Seafood Consumption, Omega-3 Fatty Acids Intake, and Life-Time Prevalence of Depression in the PREDIMED-Plus Trial
Abstract
:1. Introduction
2. Methods
2.1. Design and Participants
2.2. Exposure Assessment
2.3. Outcome Assessment
2.4. Covariate Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Mykletun, A.; Bjerkeset, O.; Overland, S.; Prince, M.; Dewey, M.; Stewart, R. Levels of anxiety and depression as predictors of mortality: The HUNT study. Br. J. Psychiatry 2009, 195, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Paul Amminger, G.; Balanzá-Martínez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. International Society for Nutritional Psychiatry Research consensus position statement: Nutritional medicine in modern psychiatry. World Psychiatry 2015, 14, 370–371. [Google Scholar] [CrossRef] [PubMed]
- Wysoczański, T.; Sokoła-Wysoczańska, E.; Pękala, J.; Lochyński, S.; Czyż, K.; Bodkowski, R.; Herbinger, G.; Patkowska-Sokoła, B.; Librowski, T. Omega-3 Fatty Acids and their Role in Central Nervous System—A Review. Curr. Med. Chem. 2016, 23, 816–831. [Google Scholar] [CrossRef] [PubMed]
- Kiecolt-Glaser, J.K.; Derry, H.M.; Fagundes, C.P. Inflammation: Depression fans the flames and feasts on the heat. Am. J. Psychiatry 2015, 172, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Mischoulon, D.; Schweitzer, I. Omega-3 for bipolar disorder: Meta-analyses of use in mania and bipolar depression. J. Clin. Psychiatry 2012, 73, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.T.; Chibnall, J.T.; Nasrallah, H.A. A meta-analysis of placebo-controlled trials of omega-3 fatty acid augmentation in schizophrenia: Possible stage-specific effects. Ann. Clin. Psychiatry 2015, 27, 289–296. [Google Scholar] [PubMed]
- Appleton, K.M.; Rogers, P.J.; Ness, A.R. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am. J. Clin. Nutr. 2010, 91, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Mocking, R.J.; Harmsen, I.; Assies, J.; Koeter, M.W.; Ruhé, H.G.; Schene, A.H. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl. Psychiatry 2016, 6, e756. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Micek, A.; Marventano, S.; Castellano, S.; Mistretta, A.; Pajak, A.; Galvano, F. Dietary n-3 PUFA, fish consumption and depression: A systematic review and meta-analysis of observational studies. J. Affect. Disord. 2016, 205, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, Y.; Je, Y. Fish consumption and risk of depression: Epidemiological evidence from prospective studies. Asia Pac. Psychiatry 2018, e12335. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Fanelli Kuczmarski, M.T.; Beydoun, H.A.; Hibbeln, J.R.; Evans, M.K.; Zonderman, A.B. ω-3 fatty acid intakes are inversely related to elevated depressive symptoms among United States women. J. Nutr. 2013, 143, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Miyake, Y.; Sasaki, S.; Tanaka, K.; Arakawa, M. Fish and n-3 polyunsaturated fatty acid intake and depressive symptoms: Ryukyus Child Health Study. Pediatrics 2010, 126, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Timonen, M.; Horrobin, D.; Jokelainen, J.; Laitinen, J.; Herva, A.; Räsänen, P. Fish consumption and depression: The Northern Finland 1966 birth cohort study. J. Affect. Disord. 2004, 82, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dai, Q.; Ekperi, LI.; Dehal, A.; Zhang, J. Fish consumption and severely depressed mood, findings from the first national nutrition follow-up study. Psychiatry Res. 2011, 190, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Je, Y. Fish consumption and depression in Korean adults: The Korea National Health and Nutrition Examination Survey, 2013-2015. Eur. J. Clin. Nutr. 2018, 72, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Sanderson, K.; McNaughton, S.A.; Gall, S.L.; Dwyer, T.; Venn, A.J. Longitudinal associations between fish consumption and depression in young adults. Am. J. Epidemiol. 2014, 179, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ballart, J.D.; Piñol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Perez-Bauer, M.; Martínez-González, M.A.; Salas-Salvadó, J.; Martí-Moreno, J.M. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mataix, V.J. Tabla De Composición De Alimentos [Food Composition Tables], 5th ed.; Universidad de Granada: Granada, Spain, 2003; ISBN 9788433849809. [Google Scholar]
- Moreiras, O.; Cabrera, L.C.A. Tablas De Composición De Alimentos [Food Composition Tables], 16th ed.; Ediciones Pirámide: Madrid, Spain, 2005; ISBN Mkt0004474369. [Google Scholar]
- Sánchez-Villegas, A.; Schlatter, J.; Ortuno, F.; Lahortiga, F.; Pla, J.; Benito, S.; Martinez-Gonzalez, M.A. Validity of a self-reported diagnosis of depression among participants in a cohort study using the Structured Clinical Interview for DSM-IV (SCID-I). BMC Psychiatry 2008, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.; Navarro, M.E.; Vázquez, C. Adaptación española para el Inventario de Depresión de Beck-II (BDI-II). 1. Propiedades psicométricas en estudiantes universitarios. Análisis y Modificación de Conducta 2003, 29, 239–288. [Google Scholar]
- Elosua, R.; Garcia, M.; Aguilar, A.; Molina, L.; Covas, M.I.; Marrugat, J. Validation of the Minnesota Leisure Time Physical Activity Questionnaire In Spanish Women. Investigators of the MARATDON Group. Med. Sci. Sports Exerc. 2000, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Elosua, R.; Marrugat, J.; Molina, L.; Pons, S.; Pujol, E. Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish men. The MARATHOM Investigators. Am. J. Epidemiol. 1994, 139, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 compendium of physical activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Villegas, A.; Henríquez, P.; Figueiras, A.; Ortuño, F.; Lahortiga, F.; Martínez-González, M.A. Long chain omega-3 fatty acids intake, fish consumption and mental disorders in the SUN cohort study. Eur. J. Nutr. 2007, 46, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, Y.J.; Sawada, N.; Mimura, M.; Shikimoto, R.; Nozaki, S.; Hamazaki, K.; Uchitomi, Y.; Tsugane, S. Dietary fish, n-3 polyunsaturated fatty acid consumption, and depression risk in Japan: A population-based prospective cohort study. Transl. Psychiatry 2017, 7, e1242. [Google Scholar] [CrossRef] [PubMed]
- Hibbeln, J.R.; Nieminen, L.R.; Blasbalg, T.L.; Riggs, J.A.; Lands, W.E. Healthy intakes of n-3 and n-6 fatty acids: Estimations considering worldwide diversity. Am. J. Clin. Nutr. 2006, 83 (Suppl. 6), 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.Y.; Cheon, Y.; Faurot, K.F.; Macintosh, B.; Majchrzak-Hong, S.F.; Mann, J.D.; Hibbeln, J.R.; Ringel, A.; Ramsden, C.E. Dietary omega-6 fatty acid lowering increases bioavailability of omega-3 polyunsaturated fatty acids in human plasma lipid pools. Prostaglandins Leukot. Essent. Fatty Acids 2014, 90, 151–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czerner, M.; Agustinelli, S.P.; Guccione, S.; Yeannes, M.I. Effect of different preservation processes on chemical composition and fatty acid profile of anchovy (Engraulis anchoita). Int. J. Food Sci. Nutr. 2015, 66, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; Pasco, J.A.; Williams, L.J.; Meyer, B.J.; Digger, R.; Berk, M. Dietary intake of fish and PUFA, and clinical depressive and anxiety disorders in women. Br. J. Nutr. 2013, 109, 2059–2066. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Mirzaei, F.; O’Reilly, E.J.; Pan, A.; Willett, W.C.; Kawachi, I.; Koenen, K.; Ascherio, A. Dietary intake of n-3 and n-6 fatty acids and the risk of clinical depression in women: A 10-y prospective follow-up study. Am. J. Clin. Nutr. 2011, 93, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Sierra, S.; Lara-Villoslada, F.; Comalada, M.; Olivares, M.; Xaus, J. Dietary eicosapentaenoic acid and docosahexaenoic acid equally incorporate as decosahexaenoic acid but differ in inflammatory effects. Nutrition 2008, 24, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Zunszain, P.A.; Hepgul, N.; Pariante, C.M. Inflammation and depression. Curr. Top Behav. Neurosci. 2013, 14, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J.; Gallacher, J.E.; Hatch, E.E. Why representativeness should be avoided. Int. J. Epidemiol. 2013, 42, 1012–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Kerr, D.A.; Zhu, K.; Devine, A.; Solah, V.A.; Wright, J.; Binns, C.W.; Prince, R.L. Under-reporting of energy intake in elderly Australian women is associated with a higher body mass index. J. Nutr. Health Aging 2013, 17, 112–118. [Google Scholar] [CrossRef] [PubMed]
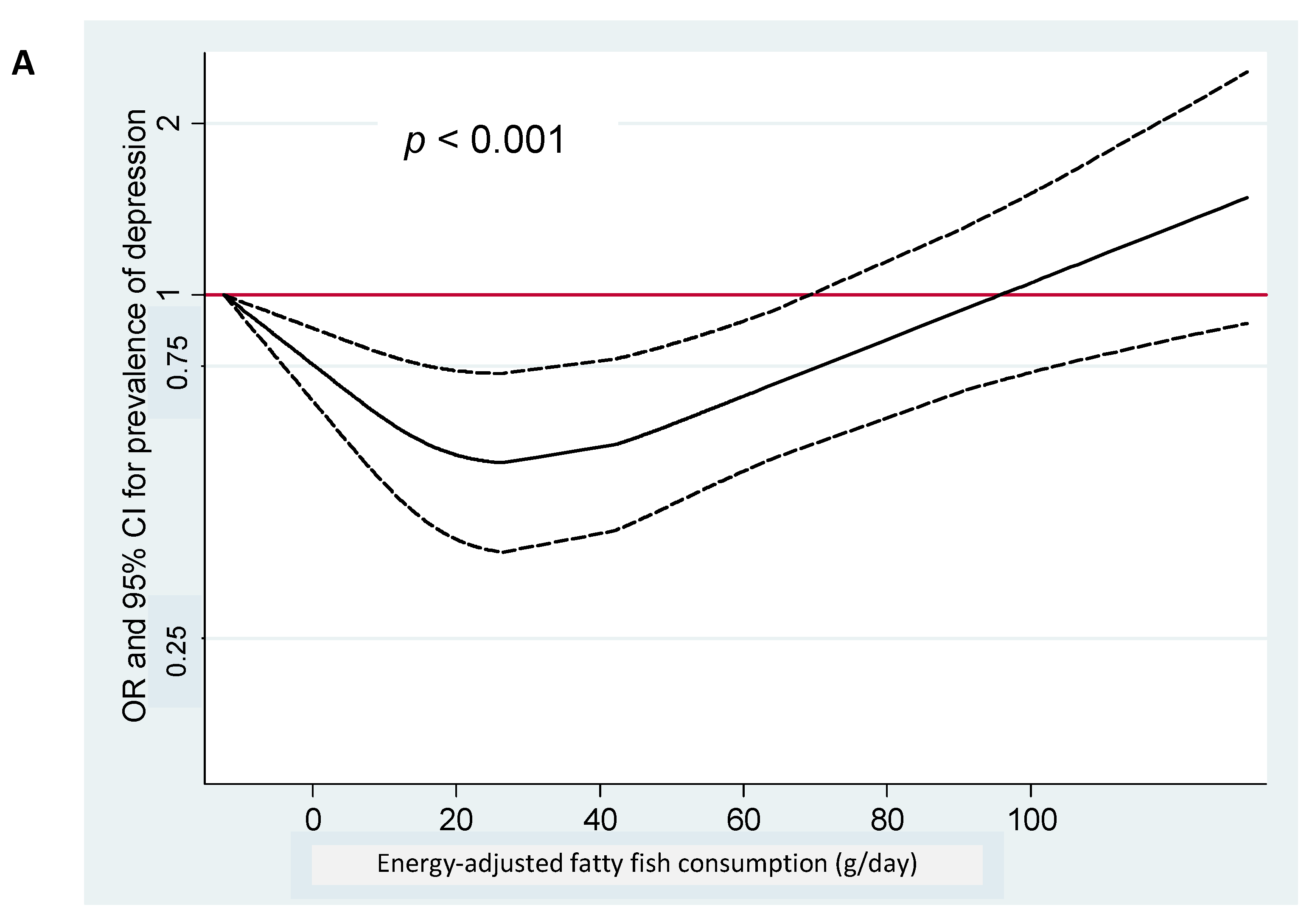
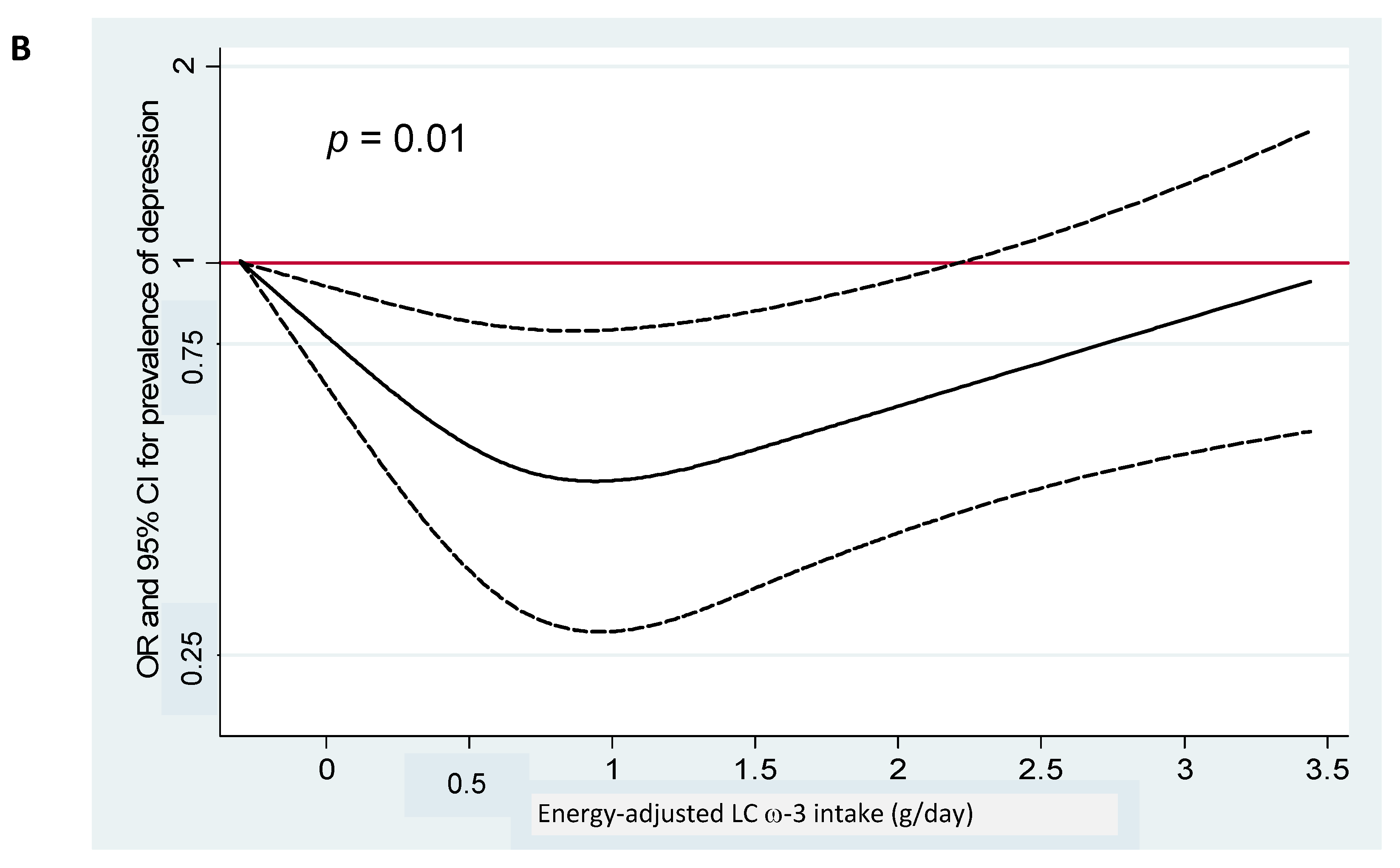
| Total Fish/Seafood | LC ω-3 PUFA (EPA + DHA + DPA) | |||||||
|---|---|---|---|---|---|---|---|---|
| Q1 | Q3 | Q5 | p | Q1 | Q3 | Q5 | p | |
| Age (mean, SD) | 64.3 (5.1) | 65.3 (4.9) | 65.1 (4.6) | <0.001 | 64.6 (5.1) | 65.0 (5.0) | 65.3 (4.7) | 0.005 |
| Sex (%) | <0.001 | <0.001 | ||||||
| Men | 65.3 | 51.8 | 41.5 | 63.3 | 51.4 | 44.4 | ||
| Educational level | 0.206 | 0.438 | ||||||
| Primary | 21.6 | 21.5 | 24.0 | 20.7 | 21.3 | 24.7 | ||
| Secondary | 28.6 | 30.1 | 29.3 | 28.9 | 30.3 | 26.7 | ||
| University | 49.2 | 47.3 | 46.1 | 49.8 | 47.6 | 47.7 | ||
| Unknown | 0.6 | 1.2 | 0.5 | 0.6 | 0.8 | 0.9 | ||
| Marital status | 0.723 | 0.647 | ||||||
| Married | 75.0 | 77.9 | 76.7 | 76.5 | 77.1 | 76.4 | ||
| Body mass index (mean, SD) | 32.7 (3.4) | 32.5 (3.4) | 32.7 (3.4) | 0.439 | 32.7 (3.4) | 32.8 (3.4) | 32.6 (3.4) | 0.420 |
| Smoking | <0.001 | <0.001 | ||||||
| Current smoker | 15.9 | 13.2 | 9.9 | 14.5 | 12.6 | 9.9 | ||
| Ex-smoker | 46.5 | 41.5 | 43.2 | 46.5 | 42.8 | 42.7 | ||
| Never smoker | 37.6 | 45.3 | 46.8 | 39.0 | 44.6 | 47.4 | ||
| Physical activity (METs-min/w) (mean, SD) | 2458 (2540) | 2410 (2314) | 2718 (2359) | 0.010 | 2388 (2501) | 2423 (2188) | 2714 (2346) | 0.002 |
| Presence of diseases | ||||||||
| Hypercholesterolemia | 66.7 | 69.4 | 69.5 | 0.044 | 66.8 | 69.4 | 70.0 | 0.002 |
| Type 2 Diabetes | 25.3 | 27.5 | 29.2 | 0.268 | 25.9 | 27.4 | 28.1 | 0.578 |
| Hypertension | 83.8 | 82.8 | 83.1 | 0.663 | 83.0 | 84.8 | 81.8 | 0.228 |
| Mediterranean diet (0–8 score) (mean, SD) | 3.6 (1.5) | 3.9 (1.5) | 4.0 (1.5) | <0.001 | 3.7 (1.5) | 3.8 (1.5) | 4.0 (1.5) | <0.001 |
| Energy intake (Kcal/day) (mean, SD) | 2476 (604) | 2332 (535) | 2356 (517) | 0.010 | 2540 (593) | 2219 (488) | 2356 (486) | <0.001 |
| Total Fish/Seafood | ||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | p for trend | |
| Median (g/day) | 40.53 | 67.95 | 90.70 | 117.26 | 155.28 | |
| Cases | 265 | 263 | 261 | 283 | 295 | |
| Model 1 | 1 (ref.) | 0.85 (0.70–1.03) | 0.84 (0.69–1.02) | 0.87 (0.72–1.06) | 0.87 (0.71–1.05) | 0.297 |
| Model 2 | 1 (ref.) | 0.87 (0.71–1.06) | 0.86 (0.71–1.05) | 0.91 (0.75–1.11) | 0.93 (0.77–1.13) | 0.777 |
| Model 3 | 1 (ref.) | 0.87 (0.71–1.06) | 0.87 (0.71–1.06) | 0.91 (0.75–1.11) | 0.93 (0.76–1.13) | 0.752 |
| Model 4 | 1 (ref.) | 0.86 (0.71–1.05) | 0.86 (0.71–1.06) | 0.91 (0.74–1.11) | 0.94 (0.77–1.14) | 0.851 |
| Fatty Fish | ||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | p for trend | |
| Median (g/day) | 2.70 | 10.01 | 15.60 | 20.39 | 53.24 | |
| Cases | 277 | 259 | 231 | 301 | 299 | |
| Model 1 | 1 (ref.) | 0.78 (0.64–0.95) | 0.69 (0.57–0.85) | 0.80 (0.66–0.97) | 0.80 (0.66–0.97) | 0.338 |
| Model 2 | 1 (ref.) | 0.80 (0.65–0.97) | 0.72 (0.59–0.88) | 0.82 (0.68–1.00) | 0.85 (0.70–1.03) | 0.701 |
| Model 3 | 1 (ref.) | 0.79 (0.65–0.97) | 0.72 (0.59–0.88) | 0.82 (0.67–1.00) | 0.85 (0.70–1.04) | 0.711 |
| Model 4 | 1 (ref.) | 0.77 (0.63–0.94) | 0.71 (0.58–0.87) | 0.78 (0.64–0.96) | 0.84 (0.69–1.03) | 0.759 |
| Lean Fish (excluding salty cod) | ||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | p for trend | |
| Median (g/day) | 5.87 | 15.33 | 21.54 | 57.80 | 63.43 | |
| Cases | 269 | 195 | 322 | 185 | 396 | |
| Model 1 | 1 (ref.) | 0.83 (0.68–1.03) | 0.76 (0.62–0.92) | 0.75 (0.60–0.92) | 0.87 (0.71–1.06) | 0.310 |
| Model 2 | 1 (ref.) | 0.86 (0.70–1.06) | 0.78 (0.64–0.95) | 0.79 (0.64–0.98) | 0.91 (0.74–1.11) | 0.614 |
| Model 3 | 1 (ref.) | 0.86 (0.70–1.06) | 0.79 (0.64–0.96) | 0.80 (0.65–0.99) | 0.91 (0.75–1.11) | 0.644 |
| Model 4 | 1 (ref.) | 0.86 (0.69–1.06) | 0.77 (0.63–0.94) | 0.83 (0.67–1.02) | 0.88 (0.72–1.08) | 0.658 |
| Canned Fish (natural and oily) | ||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | p for trend | |
| Median (g/day) | 1.55 | 4.47 | 6.91 | 14.08 | 21.99 | |
| Cases | 260 | 269 | 266 | 299 | 273 | |
| Model 1 | 1 (ref.) | 1.00 (0.82–1.22) | 0.95 (0.78–1.15) | 1.17 (0.96–1.42) | 1.02 (0.84–1.24) | 0.356 |
| Model 2 | 1 (ref.) | 1.01 (0.82–1.23) | 0.95 (0.78–1.16) | 1.16 (0.96–1.41) | 1.00 (0.82–1.22) | 0.478 |
| Model 3 | 1 (ref.) | 1.01 (0.83–1.24) | 0.95 (0.78–1.16) | 1.16 (0.95–1.41) | 1.00 (0.83–1.22) | 0.509 |
| Model 4 | 1 (ref.) | 0.97 (0.80–1.19) | 0.91 (0.75–1.12) | 1.13 (0.93–1.38) | 0.97 (0.79–1.18) | 0.625 |
| Other Seafood | ||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | p for trend | |
| Median (g/day) | 2.99 | 17.42 | 27.40 | 32.38 | 48.96 | |
| Cases | 295 | 262 | 265 | 279 | 266 | |
| Model 1 | 1 (ref.) | 0.89 (0.73–1.08) | 0.89 (0.73–1.07) | 0.93 (0.77–1.12) | 0.89 (0.74–1.08) | 0.328 |
| Model 2 | 1 (ref.) | 0.92 (0.76–1.12) | 0.92 (0.76–1.12) | 0.99 (0.82–1.20) | 0.94 (0.77–1.14) | 0.665 |
| Model 3 | 1 (ref.) | 0.91 (0.75–1.11) | 0.91 (0.75–1.11) | 0.99 (0.82–1.20) | 0.93 (0.77–1.13) | 0.622 |
| Model 4 | 1 (ref.) | 0.90 (0.74–1.10) | 0.94 (0.77–1.14) | 0.97 (0.80–1.18) | 0.94 (0.77–1.14) | 0.674 |
| Total ω-3 PUFA (LC ω-3 PUFA + ALA) | ||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | p for trend | |
| Median (g/day) | 1.305 | 1.731 | 2.072 | 2.540 | 3.316 | |
| Cases | 265 | 281 | 269 | 282 | 270 | |
| Model 1 | 1 (ref.) | 0.96 (0.79–1.16) | 0.88 (0.72–1.07) | 0.91 (0.75–1.10) | 0.85 (0.70–1.04) | 0.107 |
| Model 2 | 1 (ref.) | 0.97 (0.79–1.17) | 0.91 (0.75–1.11) | 0.94 (0.78–1.15) | 0.90 (0.74–1.10) | 0.334 |
| Model 3 | 1 (ref.) | 0.96 (0.79–1.17) | 0.92 (0.75–1.12) | 0.94 (0.77–1.15) | 0.91 (0.74–1.10) | 0.362 |
| Model 4 | 1 (ref.) | 0.92 (0.75–1.12) | 0.87 (0.71–1.06) | 0.92 (0.75–1.12) | 0.89 (0.73–1.08) | 0.368 |
| LC ω-3 PUFA (EPA + DHA + DPA) | ||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | p for trend | |
| Median (g/day) | 0.355 | 0.562 | 0.710 | 0.947 | 1.550 | |
| Cases | 286 | 255 | 266 | 274 | 286 | |
| Model 1 | 1 (ref.) | 0.76 (0.62–0.92) | 0.78 (0.64–0.95) | 0.75 (0.62–0.91) | 0.79 (0.65–0.96) | 0.126 |
| Model 2 | 1 (ref.) | 0.76 (0.66–0.93) | 0.80 (0.66–0.97) | 0.78 (0.64–0.95) | 0.84 (0.69–1.01) | 0.364 |
| Model 3 | 1 (ref.) | 0.77 (0.63–0.93) | 0.80 (0.66–0.97) | 0.77 (0.64–0.94) | 0.84 (0.69–1.02) | 0.355 |
| Model 4 | 1 (ref.) | 0.75 (0.62–0.92) | 0.77 (0.63–0.94) | 0.76 (0.62–0.92) | 0.83 (0.68–1.01) | 0.384 |
| EPA + DHA | ||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | p for trend | |
| Median (g/day) | 0.235 | 0.407 | 0.535 | 0.754 | 1.167 | |
| Cases | 270 | 247 | 284 | 274 | 292 | |
| Model 1 | 1 (ref.) | 0.77 (0.63–0.94) | 0.84 (0.69–1.02) | 0.78 (0.64–0.95) | 0.82 (0.67–0.99) | 0.179 |
| Model 2 | 1 (ref.) | 0.78 (0.64–0.95) | 0.87 (0.71–1.06) | 0.81 (0.66–0.98) | 0.87 (0.71–1.05) | 0.462 |
| Model 3 | 1 (ref.) | 0.78 (0.64–0.95) | 0.87 (0.72–1.06) | 0.80 (0.66–0.98) | 0.87 (0.71–1.05) | 0.451 |
| Model 4 | 1 (ref.) | 0.77 (0.63–0.94) | 0.85 (0.70–1.03) | 0.79 (0.65–0.97) | 0.87 (0.71–1.06) | 0.538 |
| α-Linolenic Acid (ALA) | ||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | p for trend | |
| Median (g/day) | 0.785 | 1.034 | 1.215 | 1.484 | 2.283 | |
| Cases | 266 | 286 | 275 | 279 | 261 | |
| Model 1 | 1 (ref.) | 1.00 (0.82–1.21) | 0.95 (0.78–1.15) | 0.98 (0.81–1.20) | 0.87 (0.72–1.06) | 0.144 |
| Model 2 | 1 (ref.) | 1.01 (0.83–1.22) | 0.95 (0.78–1.16) | 1.01 (0.83–1.22) | 0.91 (0.75–1.11) | 0.319 |
| Model 3 | 1 (ref.) | 1.02 (0.84–1.23) | 0.96 (0.79–1.17) | 1.02 (0.83–1.24) | 0.92 (0.76–1.13) | 0.398 |
| Model 4 | 1 (ref.) | 0.95 (0.78–1.16) | 0.87 (0.71–1.08) | 0.95 (0.77–1.17) | 0.89 (0.72–1.09) | 0.346 |
| Regression Coefficient * | 95% Confidence Interval | |
|---|---|---|
| Total seafood | ||
| Q1 | 0 (ref.) | |
| Q2 | −0.692 | −1.242 to −0.141 |
| Q3 | −0.595 | −0.648 to 0.459 |
| Q4 | −0.571 | −1.127 to −0.015 |
| Q5 | −0.478 | −1.040 to 0.083 |
| Fatty fish | ||
| Q1 | 0 (ref.) | |
| Q2 | −0.715 | −1.268 to −0.163 |
| Q3 | −0.938 | −1.494 to −0.382 |
| Q4 | −0.719 | −1.290 to −0.149 |
| Q5 | −0.504 | −1.071 to 0.064 |
| Lean fish | ||
| Q1 | 0 (ref.) | |
| Q2 | −0.528 | −1.082 to 0.025 |
| Q3 | −0.934 | −1.524 to −0.344 |
| Q4 | −0.609 | −1.164 to −0.055 |
| Q5 | −0.844 | −1.473 to −0.216 |
| LC ω-3 PUFA (EPA + DHA + DPA) | ||
| Q1 | 0 (ref.) | |
| Q2 | −0.487 | −1.038 to 0.064 |
| Q3 | −0.923 | −1.481 to −0.364 |
| Q4 | −0.413 | −0.970 to 0.144 |
| Q5 | −0.428 | −0.987 to 0.130 |
| EPA + DHA | ||
| Q1 | 0 (ref.) | |
| Q2 | −0.720 | −1.273 to −0.167 |
| Q3 | −0.777 | −1.340 to −0.213 |
| Q4 | −0.398 | −0.958 to 0.161 |
| Q5 | −0.533 | −1.097 to 0.031 |
| Fatty Fish | |||||
| Q2 | Q3 | Q4 | Q5 | p interaction | |
| Sex | 0.558 | ||||
| Men (3393) | 0.78 (0.57–1.06) | 0.71 (0.52–0.98) | 0.95 (0.69–1.32) | 0.85 (0.61–1.20) | |
| Women (3194) | 0.74 (0.57–0.97) | 0.71 (0.54–0.94) | 0.70 (0.54–0.91) | 0.82 (0.63–1.06) | |
| Diabetes ** | 0.932 | ||||
| No (4759) | 0.74 (0.59–0.94) | 0.69 (0.54–0.88) | 0.76 (0.60–0.96) | 0.81 (0.64–1.03) | |
| Yes (1810) | 0.82 (0.56–1.21) | 0.73 (0.49–1.10) | 0.81 (0.55–1.20) | 0.87 (0.59–1.28) | |
| Mediterranean diet | 0.437 | ||||
| <4 (2717) | 0.73 (0.55–0.96) | 0.65 (0.48–0.88) | 0.64 (0.48–0.85) | 0.82 (0.61–1.11) | |
| ≥4 (3870) | 0.82 (0.61–1.09) | 0.78 (0.59–1.04) | 0.93 (0.70–1.24) | 0.88 (0.67–1.16) | |
| Smoking | 0.588 | ||||
| Non-smoker (5771) | 0.74 (0.60–0.92) | 0.70 (0.57–0.87) | 0.75 (0.61–0.93) | 0.84 (0.68–1.04) | |
| Current smoker (816) | 0.93 (0.54–1.60) | 0.71 (0.39–1.28) | 1.02 (0.55–1.88) | 0.74 (0.40–1.39) | |
| LC ω-3 PUFA (EPA + DHA + DPA) | |||||
| Q2 | Q3 | Q4 | Q5 | p interaction | |
| Sex | 0.951 | ||||
| Men (3393) | 0.73 (0.54–1.00) | 0.74 (0.54–1.02) | 0.78 (0.56–1.08) | 0.77 (0.55–1.07) | |
| Women (3194) | 0.77 (0.60–1.00) | 0.79 (0.61–1.03) | 0.75 (0.58–0.97) | 0.87 (0.67–1.11) | |
| Diabetes ** | 0.951 | ||||
| No (4759) | 0.73 (0.58–0.92) | 0.75 (0.60–0.95) | 0.77 (0.61–0.97) | 0.82 (0.65–1.04) | |
| Yes (1810) | 0.82 (0.56–1.22) | 0.81 (0.55–1.20) | 0.73 (0.49–1.07) | 0.83 (0.56–1.21) | |
| Mediterranean diet | 0.821 | ||||
| <4 (2717) | 0.68 (0.51–0.91) | 0.71 (0.53–0.94) | 0.69 (0.52–0.92) | 0.81 (0.60–1.09) | |
| ≥4 (3870) | 0.83 (0.63–1.09) | 0.84 (0.64–1.12) | 0.81 (0.62–1.06) | 0.87 (0.67–1.13) | |
| Smoking | 0.234 | ||||
| Non-smoker (5771) | 0.71 (0.57–0.88) | 0.78 (0.63–0.97) | 0.75 (0.61–0.93) | 0.85 (0.69–1.05) | |
| Current smoker (816) | 1.06 (0.62–1.81) | 0.61 (0.34–1.11) | 0.78 (0.44–1.41) | 0.59 (0.31–1.12) | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Villegas, A.; Álvarez-Pérez, J.; Toledo, E.; Salas-Salvadó, J.; Ortega-Azorín, C.; Zomeño, M.D.; Vioque, J.; Martínez, J.A.; Romaguera, D.; Pérez-López, J.; et al. Seafood Consumption, Omega-3 Fatty Acids Intake, and Life-Time Prevalence of Depression in the PREDIMED-Plus Trial. Nutrients 2018, 10, 2000. https://doi.org/10.3390/nu10122000
Sánchez-Villegas A, Álvarez-Pérez J, Toledo E, Salas-Salvadó J, Ortega-Azorín C, Zomeño MD, Vioque J, Martínez JA, Romaguera D, Pérez-López J, et al. Seafood Consumption, Omega-3 Fatty Acids Intake, and Life-Time Prevalence of Depression in the PREDIMED-Plus Trial. Nutrients. 2018; 10(12):2000. https://doi.org/10.3390/nu10122000
Chicago/Turabian StyleSánchez-Villegas, Almudena, Jacqueline Álvarez-Pérez, Estefanía Toledo, Jordi Salas-Salvadó, Carolina Ortega-Azorín, Maria Dolores Zomeño, Jesús Vioque, Jose Alfredo Martínez, Dora Romaguera, Jessica Pérez-López, and et al. 2018. "Seafood Consumption, Omega-3 Fatty Acids Intake, and Life-Time Prevalence of Depression in the PREDIMED-Plus Trial" Nutrients 10, no. 12: 2000. https://doi.org/10.3390/nu10122000
APA StyleSánchez-Villegas, A., Álvarez-Pérez, J., Toledo, E., Salas-Salvadó, J., Ortega-Azorín, C., Zomeño, M. D., Vioque, J., Martínez, J. A., Romaguera, D., Pérez-López, J., López-Miranda, J., Estruch, R., Bueno-Cavanillas, A., Arós, F., Tur, J. A., Tinahones, F. J., Lecea, O., Martín, V., Ortega-Calvo, M., ... Serra-Majem, L. (2018). Seafood Consumption, Omega-3 Fatty Acids Intake, and Life-Time Prevalence of Depression in the PREDIMED-Plus Trial. Nutrients, 10(12), 2000. https://doi.org/10.3390/nu10122000


















