A Randomized Controlled Trial to Compare the Effect of Peanuts and Almonds on the Cardio-Metabolic and Inflammatory Parameters in Patients with Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Sample Size Calculation
2.4. Intervention
2.5. Diet Record
2.6. Cardio-Metabolic and Anthropometric Parameters
2.7. Hypoglycemic Episodes and Antidiabetic Medication Modification
2.8. Interleukin-6
2.9. Ratio of Urinary Albumin/Creatinine
2.10. Statistical Analysis
3. Results
3.1. Study Participants
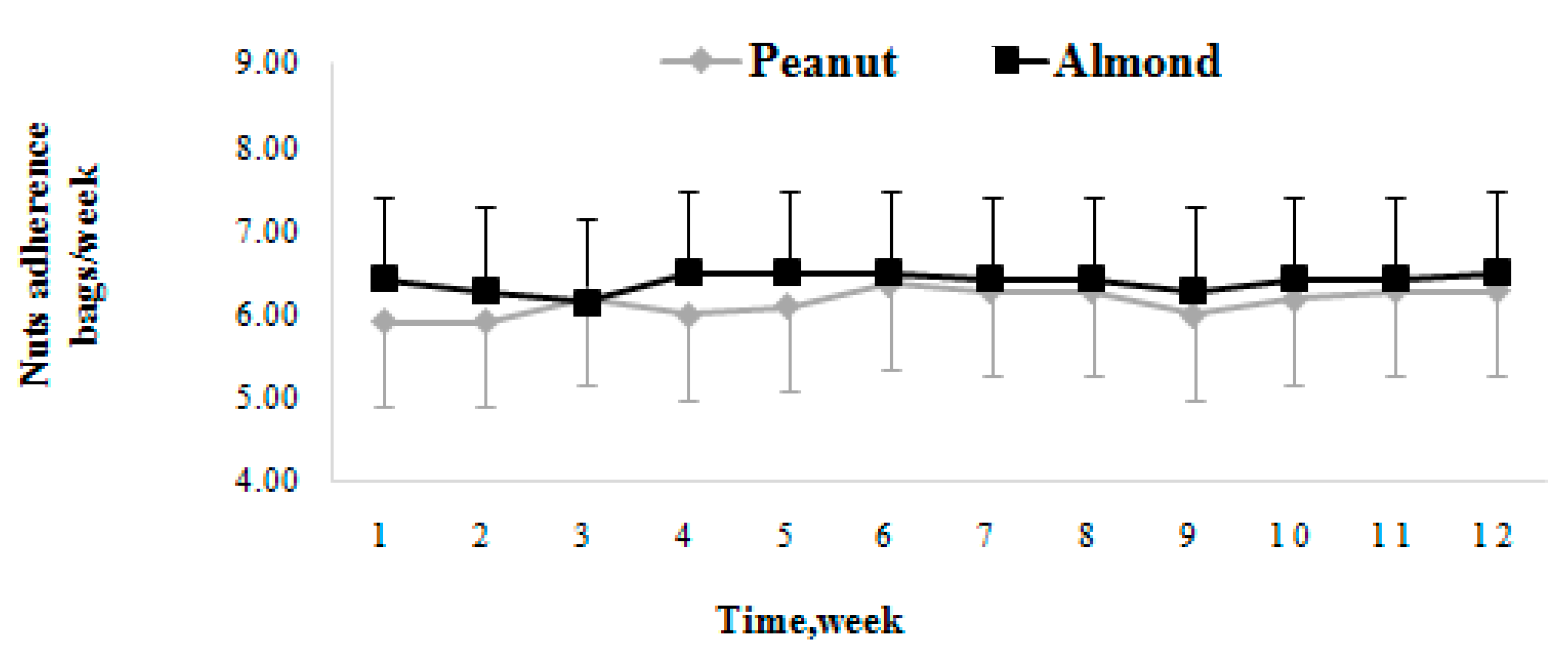
3.2. Dietary Adherence
3.3. Effect of Peanuts and Almonds on Glycemic Control
3.3.1. Fasting Blood Glucose
Changing Trends of Fasting Blood Glucose
Comparison of Fasting Blood Glucose Levels
3.3.2. Postprandial Two-Hour Blood Glucose
Trends in Postprandial Two-Hour Blood Glucose
Comparison of Postprandial Two-Hour Blood Glucose
3.3.3. Glycated Hemoglobin
3.4. Effect of Peanuts and Almonds on Other Cardio-Metabolic and Anthropometric Indicators
3.5. Hypoglycemia and Medication Changes
3.5.1. Incidence of Hypoglycemia
3.5.2. Antidiabetic Drugs Used
3.6. Effect of Peanuts and Almonds onInterleukins-6
3.7. Effect of Peanuts and Almonds on Ratio of Urinary Albumin/Creatinine
4. Discussion
4.1. Effect of Peanuts and Almonds on Glycemic Control
4.2. Other Cardio-Metabolic Indicators
4.3. Hypoglycemia and Medication Changes
4.4. Effect of Peanuts and Almonds on Interleukin-6
4.5. Effect of Peanuts and Almonds on Kidney Burden
5. Limitation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adeghate, E.; Schattner, P.; Dunn, E. An Update on the Etiology and Epidemiology of Diabetes Mellitus. Ann. N. Y. Acad. Sci. 2006, 1084, 1–29. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Organization Global Report on Diabetes: Executive Summary. 2016. Available online: http://www.who.int/diabetes/global-report (accessed on 20 May 2018).
- American Diabetes Association; Bantle, J.P.; Wylie-Rosett, J.; Albright, A.L.; Apovian, C.M.; Clark, N.G.; Franz, M.J.; Hoogwerf, B.J.; Lichtenstein, A.H.; Mayer-Davis, E.; et al. Nutrition recommendations and interventions for diabetes: A position statement of the American Diabetes Association. Diabetes Care 2010, 33, S61–S78. [Google Scholar]
- Forouhi, N.G.; Misra, A.; Mohan, V.; Taylor, R.; Yancy, W. Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ 2018, 361, k2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyson, P.A.; Kelly, T.; Deakin, T.; Duncan, A.; Frost, G.; Harrison, Z.; Khatri, D.; Kunka, D.; McArdle, P.; Mellor, D. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet. Med. 2011, 28, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.L.; Dunbar, S.A.; Jaacks, L.M.; Wahida, K.; Mayer-Davis, E.J.; Judith, W.R., Jr.; William, S. Macronutrients, Food Groups, and Eating Patterns in the Management of Diabetes. Diabetes Care 2010, 35, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.; Luscombe-Marsh, N.D.; Thompson, C.H.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Yancy, W.S., Jr.; Brinkworth, G.D. Comparison of low- and high-carbohydrate diets for type 2 diabetes management: A randomized trial. Am. J. Clin. Nutr. 2015, 102, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.G.M.; Gomes, A.C.; Naves, M.M.V.; Mota, J.F. Nuts and legume seeds for cardiovascular risk reduction: Scientific evidence and mechanisms of action. Nutr. Rev. 2015, 73, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.Y.Y.; Barnes, T. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can. J. Diabetes 2013, 373, S291–S360. [Google Scholar] [CrossRef]
- Anderson, T.J.; Gregoire, J.; Hegele, R.A.; Couture, P.; Mancini, G.B.J.; McPherson, R.; Francis, G.A.; Poirier, P.; Lau, D.C.; Grover, S.; et al. 2012 Update of the Canadian Cardiovascular Society Guidelines for the Diagnosis and Treatment of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Can. J. Cardiol. 2013, 29, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Stroes, E.S.; Thompson, P.D.; Corsini, A.; Vladutiu, G.D.; Raal, F.J.; Ray, K.K.; Roden, M.; Stein, E.; Tokgozoglu, L.; Nordestgaard, B.G.; et al. Statin-associated muscle symptoms: Impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur. Heart J. 2015, 36, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.I.; De Leeuw, I.; Hermansen, K.; Karamanos, B.; Karlstrom, B.; Katsilambros, N.; Riccardi, G.; Rivellese, A.A.; Rizkalla, S.; Slama, G.; et al. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr. Metab. Cardiovasc. 2004, 14, 373–394. [Google Scholar] [CrossRef]
- Viguiliouk, E.; Kendall, C.W.C.; Mejia, B.S.; Cozma, A.I.; Ha, V. Effect of Tree Nuts on Glycemic Control in Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Dietary Trials. PLoS ONE 2014, 9, e103376. [Google Scholar] [CrossRef] [PubMed]
- Musa-Veloso, K.; Paulionis, L.; Poon, T.; Lee, H.Y. The effects of almond consumption on fasting blood lipid levels: A systematic review and meta-analysis of randomised controlled trials. J. Nutr. Sci. 2016, 5, e34. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Chen, C.; Chang, W.; Chen, C.O. The effect of almonds on inflammation and oxidative stress in Chinese patients with type 2 diabetes mellitus: A randomized crossover controlled feeding trial. Eur. J. Nutr. 2013, 52, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Hu, F.B.; Tapsell, L.C.; Josse, A.R.; Kendall, C.W.C. Possible benefit of nuts in type 2 diabetes. J. Nutr. 2008, 138, 1752S–1756S. [Google Scholar] [CrossRef] [PubMed]
- Ros, E. Health Benefits of Nut Consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, C.E.G.; Ribeiro, D.N.; Costa, N.M.B.; Bressan, J.; Alfenas, R.C.G.; Mattes, R.D. Acute and second-meal effects of peanuts on glycaemic response and appetite in obese women with high type 2 diabetes risk: A randomised cross-over clinical trial. Brit. J. Nutr. 2013, 109, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hill, A.; West, S.; Gabauer, R.; McCrea, C.; Fleming, J.; Kris-Etherton, P. Acute Peanut Consumption Alters Postprandial Lipids and Vascular Responses in Healthy Overweight or Obese Men. J. Nutr. 2017, 147, 835–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzby, J.; Pollack, S. Almonds lead increase in tree nut consumption. Amber Waves 2008, 6, 5. [Google Scholar]
- Cohen, A.E.; Johnston, C.S. Almond ingestion at mealtime reduces postprandial glycemia and chronic ingestion reduces hemoglobin A(1c) in individuals with well-controlled type 2 diabetes mellitus. Metabolism 2011, 60, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Asghari, G.; Ghorbani, Z.; Mirmiran, P.; Azizi, F. Nut consumption is associated with lower incidence of type 2 diabetes: The Tehran Lipid and Glucose Study. Diabetes Metab. 2017, 43, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, L. A brief discussion on the increase of fasting blood glucose. Health Guide 2014, 20, 34–35. [Google Scholar]
- Wang, Q. Effect of Loose Low Carbohydrate Diet on Metabolism in Patients with Type 2 Diabetes Mellitus. Master’s Thesis, Soochow University, Suzhou, China, 2015. [Google Scholar]
- Barbour, J.A.; Howe, P.R.C.; Buckley, J.D.; Bryan, J.; Coates, A.M. Effect of 12 Weeks High Oleic Peanut Consumption on Cardio-Metabolic Risk Factors and Body Composition. Nutrients 2015, 7, 7381–7398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Liu, Y.; Liu, J.; Chang, W.; Chen, C.; Chen, C.O. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism 2011, 60, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Collier, G.; O’Dea, K. He effect of coingestion of fat on the glucose, insulin, and gastric inhibitory polypeptide responses to carbohydrate and protein. Am. J. Clin. Nutr. 1983, 37, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.P.; Clark, N.G.; Cox, D.J.; Cryer, P.E.; Davis, S.N.; Dinardo, M.M.; Kahn, R.; Kovatchev, B.; Shamoon, H. Defining and reporting hypoglycemia in diabetes—A report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005, 28, 1245–1249. [Google Scholar]
- Shi, H. The Change of Urinary Albumin-to-Creatinine Ratio in Patients with Coronary Heart Disease and the Relationship between Urinary Albumin-to-Creatinine Ratio and the Severity of Coronary Stenosis. Master’s Thesis, Fudan University, Shanghai, China, 2010. [Google Scholar]
- Chinese Diabetes Society. Guidelines for the prevention and treatment of type 2 diabetes in China. Chin. Med. J. 2017, 4–67. [Google Scholar]
- Reis, C.E.G.; Bordalo, L.A.; Rocha, A.L.C.; Freitas, D.M.O.; Da Silva, M.V.L.; de Faria, V.C.; Martino, H.S.D.; Costa, N.M.B.; Alfenas, R.C. Ground roasted peanuts leads to a lower post-prandial glycemic response than raw peanuts. Nutr. Hosp. 2011, 26, 745–751. [Google Scholar] [PubMed]
- Hernandez-Alonso, P.; Camacho-Barcia, L.; Bullo, M.; Salas-Salvado, J. Nuts and Dried Fruits: An Update of Their Beneficial Effects on Type 2 Diabetes. Nutrients 2017, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Gentilcore, D.; Chaikomin, R.; Jones, K.L.; Russo, A.; Feinle-Bisset, C.; Wishart, J.M.; Rayner, C.K.; Horowitz, M. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 2062–2067. [Google Scholar] [CrossRef] [PubMed]
- Kien, C.L. Dietary interventions for metabolic syndrome: Role of modifying dietary fats. Curr. Diabetes Rep. 2009, 9, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Riserus, U.; Willett, W.C.; Hu, F.B. Dietary fats and prevention of type 2 diabetes. Prog. Lipid Res. 2009, 48, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandalia, M.; Garg, A.; Lutjohann, D.; von Bergmann, K.; Grundy, S.M.; Brinkley, L.J. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2000, 342, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Hopping, B.N.; Etber, E.; Grandinetti, A.; Verheus, M.; Kolonel, L.N.; Maskarinec, G. Dietary Fiber, Magnesium, and Glycemic Load Alter Risk of Type 2 Diabetes in a Multiethnic Cohort in Hawaii. J. Nutr. 2010, 140, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Ketema, E.B.; Kibret, K.T. Correlation of fasting and postprandial plasma glucose with HbA1c in assessing glycemic control; systematic review and meta-analysis. Arch. Public Health 2015, 73, 43. [Google Scholar] [CrossRef] [PubMed]
- Wien, M.; Oda, K.; Sabate, J. A randomized controlled trial to evaluate the effect of incorporating peanuts into an American Diabetes Association meal plan on the nutrient profile of the total diet and cardiometabolic parameters of adults with type 2 diabetes. Nutr. J. 2014, 13, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulati, S.; Misra, A.; Pandey, R.M. Effect of Almond Supplementation on Glycemia and Cardiovascular Risk Factors in Asian Indians in North India with Type 2 Diabetes Mellitus: A 24-Week Study. Metab. Syndr. Relat. Disord. 2017, 15, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Dhillon, J.; Mattes, R.D. A review of the effects of nuts on appetite, food intake, metabolism, and body weight. Am. J. Clin. Nutr. 2014, 100, 412S–422S. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Kanazawa, A.; Hatae, C.; Makita, S.; Komiya, K.; Shimizu, T.; Ikeda, F.; Tamura, Y.; Ogihara, T.; Mita, T.; et al. One year follow-up after a randomized controlled trial of a 130 g/day low-carbohydrate diet in patients with type 2 diabetes mellitus and poor glycemic control. PLoS ONE 2017, 12, e0188892. [Google Scholar] [CrossRef] [PubMed]
- Sabate, J.; Oda, K.; Ros, E. Nut Consumption and Blood Lipid Levels A Pooled Analysis of 25 Intervention Trials. Arch. Intern. Med. 2010, 170, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Rybicka, M.; Krysiak, R.; Okopien, B. The dawn phenomenon and the Somogyi effect-two phenomena of morning hyperglycaemia. Endokrynol. Pol. 2011, 62, 276–283. [Google Scholar] [PubMed]
- Yu, Z.; Malik, V.S.; Keum, N.; Hu, F.B.; Giovannucci, E.L.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S.; Bao, Y. Associations between nut consumption and inflammatory biomarkers. Am. J. Clin. Nutr. 2016, 104, 722–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Zheng, S.; Meng, C.; Hao, J. The role of IL-6 on liver insulin resistance in type 2 diabetes mellitus rats. Chin. J. Lab. Diagn. 2013, 17, 436–439. [Google Scholar]
- When, H.J.; Yang, J.S.; Zhang, J.J. Effect of folic acid combined with pravastatin on inflammatory factors in patients with carotid artery stenosis. Chin. J. Integr. Med. Cardio-Cerebrovasc. Dis. 2013, 11, 111–112. [Google Scholar]
- Scheurig, A.C.; Thorand, B.; Fischer, B.; Heier, M.; Koenig, W. Association between the intake of vitamins and trace elements from supplements and C-reactive protein: Results of the MONICA/KORA Augsburg study. Eur. J. Clin. Nutr. 2008, 62, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Baszczuk, A.; Kopczynski, Z.; Kopczynski, J.; Cymerys, M.; Thielemann, A.; Bielawska, L.; Banaszewska, A. Impact of administration of folic acid on selected indicators of inflammation in patients with primary arterial hypertension. Postep. Hig. Med. Dosw. 2015, 69, 429–435. [Google Scholar] [CrossRef]
- Hasanato, R.M. Diagnostic efficacy of random albumin creatinine ratio for detection of micro and macro-albuminuria in type 2 diabetes mellitus. Saudi Med. J. 2016, 37, 268–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Lopez, A.; Bullo, M.; Angel Martinez-Gonzalez, M.; Guasch-Ferre, M.; Ros, E.; Basora, J.; Covas, M.; Del Carmen Lopez-Sabater, M.; Salas-Salvado, J. Effects of Mediterranean Diets on Kidney Function: A Report from the PREDIMED Trial. Am. J. Kidney Dis. 2012, 60, 380–389. [Google Scholar] [CrossRef] [PubMed]



| Variables | Peanut (n = 11) | Almond (n = 14) | t/χ2 | p | |
|---|---|---|---|---|---|
| ± SD/n (%) | ± SD/n (%) | ||||
| Demographic data | |||||
| Age (years) | 68.00 ± 5.80 | 70.86 ± 8.21 | −0.977 a | NS | |
| Gender—male | 5 (45.5) | 10 (71.4) | - b | NS | |
| Exercise (min/week) | 430.9 ± 222.2 | 421.4 ± 318.5 | 0.084 a | NS | |
| Exercise habits | Never regular exercise | 4 (36.4) | 10 (71.4) | - b | NS |
| Regular exercise | 7 (63.6) | 4 (28.6) | |||
| Like sweets or rice or noodles—no | 1 (9.1) | 1 (7.1) | - b | NS | |
| The amount of staple food, liang/day (1 liang = 50 g) | 3.77 ± 1.75 | 3.61 ± 2.02 | 0.215 a | NS | |
| Consuming nuts—yes | 9 (81.8) | 13 (92.9) | - b | NS | |
| Clinical data | |||||
| Smoking—yes | 2 (18.2) | 0 (0) | - b | NS | |
| SBP (mmHg) | 130.73 ± 7.56 | 128.00 ± 13.77 | 0.589 a | NS | |
| DBP (mmHg) | 79.55 ± 10.25 | 75.71 ± 8.89 | 1.000 a | NS | |
| Family history of diabetes—yes | 5 (45.5) | 6 (42.9) | - b | NS | |
| Diabetes duration, years | 11.27 ± 6.36 | 15.21 ± 8.82 | −1.247 a | NS | |
| Complications—yes | 4 (36.4) | 6 (42.9) | - b | NS | |
| Accompanying diseases—yes | 9 (81.8) | 7 (50.0) | - b | NS | |
| Study Period | Peanut (n = 11) | Almond (n = 14) | F | p |
|---|---|---|---|---|
| Baseline | 7.73 ± 1.19 | 8.28 ± 2.05 | 0.537 | NS |
| Third month | 6.69 ± 0.54 (adjusted: 6.77 ± 0.20) | 6.79 ± 0.92 (adjusted: 6.73 ± 0.17) | 0.016 | NS |
| F | 6.945 | 5.785 | - | - |
| p | 0.016 * | 0.024 * | - | - |
| Study Period | Peanut (n = 11) | Almond (n = 14) | F | p |
|---|---|---|---|---|
| Baseline | 10.36 ± 1.40 | 10.61 ± 2.83 | 0.072 | NS |
| Third month | 8.94 ± 1.55 (adjusted: 9.03 ± 0.38) | 8.91 ± 1.89 (adjusted: 8.85 ± 0.34) | 0.115 | NS |
| F | 5.011 | 4.487 | - | - |
| p | 0.037 * | 0.044 * | - | - |
| Study Period | Peanut (n = 11) | Almond (n = 14) | F | p |
|---|---|---|---|---|
| Baseline | 6.81 ± 0.82 | 7.39 ± 1.16 | 0.072 | NS |
| Third month | 6.76± 0.91 (adjusted: 6.97 ± 0.15) | 6.81 ± 0.73 (adjusted: 6.65 ± 0.13) | 2.453 | NS |
| F | 0.015 | 4.541 | - | - |
| p | NS | 0.043 * | - | - |
| Study Period | Peanut (n = 15) | Almond (n = 17) | F | p |
|---|---|---|---|---|
| Baseline | 6.96 ± 0.89 | 7.36 ± 1.07 | 2.119 | NS |
| Third month | 6.93 ± 0.96 (adjusted: 6.90 ± 0.18) | 6.88 ± 0.71 (adjusted: 6.65 ± 0.11) | 2.361 | NS |
| F | 0.015 | 4.210 | - | - |
| p | NS | 0.048 * | - | - |
| Variables | Study Period | Peanut (n = 11) | Almond (n = 14) | F | p |
|---|---|---|---|---|---|
| BMI (Kg/m2) | Baseline | 22.84 ± 2.48 | 24.08 ± 3.15 | 1.141 | NS |
| Third month | 22.67 ± 2.44 (adjusted: 23.30 ± 0.22) | 23.43 ± 2.90 (adjusted: 22.94 ± 0.20) | 1.482 | NS | |
| F | 0.025 | 1.141 | - | - | |
| p | NS | NS | - | - | |
| Total cholesterol (mmol/L) | Baseline | 4.48 ± 0.77 | 4.90 ± 1.00 | 1.362 | NS |
| Third month | 4.25 ± 0.93 (adjusted: 4.40 ± 0.21) | 4.51 ± 0.86 (adjusted:4.39 ± 0.19) | 0.002 | NS | |
| F | 0.398 | 1.260 | - | - | |
| p | NS | NS | - | - | |
| LDL-C (mmol/L) | Baseline | 2.48 ± 0.72 | 2.97 ± 0.84 | 2.290 | NS |
| Third month | 2.51 ± 0.84 (adjusted: 2.69 ± 0.15) | 2.74 ± 0.63 (adjusted: 2.59 ± 0.14) | 0.234 | NS | |
| F | 0.006 | 0.653 | - | - | |
| p | NS | NS | - | - | |
| HDL-C (mmol/L) | Baseline | 1.49 ± 0.28 | 1.36 ± 0.30 | 1.219 | NS |
| Third month | 1.53 ± 0.22 (adjusted: 1.49 ± 0.05) | 1.34 ± 0.26 (adjusted: 1.38 ± 0.04) | 3.123 | NS | |
| F | 0.197 | 0.029 | - | - | |
| p | NS | NS | - | - | |
| Triglycerides (mmol/L) | Baseline | 1.05 ± 0.46 | 1.87 ± 1.19 | 2.184 | NS |
| Third month | 0.96 ± 0.46 (adjusted: 0.98 ± 0.23) | 1.26 ± 0.87 (adjusted: 1.25 ± 0.20) | 0.777 | NS | |
| F | 0.207 | 1.317 | - | - | |
| p | NS | NS | - | - |
| Study Period | Peanut (n = 11) | Almond (n = 14) | F/χ2 | p | |
|---|---|---|---|---|---|
| ± SD/n (%) | ± SD/n (%) | ||||
| Baseline | No | 1 (9.1%) | 2 (14.3%) | 0.423 a | NS |
| Oral antidiabetic drugs | 7 (63.6%) | 9 (64.3%) | |||
| Insulin | 0 (0%) | 0 (0%) | |||
| Both | 3 (27.3) | 3 (21.4%) | |||
| Third month | No | 1 (9.1%) | 1 (7.1%) | 0.581 a | NS |
| Oral antidiabetic drugs | 7 (63.6%) | 10 (71.4%) | |||
| Insulin | 0 (0%) | 0 (0%) | |||
| Both | 3 (27.3) | 3 (21.4%) | |||
| Insulin dose (IU) | Baseline | 28.33 ± 11.59 | 36.00 ± 24.58 | 0.239 b | NS |
| Third month | 27.00 ± 10.82 (adjusted: 30.19 ± 1.18 d) | 33.33 ± 20.03 (adjusted: 30.14 ± 1.18 d) | 0.001 c | NS | |
| Study Period | Peanut (n = 11) | Almond (n = 14) | F | p |
|---|---|---|---|---|
| Baseline | 12.78 ± 30.62 | 2.18 ± 1.10 | 1.696 | NS |
| Third month | 10.65 ± 26.91 (adjusted: 5.44 ± 0.52) | 2.70 ± 1.83 (adjusted: 6.79 ± 0.45) | 3.761 | NS |
| F | 0.030 | 0.832 | - | - |
| p | NS | NS | - | - |
| Study Period | Peanut (n = 11) | Almond (n = 14) | F | p |
|---|---|---|---|---|
| Baseline | 25.58 ± 26.40 | 18.53 ± 16.19 | 0.679 | NS |
| Third month | 31.47 ± 48.70 (adjusted: 25.55 ± 5.26) | 17.88 ± 21.87 (adjusted: 22.54 ± 4.65) | 0.182 | NS |
| F | 0.124 | 0.008 | - | - |
| p | NS | NS | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.-Y.; Ojo, O.; Wang, L.-L.; Wang, Q.; Jiang, Q.; Shao, X.-Y.; Wang, X.-H. A Randomized Controlled Trial to Compare the Effect of Peanuts and Almonds on the Cardio-Metabolic and Inflammatory Parameters in Patients with Type 2 Diabetes Mellitus. Nutrients 2018, 10, 1565. https://doi.org/10.3390/nu10111565
Hou Y-Y, Ojo O, Wang L-L, Wang Q, Jiang Q, Shao X-Y, Wang X-H. A Randomized Controlled Trial to Compare the Effect of Peanuts and Almonds on the Cardio-Metabolic and Inflammatory Parameters in Patients with Type 2 Diabetes Mellitus. Nutrients. 2018; 10(11):1565. https://doi.org/10.3390/nu10111565
Chicago/Turabian StyleHou, Yun-Ying, Omorogieva Ojo, Li-Li Wang, Qi Wang, Qing Jiang, Xin-Yu Shao, and Xiao-Hua Wang. 2018. "A Randomized Controlled Trial to Compare the Effect of Peanuts and Almonds on the Cardio-Metabolic and Inflammatory Parameters in Patients with Type 2 Diabetes Mellitus" Nutrients 10, no. 11: 1565. https://doi.org/10.3390/nu10111565
APA StyleHou, Y.-Y., Ojo, O., Wang, L.-L., Wang, Q., Jiang, Q., Shao, X.-Y., & Wang, X.-H. (2018). A Randomized Controlled Trial to Compare the Effect of Peanuts and Almonds on the Cardio-Metabolic and Inflammatory Parameters in Patients with Type 2 Diabetes Mellitus. Nutrients, 10(11), 1565. https://doi.org/10.3390/nu10111565






