grilli, S.; Guertler, R.; Kass, G.E.N.; Koenig, J.; et al. Inability to assess the safety of selenium amino acid chelate added for nutritional purposes as a source of selenium in food supplements and the bioavailability of selenium from this source based on the supporting dossier—Scientific statement of the panel on food additives and nutrient sources added to food (ANS). EFSA J. 2009, 7, 952. [Google Scholar] [CrossRef]
- Clark, L.C.; Combs, G.F., Jr.; Turnbull, B.W.; Slate, E.H.; Chalker, D.K.; Chow, J.; Davis, L.S.; Glover, R.A.; Graham, G.F.; Gross, E.G.; et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin: A randomized controlled trial. JAMA 1996, 276, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Ponton, D.E.; Hare, L. Relating selenium concentrations in a planktivore to selenium speciation in lakewater. Environ. Pollut. 2013, 176, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Li, S.; Guo, J.; Rao, Z.; Liu, C.; Jia, Z.; Suo, D.; Wang, S.; Li, Y.; Fan, X. Application of enzymatic probe sonication for selenium speciation in animal feeds. J. Chromatogr. A 2017, 1530, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Natasha; Shahid, M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar] [CrossRef] [PubMed]
- Gosetti, F.; Frascarolo, P.; Polati, S.; Medana, C.; Gianotti, V.; Palma, P.; Aigotti, R.; Baiocchi, C.; Gennaro, M.C. Speciation of selenium in diet supplements by hplc–ms/ms methods. Food Chem. 2007, 105, 1738–1747. [Google Scholar] [CrossRef]
- Kubachka, K.M.; Hanley, T.; Mantha, M.; Wilson, R.A.; Falconer, T.M.; Kassa, Z.; Oliveira, A.; Landero, J.; Caruso, J. Evaluation of selenium in dietary supplements using elemental speciation. Food Chem. 2017, 218, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Bierla, K.; Godin, S.; Lobinski, R.; Szpunar, J. Advances in electrospray mass spectrometry for the selenium speciation: Focus on Se-rich yeast. Trends Anal. Chem. 2018, 104, 87–94. [Google Scholar] [CrossRef]
- Sakr, T.M.; Korany, M.; Katti, K.V. Selenium nanomaterials in biomedicine—An overview of new opportunities in nanomedicine of selenium. J. Drug Deliv. Sci. Technol. 2018, 46, 223–233. [Google Scholar] [CrossRef]
- Du, X.; Chao, W.; Qiong, L. Potential roles of selenium and selenoproteins in the prevention of Alzheimer’s disease. Curr. Top. Med. Chem. 2015, 16, 835–848. [Google Scholar] [CrossRef]
- Mistry, H.D.; Broughton Pipkin, F.; Redman, C.W.G.; Poston, L. Selenium in reproductive health. Am. J. Obstet. Gynecol. 2012, 206, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khiralla, G.M.; El-Deeb, B.A. Antimicrobial and antibiofilm effects of selenium nanoparticles on some foodborne pathogens. LWT-Food Sci. Technol. 2015, 63, 1001–1007. [Google Scholar] [CrossRef]
- Gangadoo, S.; Dinev, I.; Chapman, J.; Hughes, R.J.; Van, T.T.H.; Moore, R.J.; Stanley, D. Selenium nanoparticles in poultry feed modify gut microbiota and increase abundance of Faecalibacterium prausnitzii. Appl. Microbiol. Biotechnol. 2018, 102, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, S.; Epifano, F.; Preziuso, F.; Taddeo, V.A.; Genovese, S. Selenylated plant polysaccharides: A survey of their chemical and pharmacological properties. Phytochemistry 2018, 153, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.H.; Encinar, J.R.; Marchante-Gayón, J.M.; Alonso, J.I.G.; Sanz-Medel, A. Selenium bioaccessibility assessment in selenized yeast after “in vitro” gastrointestinal digestion using two-dimensional chromatography and mass spectrometry. J. Chromatogr. A 2006, 1110, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.D. Selenium speciation in human body fluids. Analyst 1998, 123, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Tiwary, A.K.; Stegelmeier, B.L.; Panter, K.E.; James, L.F.; Hall, J.O. Comparative toxicosis of sodium selenite and selenomethionine in lambs. J. Vet. Diagn. Investig. 2006, 18, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Berntssen, M.H.G.; Sundal, T.K.; Olsvik, P.A.; Amlund, H.; Rasinger, J.D.; Sele, V.; Hamre, K.; Hillestad, M.; Buttle, L.; Ørnsrud, R. Sensitivity and toxic mode of action of dietary organic and inorganic selenium in atlantic salmon (Salmo salar). Aquat. Toxicol. 2017, 192, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Herrero Latorre, C.; Barciela García, J.; García Martín, S.; Peña Crecente, R.M. Solid phase extraction for the speciation and preconcentration of inorganic selenium in water samples: A review. Anal. Chim. Acta 2013, 804, 37–49. [Google Scholar] [CrossRef] [PubMed]
- López de Arroyabe Loyo, R.; Nikitenko, S.I.; Scheinost, A.C.; Simonoff, M. Immobilization of selenite on Fe3O4 and Fe/Fe3c ultrasmall particles. Environ. Sci. Technol. 2008, 42, 2451–2456. [Google Scholar] [CrossRef]
- Niedzielski, P.; Rudnicka, M.; Wachelka, M.; Kozak, L.; Rzany, M.; Wozniak, M.; Kaskow, Z. Selenium species in selenium fortified dietary supplements. Food chem. 2016, 190, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Bierła, K.; Suzuki, N.; Ogra, Y.; Szpunar, J.; Łobiński, R. Identification and determination of selenohomolanthionine – the major selenium compound in torula yeast. Food chem. 2017, 237, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Gagandeep, K.; Anu, K.; Sodhi, H.S. Selenium biofortification of pleurotus species and its effect on yield, phytochemical profiles, and protein chemistry of fruiting bodies. J. Food Biochem. 2018, 42, e12467. [Google Scholar] [CrossRef]
- Da Silva, M.C.S.; Naozuka, J.; da Luz, J.M.R.; de Assunção, L.S.; Oliveira, P.V.; Vanetti, M.C.D.; Bazzolli, D.M.S.; Kasuya, M.C.M. Enrichment of pleurotus ostreatus mushrooms with selenium in coffee husks. Food Chem. 2012, 131, 558–563. [Google Scholar] [CrossRef]
- Maseko, T.; Callahan, D.L.; Dunshea, F.R.; Doronila, A.; Kolev, S.D.; Ng, K. Chemical characterisation and speciation of organic selenium in cultivated selenium-enriched Agaricus bisporus. Food Chem. 2013, 141, 3681–3687. [Google Scholar] [CrossRef] [PubMed]
- Mleczek, M.; Siwulski, M.; Stuper-Szablewska, K.; Rissmann, I.; Sobieralski, K.; Goliński, P. Accumulation of elements by edible mushroom species: Part i. Problem of trace element toxicity in mushrooms. J. Environ. Sci. Health B 2013, 48, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Borovička, J. Macro and trace mineral constituents and radionuclides in mushrooms: Health benefits and risks. Appl. Microbiol. Biotechnol. 2013, 97, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Pieniz, S.; Andreazza, R.; Mann, M.B.; Camargo, F.; Brandelli, A. Bioaccumulation and distribution of selenium in Enterococcus durans. J. Trace Elem. Med. Biol. 2017, 40, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.H.; Wang, T.; Regmi, N.; Chen, X.; Huang, K.; Liao, S.F. Effects of dietary supplementation of selenium-enriched probiotics on production performance and intestinal microbiota of weanling piglets raised under high ambient temperature. J. Anim. Physiol. Anim. Nutr. (Berl.) 2015, 99, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Gan, F.; Chen, X.; Liao, S.F.; Lv, C.; Ren, F.; Ye, G.; Pan, C.; Huang, D.; Shi, J.; Shi, X.; et al. Selenium-enriched probiotics improve antioxidant status, immune function, and selenoprotein gene expression of piglets raised under high ambient temperature. J. Agric. Food Chem. 2014, 62, 4502–4508. [Google Scholar] [CrossRef] [PubMed]
- Kheradmand, E.; Rafii, F.; Yazdi, M.H.; Sepahi, A.A.; Shahverdi, A.R.; Oveisi, M.R. The antimicrobial effects of selenium nanoparticle-enriched probiotics and their fermented broth against candida albicans. Daru 2014, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Nido, S.A.; Shituleni, S.A.; Mengistu, B.M.; Liu, Y.; Khan, A.Z.; Gan, F.; Kumbhar, S.; Huang, K. Effects of selenium-enriched probiotics on lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in mice fed a high-fat diet. Biol. Trace Elem. Res. 2016, 171, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Kojouri, G.A.; Sadeghian, S.; Mohebbi, A.; Mokhber Dezfouli, M.R. The effects of oral consumption of selenium nanoparticles on chemotactic and respiratory burst activities of neutrophils in comparison with sodium selenite in sheep. Biol. Trace Elem. Res. 2012, 146, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Gao, X.Y.; Zhang, L.D.; Bao, Y.P. Biological effects of a nano red elemental selenium. Biofactors 2001, 15, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Shakibaie, M.; Shahverdi, A.R.; Faramarzi, M.A.; Hassanzadeh, G.R.; Rahimi, H.R.; Sabzevari, O. Acute and subacute toxicity of novel biogenic selenium nanoparticles in mice. Pharm. Biol. 2013, 51, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Li, N.; Chen, J. A subchronic toxicity study of elemental nano-Se in sprague-dawley rats. Life Sci. 2005, 76, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Sarin, L.; Sanchez, V.C.; Yan, A.; Kane, A.B.; Hurt, R.H. Selenium-carbon bifunctional nanoparticles for the treatment of malignant mesothelioma. Adv. Mater. 2010, 22, 5207–5211. [Google Scholar] [CrossRef]
- Chen, W.; Li, Y.; Yang, S.; Yue, L.; Jiang, Q.; Xia, W. Synthesis and antioxidant properties of chitosan and carboxymethyl chitosan-stabilized selenium nanoparticles. Carbohydr. Polym. 2015, 132, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhai, X.; Zhao, G.; Ren, F.; Leng, X. Synthesis, characterization, and controlled release of selenium nanoparticles stabilized by chitosan of different molecular weights. Carbohydr. Polym. 2015, 134, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.; Hong, B.; Hong, Z.; Sun, J.; Wang, C. Selenium nanoparticles-loaded chitosan/citrate complex and its protection against oxidative stress in d-galactose-induced aging mice. J. Nanobiotechnol. 2017, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Estevez, H.; Garcia-Lidon, J.C.; Luque-Garcia, J.L.; Camara, C. Effects of chitosan-stabilized selenium nanoparticles on cell proliferation, apoptosis and cell cycle pattern in hepg2 cells: Comparison with other selenospecies. Colloids Surf. B Biointerfaces 2014, 122, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Ping, Z.; Liu, T.; Xu, H.; Meng, Y.; Li, W.; Xu, X.; Zhang, L. Construction of highly stable selenium nanoparticles embedded in hollow nanofibers of polysaccharide and their antitumor activities. Nano Res. 2017, 10, 3775–3789. [Google Scholar] [CrossRef]
- Liao, W.; Yu, Z.; Lin, Z.; Lei, Z.; Ning, Z.; Regenstein, J.M.; Yang, J.; Ren, J. Biofunctionalization of selenium nanoparticle with dictyophora indusiata polysaccharide and its antiproliferative activity through death-receptor and mitochondria-mediated apoptotic pathways. Sci. Rep. 2015, 5, 18629. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, S.; Liu, Y.; Wu, W.; Shen, Y.; Zhang, L.; Li, C.; Chen, H.; Liu, A.; Shen, L.; et al. Synthesis and antidiabetic activity of selenium nanoparticles in the presence of polysaccharides from Catathelasma ventricosum. Int. J. Biol. Macromol. 2018, 114, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, J.; Ding, D.; Zhang, L.; Muehlmann, L.A.; Deng, S.E.; Wang, X.; Li, W.; Zhang, W. Synthesis and antioxidant properties of lycium barbarum polysaccharides capped selenium nanoparticles using tea extract. Artif. Cells Nanomed. Biotechnol. 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, S.; Song, C.; Zhang, Y.; Ling, Q.; Hoffmann, P.R.; Li, J.; Chen, T.; Zheng, W.; Huang, Z. Selenium nanoparticles decorated with ulva lactuca polysaccharide potentially attenuate colitis by inhibiting NF-κB mediated hyper inflammation. J. Nanobiotechnol. 2017, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Nie, Y.; Wang, Z.; Hua, C.; Wang, R.; Gao, J. Biomacromolecule-directed synthesis and characterization of selenium nanoparticles and their compatibility with bacterial and eukaryotic cells. Nanosci. Nanotechnol. Lett. 2017, 9, 1987–1991. [Google Scholar] [CrossRef]
- Liu, C.; Fu, Y.; Li, C.-e.; Chen, T.; Li, X. Phycocyanin-functionalized selenium nanoparticles reverse palmitic acid-induced pancreatic β cell apoptosis by enhancing cellular uptake and blocking reactive oxygen species (ROS)-mediated mitochondria dysfunction. J. Agric. Food Chem. 2017, 65, 4405–4413. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yang, L.; Wang, Y.; Wang, G.; Rensing, C.; Zheng, S. Proteins enriched in charged amino acids control the formation and stabilization of selenium nanoparticles in comamonas testosteroni s44. Sci. Rep. 2018, 8, 4766. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Ma, Y.; Zhuang, M.; Luo, T.; Wang, Y.; Hong, A. Chitosan-decorated selenium nanoparticles as protein carriers to improve the in vivo half-life of the peptide therapeutic bay 55-9837 for type 2 diabetes mellitus. Int. J. Nanomed. 2014, 9, 4819–4828. [Google Scholar] [CrossRef]
- Wu, H.; Li, X.; Liu, W.; Chen, T.; Li, Y.; Zheng, W.; Man, C.W.-Y.; Wong, M.-K.; Wong, K.-H. Surface decoration of selenium nanoparticles by mushroom polysaccharides-protein complexes to achieve enhanced cellular uptake and antiproliferative activity. J. Mater. Chem. 2012, 22, 9602–9610. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, H.; Li, X.; Liu, Z.; Zheng, W.; Chen, T.; Yu, B.; Wong, K.-H. Induction of apoptosis and cell cycle arrest in a549 human lung adenocarcinoma cells by surface-capping selenium nanoparticles: An effect enhanced by polysaccharide–protein complexes from Polyporus rhinocerus. J. Agric. Food Chem. 2013, 61, 9859–9866. [Google Scholar] [CrossRef] [PubMed]
- Prateeksha; Singh, B.R.; Shoeb, M.; Sharma, S.; Naqvi, A.H.; Gupta, V.K.; Singh, B.N. Scaffold of selenium nanovectors and honey phytochemicals for inhibition of pseudomonas aeruginosa quorum sensing and biofilm formation. Front. Cell. Infect. Microbiol. 2017, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, Z.; Zhong, C.; Zhou, Y.; Bai, Y. Modulation of calcium oxalate crystallization by colloidal selenium nanoparticles–polyphenol complex. Cryst. Growth Des. 2016, 16, 2581–2589. [Google Scholar] [CrossRef]
- Mittal, A.K.; Kumar, S.; Banerjee, U.C. Quercetin and gallic acid mediated synthesis of bimetallic (silver and selenium) nanoparticles and their antitumor and antimicrobial potential. J. Colloid Interface Sci. 2014, 431, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Yan, C.; Miao, J.; Zhang, X.; Chen, J.; Sun, L.; Meng, L.; Liang, T.; Li, Q. Synthesis, characterization and antitumor properties of selenium nanoparticles coupling with ferulic acid. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Yang, L.; Liu, Y.; Zhou, X.; Sun, J.; Liu, J. Sialic acid (SA)-modified selenium nanoparticles coated with a high blood–brain barrier permeability peptide-b6 peptide for potential use in Alzheimer’s disease. Acta Biomater. 2015, 25, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-S.; Zheng, S.-Y.; Zhang, Y.-B.; Yu, B.; Zheng, W.; Yang, F.; Chen, T. Sialic acid surface decoration enhances cellular uptake and apoptosis-inducing activity of selenium nanoparticles. Colloids Surf. B Biointerfaces 2011, 83, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Li, X.; Zheng, W.; Feng, Y.; Wong, Y.-S.; Chen, T. Ph-responsive cancer-targeted selenium nanoparticles: A transformable drug carrier with enhanced theranostic effects. J. Mater. Chem. B 2014, 2, 5409–5418. [Google Scholar] [CrossRef]
- Luesakul, U.; Puthong, S.; Neamati, N.; Muangsin, N. Ph-responsive selenium nanoparticles stabilized by folate-chitosan delivering doxorubicin for overcoming drug-resistant cancer cells. Carbohydr. Polym. 2018, 181, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, A.R.; Shahverdi, F.; Faghfuri, E.; Reza khoshayand, M.; Mavandadnejad, F.; Yazdi, M.H.; Amini, M. Characterization of folic acid surface-coated selenium nanoparticles and corresponding in vitro and in vivo effects against breast cancer. Arch. Med. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Dörffel, Y.; Loening-Baucke, V.; Gille, C.; Reißhauer, A.; Göktas, O.; Krüger, M.; Neuhaus, J.; Schrödl, W. Impact of humic acids on the colonic microbiome in healthy volunteers. World J. Gastroenterol. 2017, 23, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Baigorri, R.; Zamarreño, A.M.; Garcia-Mina, J.M.; Yvin, J.-C. Glucan and humic acid: Synergistic effects on the immune system. J. Med. Food 2010, 13, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Klöcking, R.; Helbig, B. Humic substances, medical aspects and applications of. In Biopolymers Online; Steinbüchel, A., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; ISBN 9783527600038. [Google Scholar]
- Yusuke, K.; Yustiawati; Masato, T.; Sulmin, G.; Ardianor; Toshiyuki, H.; Shunitz, T.; Takeshi, S.; Masaaki, K. Mechanism of the toxicity induced by natural humic acid on human vascular endothelial cells. Environ. Toxicol. 2014, 29, 916–925. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Yang, H.-L. The effects of humic acid–arsenate complexes on human red blood cells. Environ. Res. 2002, 89, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-L.; Ho, H.-Y.; Huang, Y.-W.; Lu, F.-J.; Chiu, D.T.-Y. Humic acid induces oxidative DNA damage, growth retardation, and apoptosis in human primary fibroblasts. Exp. Biol. Med. 2003, 228, 413–423. [Google Scholar] [CrossRef]
- An, P.; Langqiu, X. The effects of humic acid on the chemical and biological properties of selenium in the environment. Sci. Total Environ. 1987, 64, 89–98. [Google Scholar] [CrossRef]
- Mal, J.; Veneman, W.J.; Nancharaiah, Y.V.; van Hullebusch, E.D.; Peijnenburg, W.J.; Vijver, M.G.; Lens, P.N. A comparison of fate and toxicity of selenite, biogenically, and chemically synthesized selenium nanoparticles to zebrafish (Danio rerio) embryogenesis. Nanotoxicology 2017, 11, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhang, C.; Xia, I.F.; Cheung, S.T.; Wong, K.S.; Wong, K.-H.; Au, D.W.T.; Hinton, D.E.; Kwok, K.W.H. Maternal dietary exposure to selenium nanoparticle led to malformation in offspring. Ecotoxicol. Environ. Saf. 2018, 156, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Bhattacharjee, S.; Daware, A.; Tribedi, P.; Krishnani, K.K.; Minhas, P.S. Selenium nanoparticles for stress-resilient fish and livestock. Nanoscale Res. Lett. 2015, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Tarrahi, R.; Khataee, A.; Movafeghi, A.; Rezanejad, F.; Gohari, G. Toxicological implications of selenium nanoparticles with different coatings along with Se4+ on Lemna minor. Chemosphere 2017, 181, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; McDonald, T.J.; Sohn, M.; Anquandah, G.A.K.; Pettine, M.; Zboril, R. Assessment of toxicity of selenium and cadmium selenium quantum dots: A review. Chemosphere 2017, 188, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, F.; Charrondiere, U.R.; Dusemund, B.; Galtier, P.; Gilbert, J.; Gott, D.M.; Grilli, S.; Guertler, R.; Kass, G.E.N.; Koenig, J.; et al. Chromium(iii)-, iron(ii)- and selenium-humic acid/fulvic acid chelate and supplemented humifulvate added for nutritional purposes to food supplements Scientific opinion of the panel on food additives and nutrient sources added to food. EFSA J. 2009, 1147, 1–36. [Google Scholar] [CrossRef]
- Alline Gomes, P.; Luanai Grazieli Luquini, G.; Letícia Satler, G.; Tércio Assunção, P.; Maria José, N. Selenized saccharomyces cerevisiae cells are a green dispenser of nanoparticles. Biomed. Phys. Eng. Express 2018, 4, 035028. [Google Scholar] [CrossRef]
- Powell, J.J.; Faria, N.; Thomas-McKay, E.; Pele, L.C. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J. Autoimmun. 2010, 34, J226–J233. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Rao, J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Roblegg, E. Models for oral uptake of nanoparticles in consumer products. Toxicology 2012, 291, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.K.; Campos, V.L.; León, C.G.; Rodríguez-Llamazares, S.M.; Rojas, S.M.; González, M.; Smith, C.; Mondaca, M.A. Biosynthesis of selenium nanoparticles by Pantoea agglomerans and their antioxidant activity. J. Nanopart. Res. 2012, 14, 1236. [Google Scholar] [CrossRef]
- Peng, D.; Zhang, J.; Liu, Q.; Taylor, E.W. Size effect of elemental selenium nanoparticles (nano-se) at supranutritional levels on selenium accumulation and glutathione s-transferase activity. J. Inorg. Biochem. 2007, 101, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Zhang, J.; Hou, J.; Chen, C. Free radical scavenging efficiency of nano-se in vitro. Free Radic. Biol. Med. 2003, 35, 805–813. [Google Scholar] [CrossRef]
- Lu, Z.; Jin, M.; Huang, M.; Wang, Y.; Wang, Y. Bioactivity of selenium-enriched exopolysaccharides produced by Enterobacter cloacae z0206 in broilers. Carbohydr. Polym. 2013, 96, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Zhang, J.; Liu, Z.; Zhang, Y.; Li, J.; Li, Y.O. Biosynthesis of selenium rich exopolysaccharide (Se-EPS) by Pseudomonas pt-8 and characterization of its antioxidant activities. Carbohydr. Polym. 2016, 142, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.-Y.; Liu, F.; Gao, H.; Sun, H.; Meng, M.; Zhang, Y.-M. Synthesis, characterization and antioxidant activity of selenium polysaccharide from Cordyceps militaris. Int. J. Biol. Macromol. 2016, 93, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, B.; Wang, X.; Yao, J.; Zhang, J. Synthesis of selenium-containing polysaccharides and evaluation of antioxidant activity in vitro. Int. J. Biol. Macromol. 2012, 51, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, G.; Zhang, J.; Zhang, G.; Jia, L.; Liu, X.; Deng, P.; Fan, K. Extraction optimization and antioxidant activity of intracellular selenium polysaccharide by Cordyceps sinensis SU-02. Carbohydr. Polym. 2011, 86, 1745–1750. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Y.; Li, D.; Liu, C.; Jin, M.; Bian, J.; Lv, M.; Sun, Y.; Zhang, L.; Gao, P. The neuroprotective and antioxidant profiles of selenium-containing polysaccharides from the fruit of Rosa laevigata. Food Funct. 2018, 9, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, X.; Bao, A.; Liu, X.; Zeng, J.; Liu, X.; Yao, J.; Zhang, J.; Lei, Z. Microwave-assisted synthesis, structure and anti-tumor activity of selenized Artemisia sphaerocephala polysaccharide. Int. J. Biol. Macromol. 2017, 95, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, E.; Krzyczkowski, W.; Herold, F.; Łapienis, G.; Ślusarczyk, J.; Suchocki, P.; Kuraś, M.; Turło, J. Biosynthesis of selenium-containing polysaccharides with antioxidant activity in liquid culture of Hericium erinaceum. Enzyme Microb. Technol. 2009, 44, 334–343. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, H. Production of intracellular selenium-enriched polysaccharides from thin stillage by Cordyceps sinensis and its bioactivities. Food Nutr. Res. 2016, 60, 30153. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, J.; Rao, S.; Su, Y.; Li, J.; Li, C.; Xu, S.; Yang, Y. Antidiabetic activity of mycelia selenium-polysaccharide from Catathelasma ventricosum in STZ-induced diabetic mice. Food Chem. Toxicol. 2013, 62, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, H.; Zhang, Y.; Zhang, N.; Fu, L. Chemical modification and antioxidant activities of polysaccharide from mushroom Inonotus obliquus. Carbohydr. Polym. 2012, 89, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Zaidman, B.-Z.; Yassin, M.; Mahajna, J.; Wasser, S.P. Medicinal mushroom modulators of molecular targets as cancer therapeutics. Appl. Microbiol. Biotechnol. 2005, 67, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Gan, D.; Ma, L.; Jiang, C.; Xu, R.; Zeng, X. Production, preliminary characterization and antitumor activity in vitro of polysaccharides from the mycelium of Pholiota dinghuensis Bi. Carbohydr. Polym. 2011, 84, 997–1003. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, L.; Zhang, M.; Chen, L.; Keung Cheung, P.C.; Oi, V.E.C.; Lin, Y. Antitumor activities of heteropolysaccharides of Poria cocos mycelia from different strains and culture media. Carbohydr. Res. 2003, 338, 1517–1521. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Guo, S.-Y.; Li, L. Bioeffects of selenite on the growth of Spirulina platensis and its biotransformation. Bioresour. Technol. 2003, 89, 171–176. [Google Scholar] [CrossRef]
- Xu, C.L.; Wang, Y.Z.; Jin, M.L.; Yang, X.Q. Preparation, characterization and immunomodulatory activity of selenium-enriched exopolysaccharide produced by bacterium Enterobacter cloacae z0206. Bioresour. Technol. 2009, 100, 2095–2097. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, Z.; Guo, M.; Xia, Y.; Zhao, M.; Wang, C.; Xu, T.; Chen, T.; Zhu, B. Inhibitory activity of selenium nanoparticles functionalized with oseltamivir on H1N1 influenza virus. Int. J. Nanomed. 2017, 12, 5733–5743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Zhang, W.; Zhao, Z.; Li, N.; Mou, Z.; Sun, D.; Cai, Y.; Wang, W.; Lin, Y. Quercetin loading CdSe/Zns nanoparticles as efficient antibacterial and anticancer materials. J. Inorg. Biochem. 2017, 167, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Webster, T.J. Nanostructured selenium for preventing biofilm formation on polycarbonate medical devices. J. Biomed. Mater. Res. A 2012, 100, 3205–3210. [Google Scholar] [CrossRef] [PubMed]
- Piacenza, E.; Presentato, A.; Zonaro, E.; Lemire, J.A.; Demeter, M.; Vallini, G.; Turner, R.J.; Lampis, S. Antimicrobial activity of biogenically produced spherical se-nanomaterials embedded in organic material against Pseudomonas aeruginosa and Staphylococcus aureus strains on hydroxyapatite-coated surfaces. Microb. Biotechnol. 2017, 10, 804–818. [Google Scholar] [CrossRef] [PubMed]
- Cremonini, E.; Zonaro, E.; Donini, M.; Lampis, S.; Boaretti, M.; Dusi, S.; Melotti, P.; Lleo, M.M.; Vallini, G. Biogenic selenium nanoparticles: Characterization, antimicrobial activity and effects on human dendritic cells and fibroblasts. Microb. Biotechnol. 2016, 9, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hao, H.; Li, Y.; Zhang, S. Selenium-substituted hydroxyapatite nanoparticles and their in vivo antitumor effect on hepatocellular carcinoma. Colloids Surf. B Biointerfaces 2016, 140, 297–306. [Google Scholar] [CrossRef]
- Yin, J.; Hou, Y.; Yin, Y.; Song, X. Selenium-coated nanostructured lipid carriers used for oral delivery of berberine to accomplish a synergic hypoglycemic effect. Int. J. Nanomed. 2017, 12, 8671–8680. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.S.; Kulkarni, A.; Khurana, A.; Kaur, J.; Tikoo, K. Selenium nanoparticles involve hsp-70 and sirt1 in preventing the progression of type 1 diabetic nephropathy. Chem. Biol. Interact. 2014, 223, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Welling, M.N.; Mantri, S.B.; Desai, K. In vitro and in vivo antioxidant, cytotoxic, and anti-chronic inflammatory arthritic effect of selenium nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Ramya, S.; Shanmugasundaram, T.; Balagurunathan, R. Biomedical potential of actinobacterially synthesized selenium nanoparticles with special reference to anti-biofilm, anti-oxidant, wound healing, cytotoxic and anti-viral activities. J. Trace Elem. Med. Biol. 2015, 32, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Shoeibi, S.; Mashreghi, M. Biosynthesis of selenium nanoparticles using enterococcus faecalis and evaluation of their antibacterial activities. J. Trace Elem. Med. Biol. 2017, 39, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Mukhopadhyay, M. Green synthesis and structural characterization of selenium nanoparticles and assessment of their antimicrobial property. Bioprocess Biosyst. Eng. 2015, 38, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.H.; Mahdavi, M.; Setayesh, N.; Esfandyar, M.; Shahverdi, A.R. Selenium nanoparticle-enriched lactobacillus brevis causes more efficient immune responses in vivo and reduces the liver metastasis in metastatic form of mouse breast cancer. Daru 2013, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, M.; Filipović, N.; Djurdjević, J.; Lukić, M.; Milenković, M.; Boccaccini, A. 45s5bioglass®-based scaffolds coated with selenium nanoparticles or with poly(lactide-co-glycolide)/selenium particles: Processing, evaluation and antibacterial activity. Colloids Surf. B Biointerfaces 2015, 132, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, J.; Yu, H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: Comparison with selenomethionine in mice. Free Radic. Biol. Med. 2007, 42, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, Z.; Zhao, M.; Xu, T.; Wang, C.; Xia, H.; Wang, H.; Zhu, B. Multifunctional selenium nanoparticles as carriers of HSP70 siRNA to induce apoptosis of HepG2 cells. Int. J. Nanomed. 2016, 11, 3065–3076. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Yao, J.; Yang, Z.; Yue, W.; Ren, Y.; Zhang, C.; Liu, X.; Wang, H.; Zhao, X.; Yuan, S.; et al. Improved fetal hair follicle development by maternal supplement of selenium at nano size (Nano-Se). Livest. Sci. 2011, 142, 270–275. [Google Scholar] [CrossRef]
- Shi, L.-G.; Yang, R.-J.; Yue, W.-B.; Xun, W.-J.; Zhang, C.-X.; Ren, Y.-S.; Shi, L.; Lei, F.-L. Effect of elemental nano-selenium on semen quality, glutathione peroxidase activity, and testis ultrastructure in male Boer goats. Anim. Reprod. Sci. 2010, 118, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.-B.; Dong, F.; Yu, M.; Qin, L.; Liu, D. Optimization of synthesis of seleno-sargassum fusiforme (harv.) setch. Polysaccharide by response surface methodology, its characterization, and antioxidant activity. J. Chem. 2013, 2013, 9. [Google Scholar] [CrossRef]
- Liu, Y.; You, Y.; Li, Y.; Zhang, L.; Yin, L.; Shen, Y.; Li, C.; Chen, H.; Chen, S.; Hu, B.; et al. The characterization, selenylation and antidiabetic activity of mycelial polysaccharides from Catathelasma ventricosum. Carbohydr. Polym. 2017, 174, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Yang, X.-Q.; Kou, M.; Lu, C.-Y.; Wang, Y.-Y.; Peng, J.; Chen, P.; Jiang, J.-H. Selenylation of polysaccharide from the sweet potato and evaluation of antioxidant, antitumor, and antidiabetic activities. J. Agric. Food Chem. 2017, 65, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Ren, Z.; Huang, Y.; Song, Y.; Lin, D.; Li, J.; Ma, Y.; Wu, X.; Qiu, F.; Xiao, Q. Selenizing Hericium erinaceus polysaccharides induces dendritic cells maturation through MAPK and NF-κB signaling pathways. Int. J. Biol. Macromol. 2017, 97, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Q.; Bao, A.; Liu, X.; Zeng, J.; Yang, X.; Yao, J.; Zhang, J.; Lei, Z. Synthesis of selenium-containing Artemisia sphaerocephala polysaccharides: Solution conformation and anti-tumor activities in vitro. Carbohydr. Polym. 2016, 152, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Oancea, F.; Szabolcs, L.; Oancea, A.O.; Radu, L.; Ábrahám, B.; Stanciu-Burileanu, M.M.; Sándor, M.; Lungu, M. Selenium biofortification biotechnologies of wheat grain in south-eastern part of Romania for a better human health. Studia Univ. Vasile Goldis Arad Seria Stiintele Vietii 2014, 24, 47–56. [Google Scholar]
- Jabłońska, E.; Reszka, E. Chapter eight: Selenium and epigenetics in cancer: Focus on DNA methylation. In Advances in Cancer Research; Tew, K.D., Galli, F., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 136, pp. 193–234. ISBN 0065–230X. [Google Scholar]
- Berglund, M.; Wieser Michael, E. Isotopic compositions of the elements 2009 (IUPAC technical report). Pure Appl. Chem. 2011, 83, 397–410. [Google Scholar] [CrossRef]
- Valiente, L.; Piccinna, M.; Romero Ale, E.; Grillo, A.; Smichowski, P. Determination of selenium in dietary supplements by ETAAS and HG-AAS: A comparative study. At. Spectrosc. 2002, 23, 129–134. [Google Scholar]
- Muñoz Olivas, R.; Donard, O.F.X. Microwave assisted reduction of Se(VI) to Se(IV) and determination by HG/FI-ICP/MS for inorganic selenium speciation. Talanta 1998, 45, 1023–1029. [Google Scholar] [CrossRef]
- Kleckner, A.E.; Kakouros, E.; Robin Stewart, A. A practical method for the determination of total selenium in environmental samples using isotope dilution-hydride generation-inductively coupled plasma-mass spectrometry. Limnol. Oceanogr. Methods 2017, 15, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Lam, J.; Sturgeon, R. Determination of as and se in seawater by flow injection vapor generation ETV-ICP-MS. At. Spectrosc. 1999, 20, 79–85. [Google Scholar]
- Krawczyk, M. Determination of macro and trace elements in multivitamin dietary supplements by high-resolution continuum source graphite furnace atomic absorption spectrometry with slurry sampling. J. Pharm. Biomed. Anal. 2014, 88, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Gajdosechova, Z.; Mester, Z.; Feldmann, J.; Krupp, E.M. The role of selenium in mercury toxicity—Current analytical techniques and future trends in analysis of selenium and mercury interactions in biological matrices. Trends Anal. Chem. 2017, 104, 95–109. [Google Scholar] [CrossRef]
- Ruszczyńska, A.; Konopka, A.; Kurek, E.; Torres Elguera, J.C.; Bulska, E. Investigation of biotransformation of selenium in plants using spectrometric methods. Spectrochim. Acta Part B At. Spectrosc. 2017, 130, 7–16. [Google Scholar] [CrossRef]
- Bryszewska, M.A.; Måge, A. Determination of selenium and its compounds in marine organisms. J. Trace Elem. Med. Biol. 2015, 29, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Forrer, R.; Gautschi, K.; Stroh, A.; Lutz, H. Direct determination of selenium and other trace elements in serum samples by ICP-MS. J. Trace Elem. Med. Biol. 1999, 12, 240–247. [Google Scholar] [CrossRef]
- Krejčová, A.; Ludvíková, I.; Černohorský, T.; Pouzar, M. Elemental analysis of nutritional preparations by inductively coupled plasma mass and optical emission spectrometry. Food Chem. 2012, 132, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Kolachi, N.F.; Kazi, T.G.; Afridi, H.I.; Khan, S.; Wadhwa, S.K.; Shah, A.Q.; Shah, F.; Baig, J.A.; Sirajuddin. Determination of selenium content in aqueous extract of medicinal plants used as herbal supplement for cancer patients. Food Chem. Toxicol. 2010, 48, 3327–3332. [Google Scholar] [CrossRef] [PubMed]
- Miksa, I.R.; Buckley, C.L.; Carpenter, N.P.; Poppenga, R.H. Comparison of selenium determination in liver samples by atomic absorption spectroscopy and inductively coupled plasma–mass spectrometry. J. Vet. Diagn. Investig. 2005, 17, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Bulska, E.; Pyrzyńska, K. Comparison of chemical modifiers for the determination of selenium by electrothermal atomic-absorption spectrometry. Spectrochim. Acta Part B At. Spectrosc. 1997, 52, 1283–1291. [Google Scholar] [CrossRef]
- Čuparigova, F.; Stafilov, T. Determination of selenium in human blood serum by electrothermal atomic absorption spectrometry. Chem. Sci. J. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Shaltout, A.A.; Castilho, I.N.B.; Welz, B.; Carasek, E.; Gonzaga Martens, I.B.; Martens, A.; Cozzolino, S.M.F. Method development and optimization for the determination of selenium in bean and soil samples using hydride generation electrothermal atomic absorption spectrometry. Talanta 2011, 85, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Tian, Y.; Zeng, W.; Hou, X.; Jiang, X. Effect of variable ultraviolet wavelength and intensity on photochemical vapor generation of trace selenium detected by atomic fluorescence spectrometry. Microchem. J. 2018, 140, 189–195. [Google Scholar] [CrossRef]
- Stosnach, H. Analytical determination of selenium in medical samples, staple food and dietary supplements by means of total reflection x-ray fluorescence spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2010, 65, 859–863. [Google Scholar] [CrossRef]
- Templeton Douglas, M.; Ariese, F.; Cornelis, R.; Danielsson, L.-G.; Muntau, H.; van Leeuwen Herman, P.; Lobinski, R. Guidelines for terms related to chemical speciation and fractionation of elements. Definitions, structural aspects, and methodological approaches (IUPAC recommendations 2000). Pure Appl. Chem. 2000, 72, 1453–1470. [Google Scholar] [CrossRef]
- Tarze, A.; Dauplais, M.; Grigoras, I.; Lazard, M.; Ha-Duong, N.-T.; Barbier, F.; Blanquet, S.; Plateau, P. Extracellular production of hydrogen selenide accounts for thiol-assisted toxicity of selenite against saccharomyces cerevisiae. J. Biol. Chem. 2007, 282, 8759–8767. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, B.R.G.; Danielson, M.; Khayat, A.; Wide, M. Comparative embryotoxicity of selenite and selenate: Uptake in murine embryonal and fetal tissues and effects on blastocysts and embryonic cells in vitro. Toxicology 1990, 63, 123–136. [Google Scholar] [CrossRef]
- Brasher, A.M.; Scott Ogle, R. Comparative toxicity of selenite and selenate to the amphipod Hyalella azteca. Arch. Environ. Contam. Toxicol. 1993, 24, 182–186. [Google Scholar] [CrossRef]
- Boehler, C.J.; Raines, A.M.; Sunde, R.A. Deletion of thioredoxin reductase and effects of selenite and selenate toxicity in caenorhabditis elegans. PLoS ONE 2013, 8, e71525. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, Z.; Kiss, I.; Kádár, I.; Bakonyi, G. Toxicity of selenate and selenite to the potworm Enchytraeus albidus (Annelida: Enchytraeidae): A laboratory test. Ecotoxicology 2007, 16, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Jain, R.; Thakur, A.; Kumar, M.; Labhsetwar, N.K.; Nayak, M.; Kumar, P. A systematic review and meta-analysis of voltammetric and optical techniques for inorganic selenium determination in water. Trends Analyt. Chem. 2017, 95, 69–85. [Google Scholar] [CrossRef]
- Vogel, M.; Fischer, S.; Maffert, A.; Hübner, R.; Scheinost, A.C.; Franzen, C.; Steudtner, R. Biotransformation and detoxification of selenite by microbial biogenesis of selenium-sulfur nanoparticles. J. Hazard. Mater. 2018, 344, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Liu, K.; Li, M.; Zhang, W.; Zhao, X.; Zhao, Z.; Liu, X. Difference of selenium uptake and distribution in the plant and selenium form in the grains of rice with foliar spray of selenite or selenate at different stages. Field Crop. Res. 2017, 211, 165–171. [Google Scholar] [CrossRef]
- Kozak, L.; Rudnicka, M.; Niedzielski, P. Determination of inorganic selenium species in dietary supplements by hyphenated analytical system HPLC-HG-AAS. Food Anal. Methods 2012, 5, 1237–1243. [Google Scholar] [CrossRef]
- Rybínová, M.; Červený, V.; Hraníček, J.; Rychlovský, P. Uv-photochemical vapor generation with quartz furnace atomic absorption spectrometry for simple and sensitive determination of selenium in dietary supplements. Microchem. J. 2016, 124, 584–593. [Google Scholar] [CrossRef]
- Barache, U.B.; Shaikh, A.B.; Lokhande, T.N.; Anuse, M.A.; Kamble, G.S.; Gurame, V.M.; Gaikwad, S.H. Acid switched efficient, cost effective, selective separation and determination of selenium(iv). J. Environ. Chem. Eng. 2017, 5, 4828–4840. [Google Scholar] [CrossRef]
- Kubachka, K.M.; Meija, J.; LeDuc, D.L.; Terry, N.; Caruso, J.A. Selenium volatiles as proxy to the metabolic pathways of selenium in genetically modified Brassica juncea. Environ. Sci. Technol. 2007, 41, 1863–1869. [Google Scholar] [CrossRef] [PubMed]
- Grant, T.D.; Wuilloud, R.G.; Wuilloud, J.C.; Caruso, J.A. Investigation of the elemental composition and chemical association of several elements in fulvic acids dietary supplements by size-exclusion chromatography UV inductively coupled plasma mass spectrometric. J. Chromatogr. A 2004, 1054, 313–319. [Google Scholar] [CrossRef]
- Drahoňovský, J.; Száková, J.; Mestek, O.; Tremlová, J.; Kaňa, A.; Najmanová, J.; Tlustoš, P. Selenium uptake, transformation and inter-element interactions by selected wildlife plant species after foliar selenate application. Environ. Exp. Bot. 2016, 125, 12–19. [Google Scholar] [CrossRef]
- Sonet, J.; Bierla, K.; Bulteau, A.-L.; Lobinski, R.; Chavatte, L. Comparison of analytical methods using enzymatic activity, immunoaffinity and selenium-specific mass spectrometric detection for the quantitation of glutathione peroxidase 1. Anal. Chim. Acta 2018, 1011, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Bianga, J.; Govasmark, E.; Szpunar, J. Characterization of selenium incorporation into wheat proteins by two-dimensional gel electrophoresis–laser ablation ICP MS followed by capillary HPLC–ICP MS and electrospray linear trap quadrupole orbitrap MS. Anal. Chem. 2013, 85, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- Cruz, E.C.S.; Susanne Becker, J.; Sabine Becker, J.; Sussulini, A. Imaging of selenium by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) in 2-d electrophoresis gels and biological tissues. In Selenoproteins: Methods and Protocols; Chavatte, L., Ed.; Springer New York: New York, NY, USA, 2018; pp. 219–227. ISBN 978–1-4939-7258-6. [Google Scholar]
- Viñas, P.; López-García, I.; Merino-Meroño, B.; Campillo, N.; Hernández-Córdoba, M. Determination of selenium species in infant formulas and dietetic supplements using liquid chromatography–hydride generation atomic fluorescence spectrometry. Anal. Chim. Acta 2005, 535, 49–56. [Google Scholar] [CrossRef]
- Barrientos, E.Y.; Wrobel, K.; Torres Guzman, J.C.; Corrales Escobosa, A.R.; Wrobel, K. Determination of semet and se(iv) in biofortified yeast by ion-pair reversed phase liquid chromatography-hydride generation-microwave induced nitrogen plasma atomic emission spectrometry (HPLC-HG-MP-AES). J. Anal. At. Spectrom. 2016, 31, 203–211. [Google Scholar] [CrossRef]
- Falandysz, J. Review: On published data and methods for selenium in mushrooms. Food Chem. 2013, 138, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, C.; Liu, H.; Zhu, Y.; Ren, Z.; Jing, H.; Li, S.; Zhang, J.; Liu, X.; Jia, L. The characteristics and antioxidation of oudemansiella radicata selenium polysaccharides on lipopolysaccharide-induced endo-toxemic mice. Int. J. Biol. Macromol. 2018, 116, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhu, Z.; Tang, Y.; Ren, Y.; Song, Q.; Tang, Y.; Zhang, Y. Structural characterization and antitumor activity of a novel se-polysaccharide from selenium-enriched cordyceps gunnii. Food Funct. 2018, 9, 2744–2754. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Chen, T.; Yan, M.; Zhao, W.; Li, F.; Cheng, W.; Yuan, L. Synthesis, characterization, antioxidant activity and neuroprotective effects of selenium polysaccharide from radix hedysari. Carbohydr. Polym. 2015, 125, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Tugarova, A.V.; Kamnev, A.A. Proteins in microbial synthesis of selenium nanoparticles. Talanta 2017, 174, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Roux, M.; Sarret, G.; Pignot-Paintrand, I.; Fontecave, M.; Coves, J. Mobilization of selenite by ralstonia metallidurans CH34. Appl. Environ. Microbiol. 2001, 67, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-L.; Hua, P.-M. Mixed surfactant template method for preparation of nanometer selenium. E-J. Chem. 2009, 6, S304–S310. [Google Scholar] [CrossRef]
- Dwivedi, S.; AlKhedhairy, A.A.; Ahamed, M.; Musarrat, J. Biomimetic synthesis of selenium nanospheres by bacterial strain js-11 and its role as a biosensor for nanotoxicity assessment: A novel se-bioassay. PLoS ONE 2013, 8, e57404. [Google Scholar] [CrossRef] [PubMed]
- Elahian, F.; Reiisi, S.; Shahidi, A.; Mirzaei, S.A. High-throughput bioaccumulation, biotransformation, and production of silver and selenium nanoparticles using genetically engineered pichia pastoris. Nanomedicine 2017, 13, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Avendaño, R.; Chaves, N.; Fuentes, P.; Sánchez, E.; Jiménez, J.I.; Chavarría, M. Production of selenium nanoparticles in pseudomonas putida KT2440. Sci. Rep. 2016, 6, 37155. [Google Scholar] [CrossRef] [PubMed]
- Lampis, S.; Zonaro, E.; Bertolini, C.; Cecconi, D.; Monti, F.; Micaroni, M.; Turner, R.J.; Butler, C.S.; Vallini, G. Selenite biotransformation and detoxification by stenotrophomonas maltophilia SeITE02: Novel clues on the route to bacterial biogenesis of selenium nanoparticles. J. Hazard. Mater. 2017, 324, 3–14. [Google Scholar] [CrossRef] [PubMed]
- M-M, P.; Somchue, W.; Shiowatana, J.; Siripinyanond, A. Flow field-flow fractionation for particle size characterization of selenium nanoparticles incubated in gastrointestinal conditions. Food Res. Int. 2014, 57, 203–209. [Google Scholar] [CrossRef]
- Palomo-Siguero, M.; Vera, P.; Echegoyen, Y.; Nerin, C.; Cámara, C.; Madrid, Y. Asymmetrical flow field-flow fractionation coupled to inductively coupled plasma mass spectrometry for sizing senps for packaging applications. Spectrochim. Acta Part B At. Spectrosc. 2017, 132, 19–25. [Google Scholar] [CrossRef]
- Farré, M.; Barceló, D. Chapter 1: Introduction to the analysis and risk of nanomaterials in environmental and food samples. In Comprehensive Analytical Chemistry; Farré, M., Barceló, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 59, pp. 1–32. ISBN 0166–526X. [Google Scholar]
- Pace, H.E.; Rogers, N.J.; Jarolimek, C.; Coleman, V.A.; Higgins, C.P.; Ranville, J.F. Determining transport efficiency for the purpose of counting and sizing nanoparticles via single particle inductively coupled plasma mass spectrometry. Anal. Chem. 2011, 83, 9361–9369. [Google Scholar] [CrossRef] [PubMed]
- Montoro Bustos, A.R.; Winchester, M.R. Single-particle-ICP-MS advances. Anal. Bioanal. Chem. 2016, 408, 5051–5052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Bi, X.; Reed, R.B.; Ranville, J.F.; Herckes, P.; Westerhoff, P. Nanoparticle size detection limits by single particle icp-ms for 40 elements. Environ. Sci. Technol. 2014, 48, 10291–10300. [Google Scholar] [CrossRef] [PubMed]
- Tanner, S.D.; Baranov, V.I.; Bandura, D.R. Reaction cells and collision cells for ICP-MS: A tutorial review. Spectrochim. Acta Part B At. Spectrosc. 2002, 57, 1361–1452. [Google Scholar] [CrossRef]
- Goossens, J.; Moens, L.; Dams, R. A mathematical correction method for spectral interferences on selenium in inductively coupled plasma mass spectrometry. Talanta 1994, 41, 187–193. [Google Scholar] [CrossRef]
- Sele, V.; Ørnsrud, R.; Sloth, J.J.; Berntssen, M.H.G.; Amlund, H. Selenium and selenium species in feeds and muscle tissue of Atlantic salmon. J. Trace Elem. Med. Biol. 2018, 47, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Trolove, S.N.; Tan, Y.; Morrison, S.C.; Feng, L.; Eason, J. Development of a method for producing selenium-enriched radish sprouts. LWT 2018, 95, 187–192. [Google Scholar] [CrossRef]
- Ponce, M.; Giraldez, I.; Calero, S.; Ruiz-Azcona, P.; Morales, E.; Fernández-Díaz, C.; Hachero-Cruzado, I. Toxicity and biochemical transformation of selenium species in rotifer (Brachionus Plicatilis) enrichments. Aquaculture 2018, 484, 105–111. [Google Scholar] [CrossRef]
- Moreda-Piñeiro, J.; Moreda-Piñeiro, A.; Romarís-Hortas, V.; Domínguez-González, R.; Alonso-Rodríguez, E.; López-Mahía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D.; Bermejo-Barrera, P. In vitro bioavailability of total selenium and selenium species from seafood. Food Chem. 2013, 139, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Fontanella, M.C.; D’Amato, R.; Regni, L.; Proietti, P.; Beone, G.M.; Businelli, D. Selenium speciation profiles in biofortified sangiovese wine. J. Trace Elem. Med. Biol. 2017, 43, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Egressy-Molnár, O.; Ouerdane, L.; Győrfi, J.; Dernovics, M. Analogy in selenium enrichment and selenium speciation between selenized yeast Saccharomyces cerevisiae and Hericium erinaceus (lion’s mane mushroom). LWT-Food Sci. Technol. 2016, 68, 306–312. [Google Scholar] [CrossRef]
- Wang, D.; Dinh, Q.T.; Anh Thu, T.T.; Zhou, F.; Yang, W.; Wang, M.; Song, W.; Liang, D. Effect of selenium-enriched organic material amendment on selenium fraction transformation and bioavailability in soil. Chemosphere 2018, 199, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Tareq, F.K.; Fayzunnesa, M.; Kabir, M.S.; Nuzat, M. Mechanism of bio molecule stabilized selenium nanoparticles against oxidation process and Clostridium botulinum. Microb. Pathogenesis 2018, 115, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Tang, X.; Xue, Y.; Lin, G.; Xiong, Y.L. Dietary linseed oil supplemented with organic selenium improved the fatty acid nutritional profile, muscular selenium deposition, water retention, and tenderness of fresh pork. Meat Sci. 2017, 131, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Silva Junior, E.C.; Wadt, L.H.O.; Silva, K.E.; Lima, R.M.B.; Batista, K.D.; Guedes, M.C.; Carvalho, G.S.; Carvalho, T.S.; Reis, A.R.; Lopes, G.; et al. Natural variation of selenium in brazil nuts and soils from the amazon region. Chemosphere 2017, 188, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, Y.; Zhang, Y.; Guo, T.; Zhang, Z.; Zhang, W.J.; Zhang, X.G.; Ashraf, M.A. The extraction of different proteins in selenium enriched peanuts and their antioxidant properties. Saudi J. Biol. Sci. 2016, 23, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Puccinelli, M.; Malorgio, F.; Rosellini, I.; Pezzarossa, B. Uptake and partitioning of selenium in basil (Ocimum Basilicum L.) plants grown in hydroponics. Sci. Hortic. (Amsterdam) 2017, 225, 271–276. [Google Scholar] [CrossRef]
- Alfthan, G.; Aspila, P.; Ekholm, P.; Eurola, M.; Hartikainen, H.; Hero, H.; Hietaniemi, V.; Root, T.; Salminen, P.; Venäl̈inen, E.R.; et al. Nationwide supplementation of sodium selenate to commercial fertilizers: History and 25-year results from the finnish selenium monitoring programme. In Combating Micronutrient Deficiencies: Food-Based Approaches; Thompson, B., Amoroso, L., Eds.; FAO & CAB Intenational: Rome, Italy, 2010; pp. 312–337. ISBN 9781845937140. [Google Scholar]
- Baltić, M.Ž.; Dokmanović Starčević, M.; Bašić, M.; Zenunović, A.; Ivanović, J.; Marković, R.; Janjić, J.; Mahmutović, H. Effects of selenium yeast level in diet on carcass and meat quality, tissue selenium distribution and glutathione peroxidase activity in ducks. Anim. Feed Sci. Technol. 2015, 210, 225–233. [Google Scholar] [CrossRef]
- Smichowski, P.; Londonio, A. The role of analytical techniques in the determination of metals and metalloids in dietary supplements: A review. Microchem. J. 2018, 136, 113–120. [Google Scholar] [CrossRef]
- Zhang, S.-Q.; Zhang, H.-B.; Zhang, Y. Quantification of selenomethionine in plasma using UPLC–MS/MS after the oral administration of selenium-enriched yeast to rats. Food Chem. 2018, 241, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hageman, S.P.W.; van der Weijden, R.D.; Stams, A.J.M.; van Cappellen, P.; Buisman, C.J.N. Microbial selenium sulfide reduction for selenium recovery from wastewater. J. Hazard. Mater. 2017, 329, 110–119. [Google Scholar] [CrossRef] [PubMed]




| Next-Generation Ingredients | Methods of (Bio)-Synthesizing SeNPs | Experimental Model | Biological Effect | Reference |
|---|---|---|---|---|
| SeNPs, chemical synthesis | (Vitamin C + Oseltamivir) (Quercetin + CdSe + ZnS), (Glutathione + NaOH) | (H1N1 influenza virus; MDCK cells), (Escherichia coli, Bacillus subtilis), (Staphylococcus aureus) | Antimicrobial activity | [163,164,165] |
| (L-cysteine or Ascorbic acid), (SDS + sodium sulfate × 5H2O or L-cysteine) | (Pseudomonas aeruginosa aeruginosa,S.aureus), (P. aeruginosa, Candida spp.) | Moderate antimicrobial activity | [166,167] | |
| (Quercetin + CdSe + ZnS), (Se-substituted hydroxyapatite NPs) | (BGC-823 cells), (human HCCLM9 cells injected in Balb/c nude mice) | Anticancer effects | [164,168] | |
| Berberine-loaded Se-coated nanostructured lipid carriers | Diabetic Sprague/Dawley rats | Enhanced hypoglycemic effect | [169] | |
| (sodium alginate + reduced glutathione) | Male Sprague/Dawley (SD) rats | Protection against diabetic nephropathy | [170] | |
| Ascorbic acid + dextrin | Wistar rats | Anti-inflammatory effect in arthritis | [171] | |
| SeNPs, biogenic synthesis | (Enterococcus faecalis), (Streptomyces minutiscleroticus), (Ralstonia eutropha), (Bacillus mycoides), (Bacillus mycoides, Stenotrophomonas maltophilia) | (Acinetobacter strains; type-1 dengue virus), (S. aureus; B. subtilis; E. coli; P. aeruginosa), (E. coli, P. aeruginosa, S. aureus, Staphylococcus pyogenes, Aspergillus clavatus), (P. aeruginosa, S. aureus), (P. aeruginosa, C. albicans) | Antimicrobial activity | [166,167,172,173,174] |
| (Enterococcus faecalis) | (DPPH assay; Phosphomolybdenum method) | Antioxidant effects | [172] | |
| (Lactobacillus brevis) | BALB/c mice | Anticancer effects | [175] | |
| (Enterococcus faecalis) | (Swiss albino rats) | Wound healing | [172] | |
| SeNPs, assisted biosynthesis | BSA + ascorbic acid-assisted biosynthesis | S. aureus, Staphylococcus epidermidis, B. subtilis, Klebsiella pneumoniae | Antimicrobial activity | [176] |
| BSA + glutathione-assisted biosynthesis | Male Kunming mice | Antioxidant effects | [177] | |
| (siRNA + vitamin C), (Polysaccharides extracted from Dictyophora indusiata + ascorbic acid) | (HepG-2 cell line), (HepG-2, A549, Hela, MCF-7, and PC3 cell lines) | Anticancer effects | [108,178] | |
| Polysaccharides from Catathelasma ventricosum + Ascorbic acid | Male ICR diabetic mice | Anti-diabetic activity | [109] | |
| SeNPs, Commercial source | Not available | cashmere goats | Improved fetal growth and hair follicle development | [179] |
| Not available | Boer goats | Enhanced semen and testicular GSH-Px activity, protection of the plasma membrane and mitochondria midpiece of spermatozoa | [180] | |
| Selenized polysaccharides | Fruits of Rosa laevigata | SH-SY5Y neuroblastoma cells | Neuroprotective effects | [152] |
| (Fruits of Rosa laevigata), (Agrocybe cylindracea), (Sargassum fusiforme) | (ABTS, DPPH, FRAP assays), (DPPH, hydroxyl radical scavenging, reducing power assays), (Kumming mice with tumor) | Antioxidant effects | [42,152,181] | |
| (Agrocybe cylindracea) | Kunming mice | Anti-ageing effects | [42] | |
| (Catathelasma ventricosum), (Sweet potato tuber) | (Male ICR diabetic mice), (Male SD diabetic rats) | Antidiabetic effects | [182,183] | |
| Hericium erinaceus | Immature dendritic cells from ICR mice | Immunostimulant (dendritic cells maturation) | [184] | |
| (Artemisia sphaerocephala), (Sweet potato tuber) | (HepG-2, A549, and Hela cell lines), (H22 hepatoma cell line, Female Kunming Mice) | Anti-tumor activity | [183,185] |
| Method | Samples | Advantages | Disadvantages |
|---|---|---|---|
| Inductively coupled plasma mass spectrometry (ICP-MS; in some case a collision cell was used) | Human plasma [22]; extracts of fish muscle, diets, and reference materials [245]; selenium nanoparticles [213]; radish sprouts [246]; rotifer tissue [247]; seafood [248]; glutathione peroxidase (Gpx) from bovine erythrocytes [221]; Se-rich yeast [73]; leaves, grapes, and wines [249]; mushrooms [250] | Can handle both simple and complex matrices; better detection limit than AAS and ICP-OES; small sample volume | Interference from plasma gas (Ar) and chlorides; high set-up and operational cost |
| Atomic fluorescence spectrophotometer (AFS)/hydride generation atomic fluorescence spectrometry (HG-AFS) | Soil samples [251]; rice [214]; Se (VI) [204] | Relatively simple equipment, the ability to analyze many samples in a short time | |
| Graphite furnace atomic absorption spectroscopy (GF-AAS)/flame atomic absorption spectrometry | Selenium nanoparticles [252]; pork meat [253]; sprouts of broccoli and white mustard [59]; brazil nuts [254]; peanuts [255] | High sensitivity, reduced analysis time | Matrix interference |
| Hydride generation atomic absorption spectrometry (HG-AAS) | Selenium nanoparticles [213], basil plants [256], meat and liver, fertilizers, and feed [257]; duck feed [258] | Minimum matrix interference | Interference of transition metals |
| Electrothermal atomic absorption spectrometry (ETAAS) | Cereals, milk, cheese, vegetables, fish, plasma, whole blood, and tissues [257]; dietary supplements [259] | Sensitive, high accuracy | Matrix interference in organic samples |
| Conjugated techniques using high-performance liquid chromatography with hydride generation atomic absorption spectrometry (HPLC–HG-AAS) | Dietary supplements [86,215]; garlic, radish sprouts, and sunflower sprouts [195] | Relatively simple | |
| Hydrophilic ion interaction chromatography (HILIC) with inductively coupled plasma mass spectrometric detection (ICP-MS) | Torula yeast [87] | ||
| Ion-pairing reversed-phase liquid chromatography HPLC–ICP-MS | Fish, seafood [196]; selenomethionine and Se-methylselenocysteine in mushrooms, Se-yeast [250] | ||
| High-performance liquid chromatography with inductively coupled plasma mass spectrometry (HPLC–ICP-MS), HPLC–Orbitrap MS | Seafood [248], leaves, grapes, and wines [249]; dietary supplements [72] | Speciation and identification of organic selenium compounds | Unknown peaks, lack of standards and reference materials |
| HPLC–ICP-MS and derivatization gas chromatography with atomic emission detection (GC–AED) | Dietary supplements [64] | ||
| Ultra-performance liquid chromatography mass spectrometry (UPLC–MS/MS) | Selenomethionine in rat plasma [260] | ||
| UV photochemical vapor generation (photo-CVG) to transform Se in its volatile species | Se (VI) [204], dietary supplements [259] | ||
| Multidimensional chromatography with dual ICP-MS and electrospray ionization ESI-MS detection | Se-rich yeast [73] | May identify peaks that are missed by ICP-MS | Sensitive to the presence of salts |
| Size exclusion chromatography (SEC) | Mushrooms, Se-yeast [250]; dietary supplements [259] | ||
| Optical emission spectrometry inductively coupled plasma (ICP-OES) | E. durans [93], wastewater [261] | Higher detection limit than ICP-MS; matrix interference |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantinescu-Aruxandei, D.; Frîncu, R.M.; Capră, L.; Oancea, F. Selenium Analysis and Speciation in Dietary Supplements Based on Next-Generation Selenium Ingredients. Nutrients 2018, 10, 1466. https://doi.org/10.3390/nu10101466
Constantinescu-Aruxandei D, Frîncu RM, Capră L, Oancea F. Selenium Analysis and Speciation in Dietary Supplements Based on Next-Generation Selenium Ingredients. Nutrients. 2018; 10(10):1466. https://doi.org/10.3390/nu10101466
Chicago/Turabian StyleConstantinescu-Aruxandei, Diana, Rodica Mihaela Frîncu, Luiza Capră, and Florin Oancea. 2018. "Selenium Analysis and Speciation in Dietary Supplements Based on Next-Generation Selenium Ingredients" Nutrients 10, no. 10: 1466. https://doi.org/10.3390/nu10101466
APA StyleConstantinescu-Aruxandei, D., Frîncu, R. M., Capră, L., & Oancea, F. (2018). Selenium Analysis and Speciation in Dietary Supplements Based on Next-Generation Selenium Ingredients. Nutrients, 10(10), 1466. https://doi.org/10.3390/nu10101466