Current Evidence about Nutrition Support in Cardiac Surgery Patients—What Do We Know?
Abstract
:1. Introduction: Cardiac Surgery, Inflammation and Current Standard of Nutrition
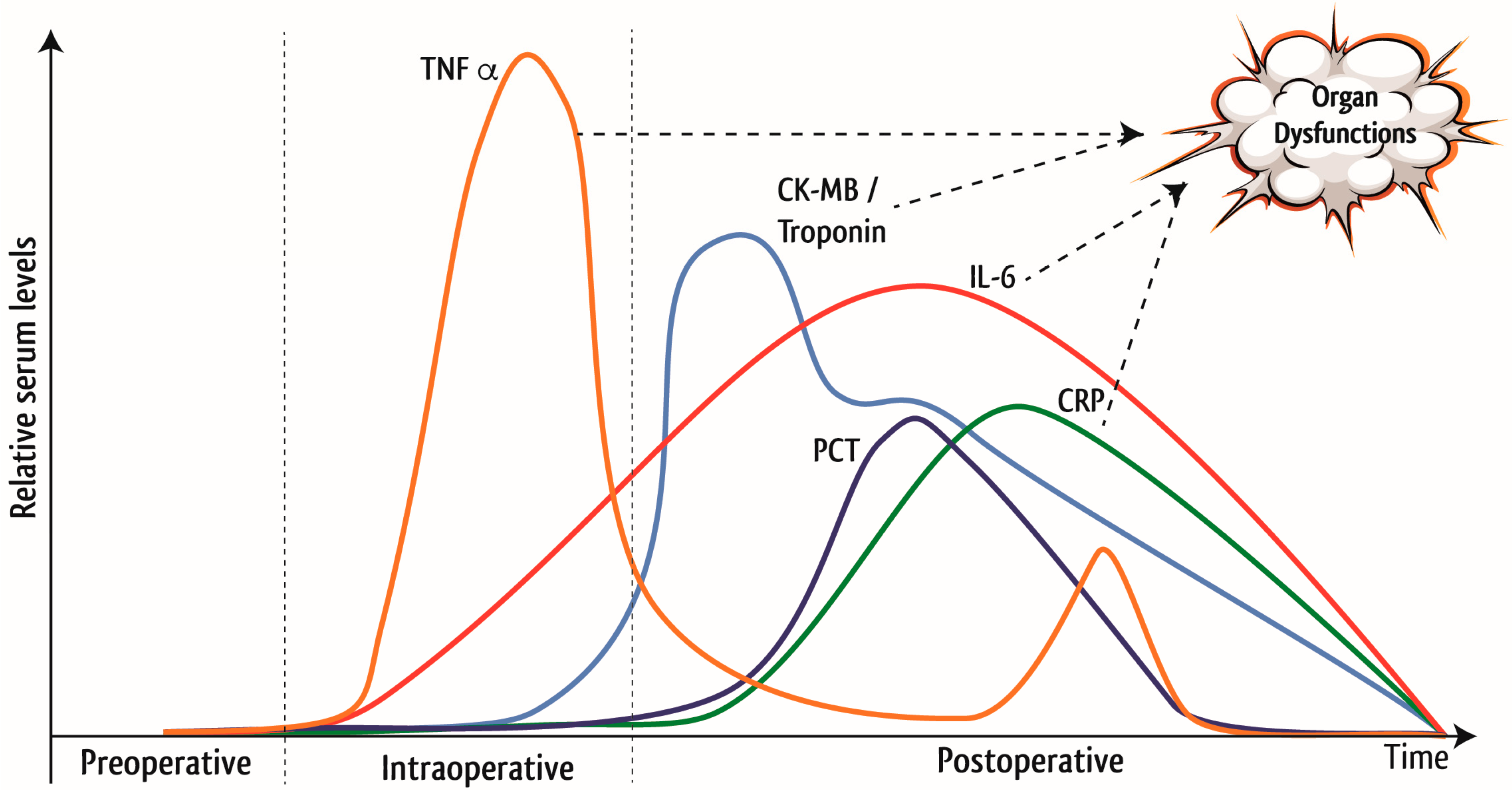
1.1. Inflammation in Cardiac Surgery
1.2. Importance of Nutrition in Cardiac Surgery
- Maintenance of metabolism
- Attenuation of catabolism
- Maintenance of gut-integrity
- Reduction of postoperative complications
- Improved wound healing
- Securing adequate hydration and euglycemia.
1.3. Current Nutritional Practice: Systematic Review, Guidelines and Observational Data
1.3.1. Systematic Review
- The low evidence received from rather small studies with from heterogeneous patient populations with different nutrition interventions and non-comparable outcome assessments
- The resulting need for adequate studies and
- The urgent need for specific guidelines for this cohort of critically ill patients, as current clinical practice may lead to malnutrition in these patients.
1.3.2. Current Nutritional Practices: Guidelines and Observational Data
2. Nutrition Screening in Cardiac Surgery Patients
3. Perioperative Nutrition Support in Cardiac Surgery Patients
3.1. Preoperative Nutritional Optimization in Cardiac Surgery Patients
3.2. Postoperative Nutrition Support in Cardiac Surgery Patients
3.2.1. Enteral Nutrition
3.2.2. Parenteral Nutrition
4. Micronutrients in Cardiac Surgery Patients
4.1. Glutamine
4.2. Selenium
4.3. Vitamins
5. Actual Recommendations and Road Map for Future Research Activities
- Assessment of preoperative nutritional status may guide health care professionals to consider early preoperative nutrition interventions in patients at elevated risk of developing postoperative complications
- Postoperative oral/enteral nutrition intake should be resumed early in the hemodynamic stable cardiac surgery patients
- Cardiac surgery patients with brief ICU stays often recover within the first 1–2 days after surgery and are considered less likely to benefit from an intense nutrition support
- Intense nutrition support with adequate caloric and protein intake is of special relevance for patients with prolonged ICU stay or patients at high nutritional risk
- Clinical significance of pharmaconutrients, fish oil and vitamins need further clarification in adequately designed clinical studies
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Atrial Fibrillation |
| ASPEN | American Society for Parenteral and Enteral Nutrition |
| BMI | Body Mass Index |
| CABG | Coronary Artery Bypass Graft |
| CK-MB | Creatine Kinase (muscle/brain) |
| CPB | Cardiopulmonary Bypass |
| CRP | C-reative Protein |
| DHA | Docosahexaenoic Acid |
| EN | Enteral Nutrition |
| EPA | Eicosapentanoic Acid |
| ERAS | Enhanced Recovery After Surgery |
| ESPEN | European Society for Parenteral and Enteral Nutrition |
| FO | Fish Oil |
| GI | Gastrointestinal |
| GRV | Gastric Residual Volume |
| ICU | Intensive Care Unit |
| IL | Interleukin |
| I/R | Ischemia/Reperfusion |
| LE | Lipid Emulsions |
| LOS | Length of Stay |
| MST | Malnutrition Screening Tool |
| MUST | Malnutrition Universal Screening Tool |
| n.a. | not available |
| NFκB | nuclear factor kappa-light-chain enhancer of activated B cells |
| NRS 2002 | Nutrition Risk Screening 2002 |
| NUTRIC | Nutrition Risk in the Critically Ill |
| PEEP | Positive End-expiratory pressure |
| PCT | Procalcitonin |
| PN | Parenteral Nutrition |
| PUFA | Polyunsaturated Fatty Acids |
| RCT | Randomized Controlled Trial |
| SGA | Subjective Global Assessment |
| SNAQ | Short Nutrition Assessment Questionnaire |
| sPN | Supplemental Parenteral Nutrition |
| TNFα | Tumor Necrosis Factor α |
References
- Paparella, D.; Yau, T.M.; Young, E. Cardiopulmonary bypass induced inflammation: Pathophysiology and treatment. An update. Eur. J. Cardio-Thorac. Surg. 2002, 21, 232–244. [Google Scholar] [CrossRef]
- Prondzinsky, R.; Knuepfer, A.; Loppnow, H.; Redling, F.; Lehmann, D.W.; Stabenow, I.; Witthaut, R.; Unverzagt, S.; Radke, J.; Zerkowski, H.-R.; et al. Surgical trauma affects the proinflammatory status after cardiac surgery to a higher degree than cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 2005, 129, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Rocker, G.M.; Westaby, S. Inflammatory Response to Cardiopulmonary Bypass. Ann. Thorac. Surg. 1993, 55, 552–559. [Google Scholar] [CrossRef]
- Hall, T.S. The pathophysiology of cardiopulmonary bypass. The risks and benefits of hemodilution. Chest 1995, 107, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.G.; Berg, G.A. Impact of off-pump coronary artery bypass surgery on systemic inflammation: Current best available evidence. J. Cardiac. Surg. 2007, 22, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Chenoweth, D.E.; Cooper, S.W.; Hugli, T.E.; Stewart, R.W.; Blackstone, E.H.; Kirklin, J.W. Complement activation during cardiopulmonary bypass: Evidence for generation of C3a and C5a anaphylatoxins. N. Engl. J. Med. 1981, 304, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Laffey, J.G.; Boylan, J.F.; Cheng, D.C.H. The systemic inflammatory response to cardiac surgery: Implications for the anesthesiologist. Anesthesiology 2002, 97, 215–252. [Google Scholar] [PubMed]
- Moore, F.D.; Warner, K.G.; Assousa, S.; Valeri, C.R.; Khuri, S.F. The effects of complement activation during cardiopulmonary bypass. Attenuation by hypothermia, heparin, and hemodilution. Ann. Surg. 1988, 208, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Bronicki, R.A.; Hall, M. Cardiopulmonary bypass-induced inflammatory response: Pathophysiology and treatment. Pediatr. Crit. Care Med. 2016, 17, S272–S278. [Google Scholar] [CrossRef] [PubMed]
- Khabar, K.S.A.; Elbarbary, M.A.; Khouqeer, F.; Devol, E.; Al-Gain, S.; Al-Halees, Z. Circulating Endotoxin and Cytokines after Cardiopulmonary Bypass: Differential Correlation with Duration of Bypass and Systemic Inflammatory Response/multiple Organ Dysfunction Syndromes. Clin. Immunol. Immunopathol. 1997, 85, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Aldous, S.J. Cardiac biomarkers in acute myocardial infarction. Int. J. Cardiol. 2013, 164, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Evora, P.R.B.; Bottura, C.; Arcencio, L.; Albuquerque, A.A.S.; Evora, P.M.; Rodrigues, A.J. Key points for curbing cardiopulmonary bypass inflammation. Acta Cir. Bras. 2016, 31 (Suppl. S1), 45–52. [Google Scholar] [CrossRef] [PubMed]
- Hall, R. Identification of inflammatory mediators and their modulation by strategies for the management of the systemic inflammatory response during cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2013, 27, 983–1033. [Google Scholar] [CrossRef] [PubMed]
- Hickey, E.; Karamlou, T.; You, J.; Ungerleider, R.M. Effects of Circuit Miniaturization in Reducing Inflammatory Response to Infant Cardiopulmonary Bypass by Elimination of Allogeneic Blood Products. Ann. Thorac. Surg. 2006, 81, S2367–S2372. [Google Scholar] [CrossRef] [PubMed]
- Jansen, P.G.; te Velthuis, H.; Bulder, E.R.; Paulus, R.; Scheltinga, M.R.; Eijsman, L.; Wildevuur, R.C. Reduction in prime volume attenuates the hyperdynamic response after cardiopulmonary bypass. Ann. Thorac. Surg. 1995, 60, 549–550. [Google Scholar] [CrossRef]
- Stephens, R.S.; Shah, A.S.; Whitman, G., Jr. Lung injury and acute respiratory distress syndrome after cardiac surgery. Ann. Thorac. Surg. 2013, 95, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Ellenberger, C.; Sologashvili, T.; Cikirikcioglu, M.; Verdon, G.; Diaper, J.; Cassina, T.; Licker, M. Risk factors of postcardiotomy ventricular dysfunction in moderate-to-high risk patients undergoing open-heart surgery. Ann. Cardiac. Anaesth. 2017, 20, 287–296. [Google Scholar] [CrossRef]
- Lomivorotov, V.V.; Efremov, S.M.; Kirov, M.Y.; Fominskiy, E.V.; Karaskov, A.M. Low-cardiac-output syndrome after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2017, 31, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Cropsey, C.; Kennedy, J.; Han, J.; Pandharipande, P. Cognitive dysfunction, delirium, stroke in cardiac surgery patients. Semin. Cardiothorac. Vasc. Anesth. 2015, 19, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Mangusan, R.F.; Hooper, V.; Denslow, S.A.; Travis, L. Outcomes associated with postoperative delirium after cardiac surgery. Am. J. Crit. Care 2015, 24, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Corredor, C.; Thomson, R.; Al-Subaie, N. Long-term consequences of acute kidney injury after cardiac surgery: A systematic review and meta-analysis. J. Cardiothorac. Vasc. Anesth. 2016, 30, 69–75. [Google Scholar] [CrossRef] [PubMed]
- The European Society for Clinical Nutrition and Metabolism. Basics in Clinical Nutrition, 4th ed.; Publishing House Galen: Somerville, NJ, USA, 2011. [Google Scholar]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic criteria for malnutrition—An espen consensus statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef] [PubMed]
- White, J.V.; Guenter, P.; Jensen, G.; Malone, A.; Schofield, M.; Academy Malnutrition Work Group; ASPEN Malnutrition Task Force; ASPEN Board of Directors. Consensus statement: Academy of nutrition and dietetics and american society for parenteral and enteral nutrition: Characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J. Parenter. Enter. Nutr. 2012, 36, 275–283. [Google Scholar] [CrossRef]
- Lomivorotov, V.V.; Efremov, S.M.; Boboshko, V.A.; Nikolaev, D.A.; Vedernikov, P.E.; Lomivorotov, V.N.; Karaskov, A.M. Evaluation of nutritional screening tools for patients scheduled for cardiac surgery. Nutrition 2013, 29, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Stoppe, C.; Goetzenich, A.; Whitman, G.; Ohkuma, R.; Brown, T.; Hatzakorzian, R.; Kristof, A.; Meybohm, P.; Mechanick, J.; Evans, A.; et al. Role of nutrition support in adult cardiac surgery: A consensus statement from an international multidisciplinary expert group on nutrition in cardiac surgery. Crit. Care 2017, 21, 131. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, G.; Tamion, F.; Coeffier, M.; Richard, V.; Bubenheim, M.; Bessou, J.-P.; Doguet, F. Modulation of mesenteric vasoreactivity and inflammatory response by protein undernutrition in cardiopulmonary bypass. Nutrition 2013, 29, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Drover, J.W.; Cahill, N.E.; Kutsogiannis, J.; Pagliarello, G.; Wischmeyer, P.; Wang, M.; Day, A.G.; Heyland, D.K. Nutrition therapy for the critically ill surgical patient: We need to do better! JPEN J. Parenter. Enter. Nutr. 2010, 34, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hubner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.; Carli, F. Promoting perioperative metabolic and nutritional care. Anesthesiology 2015, 123, 1455–1472. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of critical care medicine (SCCM) and american society for parenteral and enteral nutrition (A.S.P.E.N.). JPEN J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Agarwala, R.; Martin, C.; Nagpal, D.; Teitelbaum, M.; Heyland, D.K. Nutrition therapy in critically ill patients following cardiac surgery: Defining and improving practice. JPEN J. Parenter. Enter. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Boban, M.; Laviano, A.; Persic, V.; Rotim, A.; Jovanovic, Z.; Vcev, A. Characteristics of NRS-2002 nutritional risk screening in patients hospitalized for secondary cardiovascular prevention and rehabilitation. J. Am. Coll. Nutr. 2014, 33, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Malone, A.; Hamilton, C. The academy of nutrition and dietetics/the american society for parenteral and enteral nutrition consensus malnutrition characteristics: Application in practice. Nutr. Clin. Pract. 2013, 28, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.Z.; Harker, J.O.; SalvÃ, A.; Guigoz, Y.; Vellas, B. Screening for undernutrition in geriatric practice: Developing the short-form mini-nutritional assessment (MNA-SF). J. Gerontol. Ser. A 2001, 56, M366–M372. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the mini nutritional assessment short-form (MNA®-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.; Capra, S.; Bauer, J.; Banks, M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition 1999, 15, 458–464. [Google Scholar] [CrossRef]
- Malnutrition Advisory Group. A consistent and reliable tool for malnutrition screening. Nurs. Times 2003, 99, 26–27. [Google Scholar]
- Chermesh, I.; Hajos, J.; Mashiach, T.; Bozhko, M.; Shani, L.; Nir, R.-R.; Bolotin, G. Malnutrition in cardiac surgery: Food for thought. Eur. J. Prev. Cardiol. 2014, 21, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Wu, T.; Bricknell, R.; Muqtadir, Z.; Armstrong, D. Malnutrition matters in canadian hospitalized patients: Malnutrition risk in hospitalized patients in a tertiary care center using the malnutrition universal screening tool. Nutr. Clin. Pract. 2015, 30, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M.; Educational, European Society of Parenteral Clinical Practice Committeee and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Heyland, D.K.; Dhaliwal, R.; Jiang, X.; Day, A.G. Identifying critically ill patients who benefit the most from nutrition therapy: The development and initial validation of a novel risk assessment tool. Crit. Care 2011, 15, R268. [Google Scholar] [CrossRef] [PubMed]
- Kalaiselvan, M.S.; Renuka, M.K.; Arunkumar, A.S. Use of nutrition risk in critically ill (nutric) score to assess nutritional risk in mechanically ventilated patients: A prospective observational study. Ind. J. Crit. Care Med. 2017, 21, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Policarpo, S.; Fortuna, P.; Alves, M.; Virella, D.; Heyland, D.K.; Portuguese NUTRIC Study Group. Nutritional risk assessment and cultural validation of the modified nutric score in critically ill patients-a multicenter prospective cohort study. J. Crit. Care 2017, 37, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Hasan, R.M.; Agarwala, R.; Martin, C.; Day, A.G.; Heyland, D.K. Identifying critically-ill patients who will benefit most from nutritional therapy: Further validation of the “modified nutric” nutritional risk assessment tool. Clin. Nutr. 2016, 35, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z.; Ad Hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Baker, J.P.; Detsky, A.S.; Wesson, D.E.; Wolman, S.L.; Stewart, S.; Whitewell, J.; Langer, B.; Jeejeebhoy, K.N. Nutritional assessment: A comparison of clinical judgement and objective measurements. N. Engl. J. Med. 1982, 306, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Detsky, A.S.; McLaughlin, J.R.; Baker, J.P.; Johnston, N.; Whittaker, S.; Mendelson, R.A.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? JPEN J. Parenter. Enter. Nutr. 1987, 11, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Detsky, A.S.; Smalley, P.S.; Chang, J. The rational clinical examination. Is this patient malnourished? JAMA 1994, 271, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Sheean, P.M.; Peterson, S.J.; Gurka, D.P.; Braunschweig, C.A. Nutrition assessment: The reproducibility of subjective global assessment in patients requiring mechanical ventilation. Eur. J. Clin. Nutr. 2010, 64, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Kruizenga, H.M.; Seidell, J.C.; de Vet, H.C.; Wierdsma, N.J.; van Bokhorst-de van der Schueren, M.A. Development and validation of a hospital screening tool for malnutrition: The short nutritional assessment questionnaire (SNAQ). Clin. Nutr. 2005, 24, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Van Venrooij, L.M.W.; van Leeuwen, P.A.M.; Hopmans, W.; Borgmeijer-Hoelen, M.M.M.J.; de Vos, R.; de Mol, B.A.J.M. Accuracy of quick and easy undernutrition screening tools–short nutritional assessment questionnaire, malnutrition universal screening tool, and modified malnutrition universal screening tool–in patients undergoing cardiac surgery. J. Am. Diet. Assoc. 2011, 111, 1924–1930. [Google Scholar] [CrossRef] [PubMed]
- Van Venrooij, L.M.W.; de Vos, R.; Borgmeijer-Hoelen, M.M.M.J.; Haaring, C.; de Mol, B.A.J.M. Preoperative unintended weight loss and low body mass index in relation to complications and length of stay after cardiac surgery. Am. J. Clin. Nutr. 2008, 87, 1656–1661. [Google Scholar] [CrossRef] [PubMed]
- Jakob, S.M.; Stanga, Z. Perioperative metabolic changes in patients undergoing cardiac surgery. Nutrition 2010, 26, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Heyland, D.K.; Dhaliwal, R.; Wang, M.; Day, A.G. The prevalence of iatrogenic underfeeding in the nutritionally ‘at-risk’ critically ill patient: Results of an international, multicenter, prospective study. Clin. Nutr. 2015, 34, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Cresci, G.; Hummell, A.C.; Raheem, S.A.; Cole, D. Nutrition intervention in the critically ill cardiothoracic patient. Nutr. Clin. Pract. 2012, 27, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Ferrie, S.; Allman-Farinelli, M. Commonly used “nutrition” indicators do not predict outcome in the critically ill: A systematic review. Nutr. Clin. Pract. 2013, 28, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Bhamidipati, C.M.; LaPar, D.J.; Mehta, G.S.; Kern, J.A.; Upchurch, G.R.; Kron, I.L.; Ailawadi, G. Albumin is a better predictor of outcomes than body mass index following coronary artery bypass grafting. Surgery 2011, 150, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Thourani, V.H.; Keeling, W.B.; Kilgo, P.D.; Puskas, J.D.; Lattouf, O.M.; Chen, E.P.; Guyton, R.A. The impact of body mass index on morbidity and short- and long-term mortality in cardiac valvular surgery. J. Thorac. Cardiovasc. Surg. 2011, 142, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.-J.; Cassiere, H.A.; Dellis, S.L.; Manetta, F.; Kohn, N.; Hartman, A.R. Impact of preoperative prealbumin on outcomes after cardiac surgery. JPEN J. Parenter. Enter. Nutr. 2015, 39, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Pichette, M.; Liszkowski, M.; Ducharme, A. Preoperative optimization of the heart failure patient undergoing cardiac surgery. Can. J. Cardiol. 2017, 33, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Hage, F.G. Guidelines in review: 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: A report of the american college of cardiology/american heart association task force on practice guidelines. J. Nucl. Cardiol. 2015, 22, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Pittmann, J.G.; Cohen, P. The pathogenesis of cardiac cachexia. N. Engl. J. Med. 1964, 271, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Von Haehling, S.; Lainscak, M.; Springer, J.; Anker, S.D. Cardiac cachexia: A systematic overview. Pharmacol. Ther. 2009, 121, 227–252. [Google Scholar] [CrossRef] [PubMed]
- Von Haehling, S.; Ebner, N.; Santos, M.R.D.; Springer, J.; Anker, S.D. Muscle wasting and cachexia in heart failure: Mechanisms and therapies. Nat. Rev. Cardiol. 2017, 14, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Varadhan, K.K.; Neal, K.R.; Dejong, C.H.C.; Fearon, K.C.H.; Ljungqvist, O.; Lobo, D.N. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: A meta-analysis of randomized controlled trials. Clin. Nutr. 2010, 29, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Lassen, K.; Coolsen, M.M.E.; Slim, K.; Carli, F.; de Aguilar-Nascimento, J.E.; Schafer, M.; Parks, R.W.; Fearon, K.C.H.; Lobo, D.N.; Demartines, N.; et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced recovery after surgery (ERAS®) society recommendations. Clin. Nutr. 2012, 31, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Nygren, J.; Thacker, J.; Carli, F.; Fearon, K.C.H.; Norderval, S.; Lobo, D.N.; Ljungqvist, O.; Soop, M.; Ramirez, J.; Enhanced Recovery after Surgery Society. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced recovery after surgery (ERAS) society recommendations. Clin. Nutr. 2012, 31, 801–816. [Google Scholar] [CrossRef] [PubMed]
- Sola, M.; Ramm, C.J.; Kolarczyk, L.M.; Teeter, E.G.; Yeung, M.; Caranasos, T.G.; Vavalle, J.P. Application of a multidisciplinary enhanced recovery after surgery pathway to improve patient outcomes after transcatheter aortic valve implantation. Am. J. Cardiol. 2016, 118, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Rich, M.W.; Keller, A.J.; Schechtman, K.B.; Marshall, W.G.; Kouchoukos, N.T. Increased complications and prolonged hospital stay in elderly cardiac surgical patients with low serum albumin. Am. J. Cardiol. 1989, 63, 714–718. [Google Scholar] [CrossRef]
- Engelman, D.T.; Adams, D.H.; Byrne, J.G.; Aranki, S.F.; Collins, J.J.; Couper, G.S.; Allred, E.N.; Cohn, L.H.; Rizzo, R.J. Impact of body mass index and albumin on morbidity and mortality after cardiac surgery. J. Thorac. Cardiovasc. Surg. 1999, 118, 866–873. [Google Scholar] [CrossRef]
- ASPEN Board of Directors and the Clinical Guidelines Task Force. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J. Parenter. Enter. Nutr. 2002, 26, 1SA–138SA. [Google Scholar]
- Boban, M.; Laviano, A.; Persic, V.; Biocina, B.; Petricevic, M.; Zekanovic, D.; Rotim, C.; Aleric, I.; Vcev, A. Influence of transiently increased nutritional risk on a left ventricle myocardial mass assessed by echocardiography. Ann. Nutr. Metab. 2016, 68, 197–202. [Google Scholar] [CrossRef] [PubMed]
- DiMaria-Ghalili, R.A.; Sullivan-Marx, E.M.; Compher, C. Inflammation, functional status, and weight loss during recovery from cardiac surgery in older adults: A pilot study. Biol. Res. Nurs. 2014, 16, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Niessen, H.W.M.; Kok, W.E.M.; Cocchieri, R.; Wisselink, W.; van Leeuwen, P.A.M.; de Mol, B.A.J.M. Nutrition before and during surgery and the inflammatory response of the heart: A randomized controlled trial. J. Nutr. Metab. 2015, 2015, 123158. [Google Scholar] [CrossRef] [PubMed]
- De Waele, E.; Nguyen, D.; de Bondt, K.; Meir, M.L.; Diltoer, M.; Honore, P.M.; Spapen, H.; Pen, J.J. The cocos trial: Caloric control in cardiac surgery patients promotes survival, an interventional trial with retrospective control. Clin. Nutr. 2018, 37, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Osland, E.; Yunus, R.M.; Khan, S.; Memon, M.A. Early versus traditional postoperative feeding in patients undergoing resectional gastrointestinal surgery: A meta-analysis. JPEN J. Parenter. Enter. Nutr. 2011, 35, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.K.; Lewis, S.J.; Thomas, S. Early enteral nutrition within 24h of colorectal surgery versus later commencement of feeding for postoperative complications. Cochrane Database Syst. Rev. 2006, CD004080. [Google Scholar] [CrossRef] [PubMed]
- Martindale, R.G.; McClave, S.A.; Taylor, B.; Lawson, C.M. Perioperative nutrition: What is the current landscape? JPEN J. Parenter. Enter. Nutr. 2013, 37, 5S–20S. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Revelly, J.; Cayeux, M.; Chiolero, R.L. Enteral nutrition in critically ill patients with severe hemodynamic failure after cardiopulmonary bypass. Clin. Nutr. 2005, 24, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Revelly, J.P.; Tappy, L.; Berger, M.M.; Gersbach, P.; Cayeux, C.; Chiolero, R. Early metabolic and splanchnic responses to enteral nutrition in postoperative cardiac surgery patients with circulatory compromise. Intensiv. Care Med. 2001, 27, 540–547. [Google Scholar] [CrossRef]
- Kesek, D.R.; Akerlind, L.; Karlsson, T. Early enteral nutrition in the cardiothoracic intensive care unit. Clin. Nutr. 2002, 21, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Elke, G.; Felbinger, T.W.; Heyland, D.K. Gastric residual volume in critically ill patients: A dead marker or still alive? Nutr. Clin. Pract. 2015, 30, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Lasierra, J.L.F.; Perez-Vela, J.L.; Makikado, L.D.U.; Sanchez, E.T.; Gomez, L.C.; Rodriguez, B.M.; Lopez, P.A.; de la Camara, A.G.; Gonzalez, J.C.M. Early enteral nutrition in patients with hemodynamic failure following cardiac surgery. JPEN J. Parenter. Enter. Nutr. 2015, 39, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Chiolero, R.L. Enteral nutrition and cardiovascular failure: From myths to clinical practice. JPEN J. Parenter. Enter. Nutr. 2009, 33, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, R.; Zaki, J.; Wegner, R.; Pednekar, G.; Tse, A.; Sheinbaum, R.; Williams, G.W. Gastrointestinal complications after cardiac surgery: A nationwide population-based analysis of morbidity and mortality predictors. J. Cardiothorac. Vasc. Anesth. 2017, 31, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Viana, F.F.; Chen, Y.; Almeida, A.A.; Baxter, H.D.; Cochrane, A.D.; Smith, J.A. Gastrointestinal complications after cardiac surgery: 10-year experience of a single australian centre. ANZ J. Surg. 2013, 83, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Abboud, B.; Daher, R.; Boujaoude, J. Acute mesenteric ischemia after cardio-pulmonary bypass surgery. World J. Gastroenterol. 2008, 14, 5361–5370. [Google Scholar] [CrossRef] [PubMed]
- Faisy, C.; Lerolle, N.; Dachraoui, F.; Savard, J.-F.; Abboud, I.; Tadie, J.; Fagon, J. Impact of energy deficit calculated by a predictive method on outcome in medical patients requiring prolonged acute mechanical ventilation. Br. J. Nutr. 2009, 101, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Paccagnella, A.; Calò, M.A.; Caenaro, G.; Salandin, V.; Jus, P.; Simini, G.; Heymsfield, S.B. Cardiac cachexia: Preoperative and postoperative nutrition management. J. Parenter. Enter. Nutr. 1994, 18, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.E.; Parrott, F.; Harrison, D.A.; Bear, D.E.; Segaran, E.; Beale, R.; Bellingan, G.; Leonard, R.; Mythen, M.G.; Rowan, K.M. CALORIES Trial Investigators. Trial of the route of early nutritional support in critically ill adults. N. Engl. J. Med. 2014, 371, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Wischmeyer, P.E.; Puthucheary, Z.; Millan, I.S.; Butz, D.; Grocott, M.P.W. Muscle mass and physical recovery in ICU: Innovations for targeting of nutrition and exercise. Curr. Opin. Crit. Care 2017, 23, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Simpson, F.; Doig, G.S. Parenteral vs. enteral nutrition in the critically ill patient: A meta-analysis of trials using the intention to treat principle. Intensiv. Care Med. 2005, 31, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Casaer, M.P.; Mesotten, D.; Hermans, G.; Wouters, P.J.; Schetz, M.; Meyfroidt, G.; van Cromphaut, S.; Ingels, C.; Meersseman, P.; Muller, J.; et al. Early versus late parenteral nutrition in critically ill adults. N. Engl. J. Med. 2011, 365, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Heidt, M.C.; Vician, M.; Stracke, S.K.H.; Stadlbauer, T.; Grebe, M.T.; Boening, A.; Vogt, P.R.; Erdogan, A. Beneficial effects of intravenously administered n-3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: A prospective randomized study. Thorac. Cardiovasc. Surg. 2009, 57, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Delodder, F.; Liaudet, L.; Tozzi, P.; Schlaepfer, J.; Chiolero, R.L.; Tappy, L. Three short perioperative infusions of n-3 pufas reduce systemic inflammation induced by cardiopulmonary bypass surgery: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, R.G.; James, M.J.; Gibson, R.A.; Edwards, J.R.; Stubberfield, J.; Stuklis, R.; Roberts-Thomson, K.; Young, G.D.; Cleland, L.G. Effects of fish-oil supplementation on myocardial fatty acids in humans. Am. J. Clin. Nutr. 2007, 85, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Lomivorotov, V.V.; Efremov, S.M.; Pokushalov, E.A.; Romanov, A.B.; Ponomarev, D.N.; Cherniavsky, A.M.; Shilova, A.N.; Karaskov, A.M.; Lomivorotov, V.N. Randomized trial of fish oil infusion to prevent atrial fibrillation after cardiac surgery: Data from an implantable continuous cardiac monitor. J. Cardiothorac. Vasc. Anesth. 2014, 28, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wu, J.H.Y.; Otto, M.C.D.; Sandesara, C.M.; Metcalf, R.G.; Latini, R.; Libby, P.; Lombardi, F.; O’Gara, P.T.; Page, R.L.; et al. Fish oil and post-operative atrial fibrillation: A meta-analysis of randomized controlled trials. J. Am. Coll. Cardiol. 2013, 61, 2194–2196. [Google Scholar] [CrossRef] [PubMed]
- Christou, G.A.; Christou, K.A.; Korantzopoulos, P.; Rizos, E.C.; Nikas, D.N.; Goudevenos, J.A. The current role of omega-3 fatty acids in the management of atrial fibrillation. Int. J. Mol. Sci. 2015, 16, 22870–22887. [Google Scholar] [CrossRef] [PubMed]
- Sandesara, C.M.; Chung, M.K.; van Wagoner, D.R.; Barringer, T.A.; Allen, K.; Ismail, H.M.; Zimmerman, B.; Olshansky, B. A randomized, placebo-controlled trial of omega-3 fatty acids for inhibition of supraventricular arrhythmias after cardiac surgery: The fish trial. J. Am. Heart Assoc. 2012, 1, e000547. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kajikawa, Y.; Otani, S.; Yamada, Y.; Takemoto, S.; Hirota, M.; Ikeda, M.; Iwagaki, H.; Saito, S.; Fujiwara, T. Protective effect of eicosapentaenoic acid on insulin resistance in hyperlipidemic patients and on the postoperative course of cardiac surgery patients: The possible involvement of adiponectin. Acta Med. Okayama 2014, 68, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Sorice, M.; Tritto, F.P.; Sordelli, C.; Gregorio, R.; Piazza, L. N-3 polyunsaturated fatty acids reduces post-operative atrial fibrillation incidence in patients undergoing “on-pump” coronary artery bypass graft surgery. Monaldi Arch. Chest Dis. 2011, 76, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yan, X.; Chen, Y.; Tang, R.; Du, X.; Dong, J.; Ma, C. Omega-3 fatty acids for postoperative atrial fibrillation: Alone or in combination with antioxidant vitamins? Heart Lung Circ. 2014, 23, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhen, Y.; Tao, A.; Bao, Z.; Zhang, G. Polyunsaturated fatty acids for the prevention of atrial fibrillation after cardiac surgery: An updated meta-analysis of randomized controlled trials. J. Cardiol. 2014, 63, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Heidarsdottir, R.; Arnar, D.O.; Skuladottir, G.V.; Torfason, B.; Edvardsson, V.; Gottskalksson, G.; Palsson, R.; Indridason, O.S. Does treatment with n-3 polyunsaturated fatty acids prevent atrial fibrillation after open heart surgery? Europace Eur. Pac. Arrhythm. Cardiac. Electrophysiol. 2010, 12, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Farquharson, A.L.; Metcalf, R.G.; Sanders, P.; Stuklis, R.; Edwards, J.R.M.; Gibson, R.A.; Cleland, L.G.; Sullivan, T.R.; James, M.J.; et al. Effect of dietary fish oil on atrial fibrillation after cardiac surgery. Am. J. Cardiol. 2011, 108, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.; Davidson, N.C.; Schmidt, E.B.; Calder, P.C. Cardiovascular effects of marine omega-3 fatty acids. Lancet 2010, 376, 540–550. [Google Scholar] [CrossRef]
- Calder, P.C.; Adolph, M.; Deutz, N.E.; Grau, T.; Innes, J.K.; Klek, S.; Lev, S.; Mayer, K.; Michael-Titus, A.T.; Pradelli, L.; et al. Lipids in the intensive care unit: Recommendations from the espen expert group. Clin. Nutr. 2018, 37, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lomivorotov, V.V.; Efremov, S.M.; Shmirev, V.A.; Ponomarev, D.N.; Lomivorotov, V.N.; Karaskov, A.M. Glutamine is cardioprotective in patients with ischemic heart disease following cardiopulmonary bypass. Heart Surg. Forum 2011, 14, E384–E388. [Google Scholar] [CrossRef] [PubMed]
- Sufit, A.; Weitzel, L.B.; Hamiel, C.; Queensland, K.; Dauber, I.; Rooyackers, O.; Wischmeyer, P.E. Pharmacologically dosed oral glutamine reduces myocardial injury in patients undergoing cardiac surgery: A randomized pilot feasibility trial. JPEN J. Parenter. Enter. Nutr. 2012, 36, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Benstoem, C.; Goetzenich, A.; Kraemer, S.; Borosch, S.; Manzanares, W.; Hardy, G.; Stoppe, C. Selenium and its supplementation in cardiovascular disease–what do we know? Nutrients 2015, 7, 3094–3118. [Google Scholar] [CrossRef] [PubMed]
- Stoppe, C.; Schaelte, G.; Rossaint, R.; Coburn, M.; Graf, B.; Spillner, J.; Marx, G.; Rex, S. The intraoperative decrease of selenium is associated with the postoperative development of multiorgan dysfunction in cardiac surgical patients. Crit. Care Med. 2011, 39, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Stoppe, C.; Spillner, J.; Rossaint, R.; Coburn, M.; Schalte, G.; Wildenhues, A.; Marx, G.; Rex, S. Selenium blood concentrations in patients undergoing elective cardiac surgery and receiving perioperative sodium selenite. Nutrition 2013, 29, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Pargger, H.; Seeberger, E.; Eckhart, F.; von Felten, S.; Haberthuer, C. Effect of high-dose sodium selenite in cardiac surgery patients: A randomized controlled bi-center trial. Clin. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Stoppe, C.; McDonald, B.; Rex, S.; Manzanares, W.; Whitlock, R.; Fremes, S.; Fowler, R.; Lamarche, Y.; Meybohm, P.; Haberthür, C.; et al. Sodium selenite adminstration in cardiac surgery (sustain CSX-trial): Study design of an international multicenter randomized double-blinded controlled trial of high dose sodium-selenite administration in high-risk cardiac surgical patients. Trials 2014, 15, 339. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.W.; Liu, X.; Peng, T.J.; Giberson, T.A.; Khabbaz, K.R.; Donnino, M.W. Pyruvate dehydrogenase activity and quantity decreases after coronary artery bypass grafting: A prospective observational study. Shock 2015, 43, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Donnino, M.W.; Cocchi, M.N.; Smithline, H.; Carney, E.; Chou, P.P.; Salciccioli, J.; Salciccoli, J. Coronary artery bypass graft surgery depletes plasma thiamine levels. Nutrition 2010, 26, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.W.; Holmberg, M.J.; Doherty, M.; Khabbaz, K.; Lerner, A.; Berg, K.M.; Donnino, M.W. Postoperative lactate levels and hospital length of stay after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2015, 29, 1454–1460. [Google Scholar] [CrossRef] [PubMed]
- Badreldin, A.M.A.; Doerr, F.; Elsobky, S.; Brehm, B.R.; Abul-dahab, M.; Lehmann, T.; Bayer, O.; Wahlers, T.; Hekmat, K. Mortality prediction after cardiac surgery: Blood lactate is indispensible. Thorac. Cardiovasc. Surg. 2013, 61, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.W.; Holmberg, M.J.; Berg, K.M.; Chase, M.; Cocchi, M.N.; Sulmonte, C.; Balkema, J.; MacDonald, M.; Montissol, S.; Senthilnathan, V.; et al. Thiamine as an adjunctive therapy in cardiac surgery: A randomized, double-blind, placebo-controlled, phase ii trial. Crit. Care 2016, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Luger, M.; Hiesmayr, M.; Koeppel, P.; Sima, B.; Ranz, I.; Weiss, C.; Koenig, J.; Luger, E.; Kruschitz, R.; Ludvik, B.; et al. Influence of intravenous thiamine supplementation on blood lactate concentration prior to cardiac surgery: A double-blinded, randomised controlled pilot study. Eur. J. Anaesthesiol. 2015, 32, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Chung, M.; Trikalinos, T.; Mitri, J.; Brendel, M.; Patel, K.; Lichtenstein, A.H.; Lau, J.; Balk, E.M. Systematic review: Vitamin d and cardiometabolic outcomes. Ann. Inter. Med. 2010, 152, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Turan, A.; Grady, M.; You, J.; Mascha, E.J.; Keeyapaj, W.; Komatsu, R.; Bashour, C.A.; Sessler, D.I.; Saager, L.; Kurz, A. Low vitamin d concentration is not associated with increased mortality and morbidity after cardiac surgery. PLoS ONE 2013, 8, e63831. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Shaw, G.M.; Fowler, A.A.; Natarajan, R. Ascorbate-dependent vasopressor synthesis: A rationale for vitamin c administration in severe sepsis and septic shock? Crit. Care 2015, 19, 418. [Google Scholar] [CrossRef] [PubMed]
- Antonic, M. Effect of ascorbic acid on postoperative acute kidney injury in coronary artery bypass graft patients: A pilot study. Heart Surg. Forum 2017, 20, E214–E218. [Google Scholar] [CrossRef] [PubMed]
- Polymeropoulos, E.; Bagos, P.; Papadimitriou, M.; Rizos, I.; Patsouris, E.; Toumpoulis, I. Vitamin c for the prevention of postoperative atrial fibrillation after cardiac surgery: A meta-analysis. Adv. Pharm. Bull. 2016, 6, 243. [Google Scholar] [CrossRef] [PubMed]





| Form of Nutrition | Percentage of Patients | Caloric Adequacy | Protein Adequacy |
|---|---|---|---|
| EN | 78% | 25.5% | 24.9% |
| EN + PN | 17% | 32.4% | 28.8% |
| Tool | Parameters | Source |
|---|---|---|
| ASPEN Guideline |
| [24,31,34] |
| ESPEN Guideline |
| [23,29] |
| MNA-SF |
| [35,36] |
| MST |
| [37] |
| MUST |
| [38,39,40,41] |
| NUTRIC Score |
| [42,43,44,45] |
| NRS-2002 |
| [41,46] |
| SGA |
| [47,48,49,50] |
| SNAQ |
| [51] |
| Period of Illness | Possible Outcome Parameters |
|---|---|
| Acute illness | Nutrition tolerance |
| Protein balance | |
| Muscle mass | |
| Muscle biopsies | |
| Physical function | Handgrip strength |
| Quadriceps strength | |
| 6-min walk distance | |
| Timed up and go test | |
| 4-m gait speed | |
| Participation in life | Activities of daily living |
| Clinical frailty score | |
| Quality of life | Short Form 36 |
| EQ-5D |
| Author, Year | No. of Patients | Time to Start of EN | Mean Energy Delivery | Vasopressor or Inotropic Drugs | Intestinal Tolerance |
|---|---|---|---|---|---|
| Berger 2005, [80] | 70 | <72 h | 1360 ± 620 kcal/day | Median 5 days |
|
| Revelly 2001, [81] | 9 | 12–16 h | 1.1 ± 0.25 kcal/kg/h | dobutamine (mean 420 µg/min) and norepinephrine (6–30 µg/min) | Hemodynamic response
|
| Kesek 2002, [82] | 62 | <72 h | Depended individually as calculated by REE | n.a. 1 |
|
| Flordelís Lasierra 2015, [84] | 37 | n.a. | 1228.4 kcal/day | 3 drugs: 38% 4 drugs: 24% 4 drugs + mechanical assistance in 16% |
|
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, A.; Nesterova, E.; Lomivorotov, V.; Efremov, S.; Goetzenich, A.; Benstoem, C.; Zamyatin, M.; Chourdakis, M.; Heyland, D.; Stoppe, C. Current Evidence about Nutrition Support in Cardiac Surgery Patients—What Do We Know? Nutrients 2018, 10, 597. https://doi.org/10.3390/nu10050597
Hill A, Nesterova E, Lomivorotov V, Efremov S, Goetzenich A, Benstoem C, Zamyatin M, Chourdakis M, Heyland D, Stoppe C. Current Evidence about Nutrition Support in Cardiac Surgery Patients—What Do We Know? Nutrients. 2018; 10(5):597. https://doi.org/10.3390/nu10050597
Chicago/Turabian StyleHill, Aileen, Ekaterina Nesterova, Vladimir Lomivorotov, Sergey Efremov, Andreas Goetzenich, Carina Benstoem, Mikhail Zamyatin, Michael Chourdakis, Daren Heyland, and Christian Stoppe. 2018. "Current Evidence about Nutrition Support in Cardiac Surgery Patients—What Do We Know?" Nutrients 10, no. 5: 597. https://doi.org/10.3390/nu10050597
APA StyleHill, A., Nesterova, E., Lomivorotov, V., Efremov, S., Goetzenich, A., Benstoem, C., Zamyatin, M., Chourdakis, M., Heyland, D., & Stoppe, C. (2018). Current Evidence about Nutrition Support in Cardiac Surgery Patients—What Do We Know? Nutrients, 10(5), 597. https://doi.org/10.3390/nu10050597






