In Vitro and In Vivo Functional Characterization of Essence of Chicken as An Ergogenic Aid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Groupings
2.3. Sample Collection
2.4. Weight-Loaded Forced Swimming Test (WFST)
2.5. Forelimb Group Strength
2.6. Cell culture and Treatment
2.7. Blood Biochemical Indices upon Acute 10-min Free Swimming Test
2.8. The 90-min Free Swimming Test with Serum Biochemical Measurements
2.9. Measurement of Antioxidant Status in Liver and Muscle
2.10. Tissue Glycogen Concentration
2.11. Histological Staining of Tissues
2.12. Statistical Analyses
3. Results
3.1. Effect of EC on Food and Water Consumption, Body Weight and Organ Weight
3.2. Effect of Four-Week EC Supplementation on Weight Loaded Forced Swimming Test
3.3. Effect of EC Supplementation on Forelimb Grip Strength
3.4. Effect of EC Treatment on C2C12 Myotubes
3.5. Effect of EC Supplementation on Lactate after a 10-min Swimming Test
3.6. Effect of EC Supplementation on Serum Ammonia, Creatine Kinase and Glucose after a 10-min Swimming Test
3.7. Effect of 4-Week EC Supplementation on BUN and after a 90-min Swimming Test
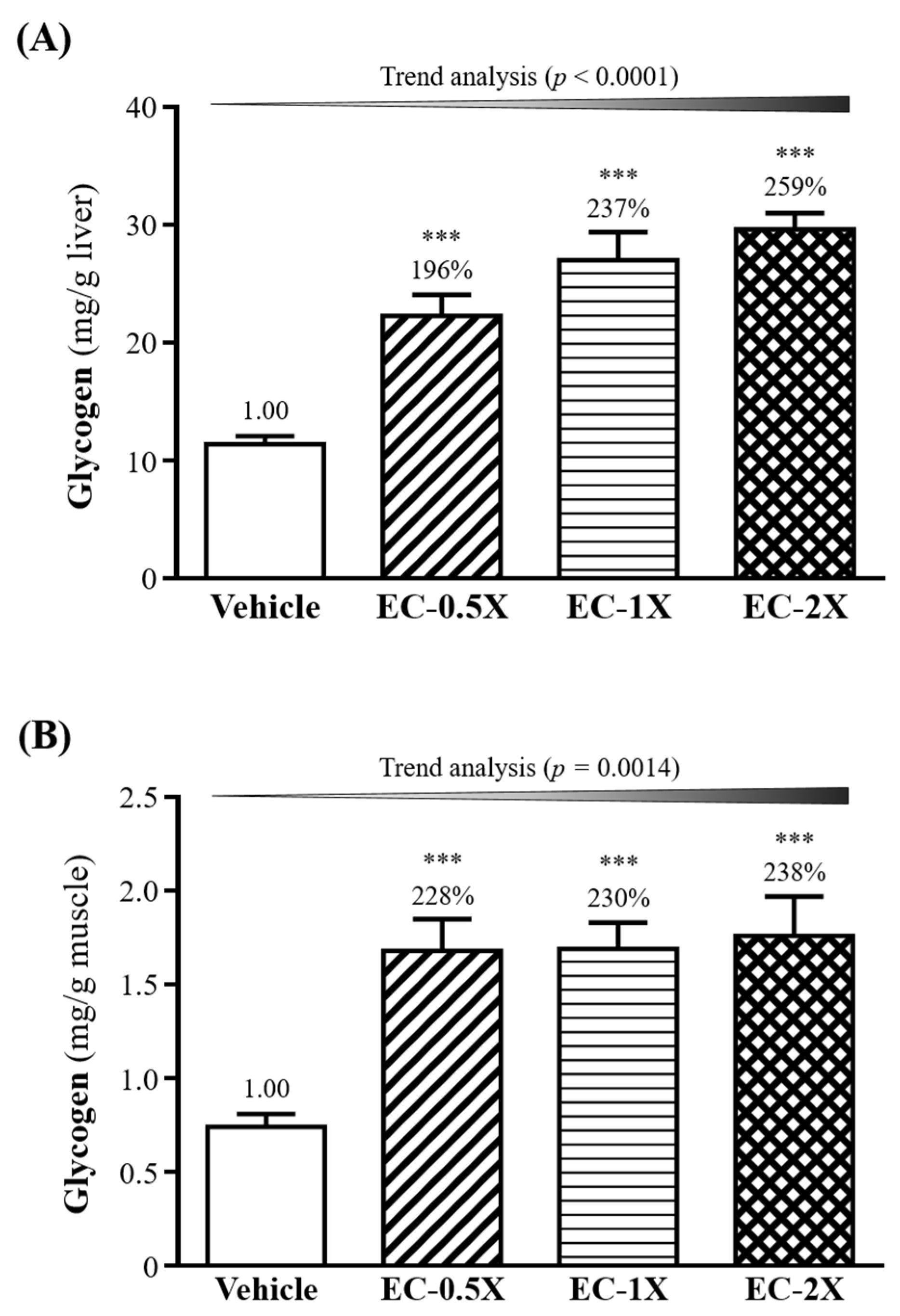
3.8. Effect of EC Supplementation on Liver and Muscle Glycogen
3.9. Effect of EC Supplementation on Liver and Muscle Antioxidant Status
3.10. Effect of EC Supplementation on Biochemical Variables at the End of the Experiment
3.11. Effect of EC Supplementation on Histopathological Evaluation of Tissues
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Y.F.; He, R.R.; Tsoi, B.; Kurihara, H. Bioactivities of chicken essence. J. Food Sci. 2012, 77, R105–R110. [Google Scholar] [CrossRef]
- Ikeda, T.; Nishijima, Y.; Kiso, Y.; Shibata, H.; Ono, H.; Moritani, T. Effects of Chicken Essence Tablets on Resting Metabolic Rate. Biosci. Biotechnol. Biochem. 2001, 65, 2083–2086. [Google Scholar] [CrossRef]
- Geissler, C.; Boroumand-Naini, M.; Tomassen, C. Large acute thermic response to chicken essence in humans. Nutr. Rep. Int. USA 1989. [Google Scholar]
- Soong, Y.Y.; Lim, J.; Sun, L.; Henry, C.J. Effect of co-ingestion of amino acids with rice on glycaemic and insulinaemic response. Br. J. Nutr. 2015, 114, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wei Jie Tan, K.; Jeyakumar Henry, C. Co-ingestion of essence of chicken to moderate glycaemic response of bread. Int. J. Food Sci Nutr 2015, 66, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.-I.; Tsi, D.; Tan, A.C.L.; Wang, S.-W.; Hsu, M.-C. Effects of postexercise supplementation of chicken essence on the elimination of exercise-induced plasma lactate and ammonia. Chin. J. Physiol. 2005, 48, 187–192. [Google Scholar] [PubMed]
- Young, H.; Benton, D.; Carter, N. The Effect of Chicken Extract on Mood, Cognition and Heart Rate Variability. Nutrients 2015, 7, 887–904. [Google Scholar] [CrossRef]
- Azhar, M.Z.; Abdul Razak, K.; Mohsin, S. The Use of Chicken Essence as an Adjunct to Psychotherapy in Anxious Subject—A Clinical Assessment. Malays. J. Psychiatry 2001, 9, 13–22. [Google Scholar]
- Nagai, H.; Harada, M.; Nakagawa, M.; Tanaka, T.; Gunadi, B.; Setiabudi, M.L.; Uktolseja, J.L.; Miyata, Y. Effects of chicken extract on the recovery from fatigue caused by mental workload. Appl. Human Sci. 1996, 15, 281–286. [Google Scholar] [CrossRef]
- Yamano, E.; Tanaka, M.; Ishii, A.; Tsuruoka, N.; Abe, K.; Watanabe, Y. Effects of chicken essence on recovery from mental fatigue in healthy males. Med. Sci. Monit. 2013, 19, 540–547. [Google Scholar] [CrossRef]
- Zain, A.M.; Syedsahiljamalulail, S. Effect of taking chicken essence on stress and cognition of human volunteers. Malays. J. Nutr. 2003, 9, 19–29. [Google Scholar] [PubMed]
- Geissler, C.; Boroumand-Naini, M.; Harada, M.; Iino, T.; Hirai, K.; Suwa, Y.; Tanaka, T.; Iwata, S. Chicken extract stimulates haemoglobin restoration in iron deficient rats. Int. J. Food Sci. Nutr. 1996, 47, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Migita, T.; Mitsuzono, R.; Komiya, S. Effect of BEC (Brands Essence of Chicken) Ingestion on Physical and Mental Fatigue During Intensive Long Distance Running Training. Kurume J. Health Phys. Educ. 1998, 6, 9–14. [Google Scholar]
- Azizbeigi, K.; Stannard, S.; Atashak, S.; Mosalman Haghighi, M. Antioxidant enzymes and oxidative stress adaptation to exercise training: Comparison of endurance, resistance, and concurrent training in untrained males. J. Exerc. Sci. Fitness 2014, 12. [Google Scholar] [CrossRef]
- Reid, M.B. Free radicals and muscle fatigue: Of ROS, canaries, and the IOC. Free Radic. Biol. Med. 2008, 44, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Corbi, G.; Conti, V.; Davinelli, S.; Scapagnini, G.; Filippelli, A.; Ferrara, N. Dietary Phytochemicals in Neuroimmunoaging: A New Therapeutic Possibility for Humans? Front. Pharmacol. 2016, 7, 364. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Corbi, G.; Zarrelli, A.; Arisi, M.; Calzavara-Pinton, P.; Grassi, D.; De Vivo, I.; Scapagnini, G. Short-term supplementation with flavanol-rich cocoa improves lipid profile, antioxidant status and positively influences the AA/EPA ratio in healthy subjects. J. Nutr. Biochem. 2018, 61, 33–39. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic. Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Kan, N.-W.; Ho, C.-S.; Chiu, Y.-S.; Huang, W.-C.; Chen, P.-Y.; Tung, Y.-T.; Huang, C.-C. Effects of Resveratrol Supplementation and Exercise Training on Exercise Performance in Middle-Aged Mice. Molecules 2016, 21, 661. [Google Scholar] [CrossRef]
- Huang, C.-C.; Hsu, M.-C.; Huang, W.-C.; Yang, H.-R.; Hou, C.-C. Triterpenoid-Rich Extract from Antrodia camphorata Improves Physical Fatigue and Exercise Performance in Mice. Evid Based Complement. Alternat. Med. 2012, 2012, 364741. [Google Scholar] [CrossRef]
- Chen, W.-C.; Huang, W.-C.; Chiu, C.-C.; Chang, Y.-K.; Huang, C.-C. Whey protein improves exercise performance and biochemical profiles in trained mice. Med. Sci. Sports Exerc. 2014, 46, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.C.; Ito, H.; Blau, H.M.; Torti, F.M. Tumor necrosis factor inhibits human myogenesis in vitro. Mol. Cell. Biol. 1988, 8, 2295–2301. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.M.; Kiely, M.; Jakeman, P.M.; Kiely, P.A.; Carson, B.P. Optimization of an in vitro bioassay to monitor growth and formation of myotubes in real time. Biosci. Rep. 2016, 36. [Google Scholar] [CrossRef] [PubMed]
- Fouré, A.; Bendahan, D. Is Branched-Chain Amino Acids Supplementation an Efficient Nutritional Strategy to Alleviate Skeletal Muscle Damage? A Systematic Review. Nutrients 2017, 9, 47. [Google Scholar] [CrossRef]
- Brown, D.R.; Gough, L.A.; Deb, S.K.; Sparks, S.A.; McNaughton, L.R. Astaxanthin in Exercise Metabolism, Performance and Recovery: A Review. Front. Nutr. 2017, 4, 76. [Google Scholar] [CrossRef] [PubMed]
- Funes, L.; Carrera-Quintanar, L.; Cerdán-Calero, M.; Ferrer, M.D.; Drobnic, F.; Pons, A.; Roche, E.; Micol, V. Effect of lemon verbena supplementation on muscular damage markers, proinflammatory cytokines release and neutrophils’ oxidative stress in chronic exercise. Eur. J. Appl. Physiol. 2011, 111, 695–705. [Google Scholar] [CrossRef]
- Burattini, S.; Ferri, P.; Battistelli, M.; Curci, R.; Luchetti, F.; Falcieri, E. C2C12 murine myoblasts as a model of skeletal muscle development: Morpho-functional characterization. Eur. J. Histochem. 2004, 48, 223–233. [Google Scholar]
- Girgis, C.M.; Clifton-Bligh, R.J.; Mokbel, N.; Cheng, K.; Gunton, J.E. Vitamin D signaling regulates proliferation, differentiation, and myotube size in C2C12 skeletal muscle cells. Endocrinology 2014, 155, 347–357. [Google Scholar] [CrossRef]
- Maki, T.; Yamamoto, D.; Nakanishi, S.; Iida, K.; Iguchi, G.; Takahashi, Y.; Kaji, H.; Chihara, K.; Okimura, Y. Branched-chain amino acids reduce hindlimb suspension-induced muscle atrophy and protein levels of atrogin-1 and MuRF1 in rats. Nutr. Res. 2012, 32, 676–683. [Google Scholar] [CrossRef]
- Yamamoto, D.; Maki, T.; Herningtyas, E.H.; Ikeshita, N.; Shibahara, H.; Sugiyama, Y.; Nakanishi, S.; Iida, K.; Iguchi, G.; Takahashi, Y.; et al. Branched-chain amino acids protect against dexamethasone-induced soleus muscle atrophy in rats. Muscle Nerve 2010, 41, 819–827. [Google Scholar] [CrossRef]
- Oelkrug, C.; Horn, K.; Makert, G.R.; Schubert, A. Novel In Vitro Platform to Investigate Myotube Atrophy. Anticancer Res. 2015, 35, 2085–2091. [Google Scholar] [PubMed]
- Cairns, S.P. Lactic acid and exercise performance: Culprit or friend? Sports Med. 2006, 36, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Peixoto, J.; Alves, R.C.; Cameron, L.-C. Glutamine and carbohydrate supplements reduce ammonemia increase during endurance field exercise. Appl. Physiol. Nutr. Metab. 2007, 32, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Drory, V.E. Wet, volatile, and dry biomarkers of exercise-induced muscle fatigue. BMC Musculoskelet. Disord. 2016, 17, 40. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine kinase monitoring in sport medicine. Br. Med. Bull. 2007, 81–82, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Baird, M.F.; Graham, S.M.; Baker, J.S.; Bickerstaff, G.F. Creatine-Kinase- and Exercise-Related Muscle Damage Implications for Muscle Performance and Recovery. Available online: https://www.hindawi.com/journals/jnme/2012/960363/ (accessed on 21 May 2018).
- Koo, H.-N.; Lee, J.-K.; Hong, S.-H.; Kim, H.-M. Herbkines increases physical stamina in mice. Biol. Pharm. Bull. 2004, 27, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yao, X.; Bao, B.; Zhang, Y. Anti-fatigue activity of a triterpenoid-rich extract from Chinese bamboo shavings (Caulis bamfusae in taeniam). Phytother. Res. 2006, 20, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Ørtenblad, N.; Westerblad, H.; Nielsen, J. Muscle glycogen stores and fatigue. J. Physiol. (Lond.) 2013, 591, 4405–4413. [Google Scholar] [CrossRef]
- Jensen, J.; Rustad, P.I.; Kolnes, A.J.; Lai, Y.-C. The Role of Skeletal Muscle Glycogen Breakdown for Regulation of Insulin Sensitivity by Exercise. Front. Physiol. 2011, 2. [Google Scholar] [CrossRef]
- Dohm, G.L.; Tapscott, E.B.; Barakat, H.A.; Kasperek, G.J. Influence of fasting on glycogen depletion in rats during exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 830–833. [Google Scholar] [CrossRef]
- Holloszy, J.O.; Kohrt, W.M.; Hansen, P.A. The regulation of carbohydrate and fat metabolism during and after exercise. Front. Biosci. 1998, 3, D1011–D1027. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; DeRuisseau, K.C.; Quindry, J.; Hamilton, K.L. Dietary antioxidants and exercise. J. Sports Sci. 2004, 22, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Suzuki, K.; Coombes, J.S. The influence of antioxidant supplementation on markers of inflammation and the relationship to oxidative stress after exercise. J. Nutr. Biochem. 2007, 18, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Hamilton, K. Antioxidants and exercise. Clin. Sports Med. 1999, 18, 525–536. [Google Scholar] [CrossRef]
- Zhai, Y.-J.; He, R.-R.; Tsoi, B.; Li, Y.-F.; Li, X.-D.; Tsuruoka, N.; Abe, K.; Kurihara, H. Protective effect of extract of chicken meat on restraint stress-induced liver damage in mice. Food Funct. 2012, 3, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-C.; Lin, S.-H. Effects of chicken extract on antioxidative status and liver protection under oxidative stress. J. Nutr. Sci. Vitaminol. 2004, 50, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-R.; Peng, H.-C.; Lin, S.-H. Effects of chicken extract on plasma antioxidative status and lipid oxidation in healthy rats. J. Nutr. Sci. Vitaminol. 2004, 50, 320–324. [Google Scholar] [CrossRef]
- Atmaca, G. Antioxidant effects of sulfur-containing amino acids. Yonsei Med. J. 2004, 45, 776–788. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Uchida, K.; Kawakishi, S. Sequence-dependent reactivity of histidine-containing peptides with copper(II)/ascorbate. J. Agric. Food Chem. 1992, 40, 13–16. [Google Scholar] [CrossRef]
- Girgih, A.T.; He, R.; Malomo, S.; Offengenden, M.; Wu, J.; Aluko, R.E. Structural and functional characterization of hemp seed (Cannabis sativa L.) protein-derived antioxidant and antihypertensive peptides. J. Funct. Foods 2014, 6, 384–394. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Dávalos, A.; Bartolomé, B.; Amigo, L. Preparation of antioxidant enzymatic hydrolysates from alpha-lactalbumin and beta-lactoglobulin. Identification of active peptides by HPLC-MS/MS. J. Agric. Food Chem. 2005, 53, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Lee, J.H.; Kim, D.H.; Kim, K.-T.; Lee, G.W.; Choi, S.J.; Chang, P.-S.; Paik, H.-D. Antioxidative and nitric oxide scavenging activity of branched-chain amino acids. Food Sci. Biotechnol. 2015, 24, 1555–1558. [Google Scholar] [CrossRef]
- Coombes, J.S.; McNaughton, L.R. Effects of branched-chain amino acid supplementation on serum creatine kinase and lactate dehydrogenase after prolonged exercise. J. Sports Med. Phys. Fitness 2000, 40, 240–246. [Google Scholar] [PubMed]
- Sun, Y.; Pan, D.; Guo, Y.; Li, J. Purification of chicken breast protein hydrolysate and analysis of its antioxidant activity. Food Chem. Toxicol. 2012, 50, 3397–3404. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Pan, B.S.; Chang, C.L.; Shiau, C.Y. Low Molecular Weight Peptides as Related to Antioxidant Properties of Chicken Essence. J. Food Drug Anal. 2005, 13, 176–183. [Google Scholar]
- Chan, K.M.; Decker, E.A. Endogenous skeletal muscle antioxidants. Crit. Rev. Food Sci. Nutr. 1994, 34, 403–426. [Google Scholar] [CrossRef]
- Kohen, R.; Yamamoto, Y.; Cundy, K.C.; Ames, B.N. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc. Natl. Acad. Sci. USA. 1988, 85, 3175–3179. [Google Scholar] [CrossRef]
- Avena, R.M.; Bowen, W.J. Effects of carnosine and anserine on muscle adenosine triphosphatases. J. Biol. Chem. 1969, 244, 1600–1604. [Google Scholar]
- Sato, M.; Suzuki, Y.; Morimatsu, F.; Takamatsu, K. Effect of carnosine concentration in muscle and improvement of exercise performances due to long-term intake of chicken breast extract. Jpn. J. Phys. Fitness Sports Med. 2003, 52, 255–263. [Google Scholar] [CrossRef]






| Ingredients | Amount |
|---|---|
| Proteins and amino acids (mg/mL) | |
| Protein and peptides | 83.0 |
| Free amino acid | 3.1 |
| l-anserine | 2.3 |
| l-carnosine | 0.8 |
| Taurine | 0.7 |
| Carbohydrate (mg/mL) | |
| Hexose | 0.8 |
| Lipid (mg/mL) | |
| Lipids | 0.4 |
| Minerals (µg/mL) | |
| Calcium | 26 |
| Iron | 1 |
| Zinc | 2 |
| Magnesium | 32 |
| Potassium | 1740 |
| Sodium | 550 |
| Chlorine | 1340 |
| Phosphorus | 480 |
| Sulfur | 500 |
| Copper | 2 |
| Manganese | 5 |
| Selenium | 0.05 |
| Vitamins (µg/mL) | |
| Vitamin B2 | 1.0 |
| Vitamin B6 | 0.37 |
| Vitamin B12 | 0.002 |
| Niacin | 6.4 |
| Folic acid | 0.15 |
| Vitamin C | 15 |
| Characteristic | Vehicle | EC-0.5X | EC-1X | EC-2X | Trend Analysis |
|---|---|---|---|---|---|
| Initial BW (g) | 37.30 ± 1.15 | 37.76 ± 0.60 | 37.37 ± 0.99 | 37.58 ± 0.90 | 0.9082 |
| 1st week BW (g) | 38.61 ± 1.07 | 38.55 ± 0.86 | 38.43 ± 0.99 | 38.86 ± 0.86 | 0.8380 |
| 2nd week BW (g) | 38.76 ± 1.08 | 38.84 ± 0.86 | 38.62 ± 1.01 | 39.25 ± 0.99 | 0.7187 |
| 3rd week BW (g) | 39.58 ± 0.98 | 39.68 ± 0.75 | 39.13 ± 1.10 | 39.84 ± 0.90 | 0.8847 |
| 4th week BW (g) | 39.81 ± 0.96 | 40.05 ± 0.70 | 39.64 ± 1.07 | 40.36 ± 0.98 | 0.7102 |
| 5th week BW (g) | 40.14 ± 0.96 | 40.34 ± 0.70 | 40.06 ± 1.05 | 40.60 ± 1.99 | 0.7501 |
| Final BW (g) | 40.41 ± 0.86 | 41.58 ± 0.78 | 41.04 ± 1.01 | 40.95 ± 1.02 | 0.8552 |
| Food intake (g/day) | 8.95 ± 0.18 | 8.75 ± 0.19 | 8.72 ± 0.16 | 8.55 ± 0.14 | 0.1054 |
| Water intake (mL/day) | 11.02 ± 0.11 | 11.00 ± 0.16 | 11.03 ± 0.14 | 11.03 ± 0.16 | 0.9389 |
| Liver (g) | 2.310 ± 0.047 | 2.328 ± 0.056 | 2.327 ± 0.053 | 2.331 ± 0.081 | 0.8289 |
| Kidney (g) | 0.661 ± 0.021 | 0.663 ± 0.027 | 0.662 ± 0.018 | 0.653 ± 0.019 | 0.7564 |
| Heart (g) | 0.227 ± 0.012 | 0.229 ± 0.010 | 0.229 ± 0.011 | 0.230 ± 0.007 | 0.8496 |
| Lung (g) | 0.232 ± 0.007 | 0.232 ± 0.006 | 0.238 ± 0.007 | 0.230 ± 0.012 | 0.8190 |
| Muscle (g) | 0.371 ± 0.009 | 0.382 ± 0.007 | 0.379 ± 0.008 | 0.375 ± 0.009 | 0.8743 |
| EFP (g) | 0.261 ± 0.020 | 0.264 ± 0.029 | 0.262 ± 0.016 | 0.266 ± 0.011 | 0.6049 |
| BAT (g) | 0.095 ± 0.011 | 0.104 ± 0.010 | 0.101 ± 0.009 | 0.104 ± 0.009 | 0.9125 |
| Relative liver weight (%) | 5.719 ± 0.052 | 5.698 ± 0.081 | 5.672 ± 0.050 | 5.688 ± 0.114 | 0.9683 |
| Relative kidney weight (%) | 1.635 ± 0.036 | 1.592 ± 0.043 | 1.615 ± 0.027 | 1.594 ± 0.028 | 0.5164 |
| Relative Heart weight (%) | 0.562 ± 0.030 | 0.553 ± 0.024 | 0.561 ± 0.026 | 0.563 ± 0.020 | 0.8790 |
| Relative Lung weight (%) | 0.575 ± 0.015 | 0.559 ± 0.006 | 0.557 ± 0.013 | 0.566 ± 0.030 | 0.8130 |
| Relative Muscle weight (%) | 0.918 ± 0.013 | 0.919 ± 0.011 | 0.925 ± 0.013 | 0.916 ± 0.018 | 0.9830 |
| Relative EFP weight (%) | 0.646 ± 0.048 | 0.641 ± 0.078 | 0.634 ± 0.025 | 0.647 ± 0.019 | 0.5667 |
| Relative BAT weight (%) | 0.231 ± 0.023 | 0.252 ± 0.025 | 0.245 ± 0.019 | 0.253 ± 0.021 | 0.9317 |
| Time Point | Vehicle | EC-0.5X | EC-1X | EC-2X | Trend Analysis |
|---|---|---|---|---|---|
| Lactate (mmol/L) | |||||
| Before swimming [A] | 3.0 ± 0.2 | 3.0 ± 0.2 | 3.0 ± 0.2 | 3.0 ± 0.1 | 0.9906 |
| After 10 min swim [B] | 6.2 ± 0.1 | 4.8 ± 0.1 *** | 4.8 ± 0.2 *** | 4.3 ± 0.1 *** | <0.0001(↓) |
| At rest for 20 min [C] | 5.0 ± 0.2 | 3.3 ± 0.1 *** | 3.3 ± 0.1 *** | 3.0 ± 0.1 *** | <0.0001(↓) |
| Increase ratio [B/A] | 2.16 ± 0.14 | 1.68 ± 0.10 ** | 1.66 ± 0.10 ** | 1.47 ± 0.07 *** | 0.0002(↓) |
| Clearance (%) [(B-C)/B] | 0.20 ± 0.03 | 0.31 ± 0.03 ** | 0.30 ± 0.03 * | 0.30 ± 0.02 * | 0.0717 (↑) |
| Characteristic | Vehicle | EC-0.5X | EC-1X | EC-2X | Trend Analysis |
|---|---|---|---|---|---|
| Liver tissues | |||||
| SOD (U/mg) | 2.27 ± 0.10 | 3.01 ± 0.12 ** | 3.09 ± 0.20 ** | 3.25 ± 0.27 *** | 0.0024↑) |
| GPX (nmol/min/mg protein) | 2.04 ± 0.24 | 2.13 ± 0.17 | 2.56 ± 0.24 | 2.88 ± 0.24 * | 0.0048(↑) |
| GR (nmol/min/mg protein) | 1.79 ± 0.12 | 2.07 ± 0.13 | 2.08 ± 0.24 | 2.14 ± 0.24 | 0.2522 |
| CAT (nmol/min/mg protein) | 7.28 ± 0.39 | 9.78 ± 0.31 *** | 10.86 ± 0.33 *** | 12.12 ± 0.32 *** | <0.0001(↑) |
| GSH (µM/mg protein) | 1.57 ± 0.12 a | 1.78 ± 0.13 b | 1.81 ± 0.13 b | 1.81 ± 0.12 b | 0.0015(↑) |
| Muscle tissues | |||||
| SOD (U/mg) | 63 ± 4 | 100 ± 5 *** | 101±6 *** | 105 ± 5 *** | 0.0002(↑) |
| GPX (nmol/min/mg protein) | 201 ± 11 | 206 ± 10 | 206 ± 8 | 207 ± 11 | 0.6676 |
| GR (nmol/min/mg protein) | 122 ± 5 | 122 ± 6 | 126 ± 4 | 129 ± 6 | 0.2710 |
| CAT (nmol/min/mg protein) | 24 ± 1 | 25 ± 1 | 25 ± 1 | 25 ± 2 | 0.6670 |
| GSH (µM/mg protein) | 0.18 ± 0.01 | 0.19 ± 0.02 | 0.19 ± 0.02 | 0.19 ± 0.02 | 0.8085 |
| Characteristic | Vehicle | EC-0.5X | EC-1X | EC-2X | Trend Analysis |
|---|---|---|---|---|---|
| AST (U/L) | 72 ± 3 | 72 ± 3 | 71 ± 3 | 71 ± 2 | 0.7116 |
| ALT (U/L) | 45 ± 3 | 44 ± 3 | 45 ± 3 | 43 ± 3 | 0.6582 |
| CK (U/L) | 124 ± 7 | 120 ± 7 | 114 ± 8 | 111 ± 7 | 0.1858 |
| TP (g/dL) | 4.9 ± 0.1 | 5.0 ± 0.1 | 5.0 ± 0.0 | 5.0 ± 0.1 | 0.5979 |
| Albumin (g/dL) | 2.7 ± 0.1 | 2.8 ± 0.0 | 2.8 ± 0.0 | 2.8 ± 0.1 | 0.6893 |
| BUN (mg/dL) | 20.3 ± 0.5 | 20.2 ± 0.7 | 20.4 ± 0.3 | 20.5 ± 0.5 | 0.7425 |
| Creatinine (mg/dL) | 0.23 ± 0.01 | 0.23 ± 0.01 | 0.23 ± 0.01 | 0.23 ± 0.01 | 0.7530 |
| UA (mg/dL) | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.0 | 0.9 ± 0.1 | 0.8945 |
| TC (mg/dL) | 144 ± 4 | 144 ± 3 | 141 ± 4 | 141 ± 3 | 0.4556 |
| TG (mg/dL) | 148 ± 3 | 144 ± 3 | 144 ± 2 | 142 ± 3 | 0.2008 |
| Glucose (mg/dL) | 138 ± 5 | 144 ± 2 | 143 ± 2 | 144 ± 3 | 0.2959 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-W.; Hsu, Y.-J.; Lee, M.-C.; Li, H.-S.; Yeo, P.C.W.; Lim, A.L.; Huang, C.-C. In Vitro and In Vivo Functional Characterization of Essence of Chicken as An Ergogenic Aid. Nutrients 2018, 10, 1943. https://doi.org/10.3390/nu10121943
Huang S-W, Hsu Y-J, Lee M-C, Li H-S, Yeo PCW, Lim AL, Huang C-C. In Vitro and In Vivo Functional Characterization of Essence of Chicken as An Ergogenic Aid. Nutrients. 2018; 10(12):1943. https://doi.org/10.3390/nu10121943
Chicago/Turabian StyleHuang, Shih-Wei, Yi-Ju Hsu, Mon-Chien Lee, Hua-Shuai Li, Paul Chee Wei Yeo, Ai Lin Lim, and Chi-Chang Huang. 2018. "In Vitro and In Vivo Functional Characterization of Essence of Chicken as An Ergogenic Aid" Nutrients 10, no. 12: 1943. https://doi.org/10.3390/nu10121943
APA StyleHuang, S.-W., Hsu, Y.-J., Lee, M.-C., Li, H.-S., Yeo, P. C. W., Lim, A. L., & Huang, C.-C. (2018). In Vitro and In Vivo Functional Characterization of Essence of Chicken as An Ergogenic Aid. Nutrients, 10(12), 1943. https://doi.org/10.3390/nu10121943







