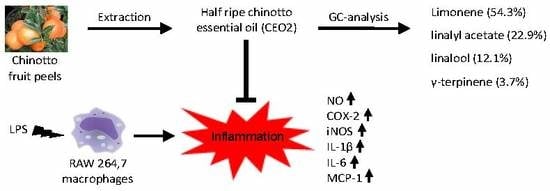
In Vitro Anti-Inflammatory and Radical Scavenging Properties of Chinotto (Citrus myrtifolia Raf.) Essential Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Extraction of the Oils
2.3. GC Analyses of CEOs
2.4. In Vitro Radical Scavenging Activity
2.4.1. DPPH Assay
2.4.2. ABTS Assay
2.5. Cell Culture
2.6. XTT Viability Assay
2.7. Nitric Oxide Quantification
2.8. RNA Extraction, Purification, and Quantitative Reverse Transcription Real-Time PCR
2.9. Statistical Analysis
3. Results
3.1. Composition of CEOs
3.2. In Vitro Radical Scavenging Properties of CEOs
3.3. CEOs Have No Effect on Cell Proliferation
3.4. CEO2 Reduces No Concentration in LPS-Stimulated Macrophages
3.5. CEO2 Attenuates LPS-Induced COX-2, iNOS, IL-1β, IL6, and MCP-1 Expression
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Valledor, A.F.; Comalada, M.; Santamaria-Babi, L.F.; Lloberas, J.; Celada, A. Macrophage proinflammatory activation and deactivation: A question of balance. Adv. Immunol. 2010, 108, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lian, L.H.; Bai, T.; Wu, Y.L.; Wan, Y.; Xie, W.X.; Jin, X.; Nan, J.X. Cryptotanshinone inhibits LPS-induced proinflammatory mediators via TLR4 and TAK1 signaling pathway. Int. Immunopharmacol. 2011, 11, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, W. TLR4-mediated activation of macrophages by the polysaccharide fraction from Polyporus umbellatus(pers) Fries. J. Ethnopharmacol. 2011, 135, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Barton, G.M. A calculated response: Control of inflammation by the innate immune system. J. Clin. Investig. 2008, 118, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Bosca, L.; Zeini, M.; Traves, P.G.; Hortelano, S. Nitric oxide and cell viability in inflammatory cells: A role for NO in macrophage function and fate. Toxicology 2005, 208, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Sergent, T.; Piront, N.; Meurice, J.; Toussaint, O.; Schneider, Y.J. Anti-inflammatory effects of dietary phenolic compounds in an in vitro model of inflamed human intestinal epithelium. Chem.-Biol. Int. 2010, 188, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla, P.; Rodriguez-Nogales, A.; Algieri, F.; Garrido-Mesa, N.; Olivares, M.; Rondon, D.; Zarzuelo, A.; Utrilla, M.P.; Galvez, J.; Rodriguez-Cabezas, M.E. Intestinal anti-inflammatory activity of the polyphenolic-enriched extract Amanda® in the trinitrobenzenesulphonic acid model of rat colitis. J. Funct. Foods 2014, 11, 449–459. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.-T.; Lai, C.-P.; Lin, C.-C.; Shih, Y. Study of the chemical composition, antioxidant activity and anti-inflammatory activity of essential oil from Vetiveria zizanioides. Food Chem. 2012, 134, 262–268. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Jo, C. Essential oils as potential antimicrobial agents in meat and meat products: A review. Trends Food Sci. Technol. 2013, 34, 96–108. [Google Scholar] [CrossRef]
- Dugo, G.; Di Giacomo, A. Citrus—The Genus Citrus; Taylor & Francis: London, UK, 2002; ISBN 9780415284912. [Google Scholar]
- Liu, Y.; Heying, E.; Tanumihardjo, S.A. History, global distribution, and nutritional importance of citrus fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Flavonoid composition and antioxidant activity of juices from chinotto (Citrus x myrtifolia Raf.) fruits at different ripening stages. J. Agric. Food Chem. 2010, 58, 3031–3036. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, G.; Barreca, D.; Caristi, C.; Gargiulli, C.; Leuzzi, U. Distribution of flavonoids and furocoumarins in juices from cultivars of Citrus bergamia Risso. J. Agric. Food Chem. 2007, 55, 9921–9927. [Google Scholar] [CrossRef] [PubMed]
- Taverna, D.; Di Donna, L.; Mazzotti, F.; Tagarelli, A.; Napoli, A.; Furia, E.; Sindona, G. Rapid discrimination of bergamot essential oil by paper spray mass spectrometry and chemometric analysis. J. Mass Spectrom. 2016, 51, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Borgatti, M.; Mancini, I.; Bianchi, N.; Guerrini, A.; Lampronti, I.; Rossi, D.; Sacchetti, G.; Gambari, R. Bergamot (Citrus bergamia Risso) fruit extracts and identified components alter expression of interleukin 8 gene in cystic fibrosis bronchial epithelial cell lines. BMC Biochem. 2011, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, N.; Cirmi, S.; Calapai, G.; Ventura-Spagnolo, E.; Gangemi, S.; Navarra, M. Anti-Inflammatory Activity of Citrus bergamia Derivatives: Where Do We Stand? Molecules 2016, 21, 1273. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Cordaro, M.; Campolo, M.; Gugliandolo, E.; Esposito, E.; Benedetto, F.; Cuzzocrea, S.; Navarra, M. Anti-inflammatory and antioxidant effects of flavonoid-rich fraction of bergamot juice (BJe) in a mouse model of intestinal ischemia/reperfusion injury. Front. Pharmacol. 2016, 7, 203. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Bruschetta, G.; Di Paola, R.; Ahmad, A.; Campolo, M.; Cuzzocrea, S.; Esposito, E.; Navarra, M. The anti-inflammatory and antioxidant effects of bergamot juice extract (BJe) in an experimental model of inflammatory bowel disease. Clin. Nutr. 2015, 34, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Navarra, M.; Mannucci, C.; Delbò, M.; Calapai, G. Citrus bergamia essential oil: From basic research to clinical application. Front. Pharmacol. 2015, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Risitano, R.; Currò, M.; Cirmi, S.; Ferlazzo, N.; Campiglia, P.; Caccamo, D.; Ientile, R.; Navarra, M. Flavonoid fraction of bergamot juice reduces LPS-induced inflammatory response through SIRT1-mediated NF-κB inhibition in THP-1 monocytes. PLoS ONE 2014, 9, e107431. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Pepe, G.; Pagano, F.; Tenore, G.C.; Marzocco, S.; Manfra, M.; Calabrese, G.; Aquino, R.P.; Campiglia, P. UHPLC profiling and effects on LPS-stimulated J774A.1 macrophages of flavonoids from bergamot (Citrus bergamia) juice, an underestimated waste product with high anti-inflammatory potential. J. Funct. Foods 2014, 7, 641–649. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Distribution of C- and O-glycosyl flavonoids, (3-hydroxy-3-methylglutaryl)glycosyl flavanones and furocoumarins in Citrus aurantium L. Juice. Food Chem. 2011, 124, 576–582. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Elucidation of the flavonoid and furocoumarin composition and radical-scavenging activity of green and ripe chinotto (Citrus myrtifolia Raf.) fruit tissues, leaves and seeds. Food Chem. 2011, 129, 1504–1512. [Google Scholar] [CrossRef]
- Protti, M.; Valle, F.; Poli, F.; Raggi, M.A.; Mercolini, L. Bioactive molecules as authenticity markers of Italian Chinotto (Citrus × myrtifolia) fruits and beverages. J. Pharm. Biomed. Anal. 2015, 104, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Scordino, M.; Sabatino, L.; Belligno, A.; Gagliano, G. Preliminary Study on Bioactive Compounds of Citrus × myrtifolia Rafinesque (Chinotto) to Its Potential Application in Food Industry. Food Nutr. Sci. 2011, 2, 685–691. [Google Scholar] [CrossRef]
- Lota, M.-L.; de Rocca Serra, D.; Jacquemond, C.; Tomi, F.; Casanova, J. Chemical variability of peel and leaf essential oils of sour orange. Flavour Fragr. J. 2001, 16, 89–96. [Google Scholar] [CrossRef]
- Chialva, F.; Doglia, G. Essential Oil Constituents of Chinotto (Citrus aurantium L. var. myrtifolia Guill.). J. Essent. Oil Res. 1990, 2, 33–35. [Google Scholar] [CrossRef]
- Gabriele, B.; Fazio, A.; Dugo, P.; Costa, R.; Mondello, L. Essential oil composition of Citrus medica L. Cv. Diamante (Diamante citron) determined after using different extraction methods. J. Sep. Sci. 2009, 32, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Fazio, A.; Caroleo, M.C.; Cione, E.; Plastina, P. Novel acrylic polymers for food packaging: Synthesis and antioxidant properties. Food Packag. Shelf Life 2017, 11, 84–90. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1996, 26, 1231–1237. [Google Scholar] [CrossRef]
- Wang, Y.; Plastina, P.; Vincken, J.-P.; Jansen, R.; Balvers, M.; ten Klooster, J.P.; Gruppen, H.; Witkamp, R.; Meijerink, J. N-Docosahexaenoyl Dopamine, an Endocannabinoid-like Conjugate of Dopamine and the n-3 Fatty Acid Docosahexaenoic Acid, Attenuates Lipopolysaccharide-Induced Activation of Microglia and Macrophages via COX-2. ACS Chem. Neurosci. 2017, 8, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Higgs, A. The l-arginine-nitric oxide pathway. N. Eng. J. Med. 1993, 329, 2002–2012. [Google Scholar] [CrossRef]
- Meijerink, J.; Poland, M.; Balvers, M.G.J.; Plastina, P.; Lute, C.; Dwarkasing, J.; van Norren, K.; Witkamp, R.F. Inhibition of COX-2-mediated eicosanoid production plays a major role in the anti-inflammatory effects of the endocannabinoid N-docosahexaenoylethanolamine (DHEA) in macrophages. Br. J. Pharmacol. 2015, 172, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Li, P.C.; Chen, B.C.; Chang, M.S.; Wang, J.L.; Chiu, W.T.; Lin, C.H. Lipoteichoic acid-induced nitric oxide synthase expression in RAW 264.7 macrophages is mediated by cyclooxygenase-2, prostaglandin E2, protein kinase A, p38 MAPK, and nuclear factor-kappaB pathways. Cell Signal. 2006, 18, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Cerella, C.; Sobolewski, C.; Dicato, M.; Diederich, M. Targeting COX-2 expression by natural compounds: A promising alternative strategy to synthetic COX-2 inhibitors for cancer chemoprevention and therapy. Biochem. Pharmacol. 2010, 80, 1801–1815. [Google Scholar] [CrossRef] [PubMed]
- Furia, E.; Napoli, A.; Tagarelli, A.; Sindona, G. Speciation of 2-hydroxybenzoic acid with calcium(II), magnesium(II), and nickel(II) cations in self-medium. J. Chem. Eng. Data 2013, 58, 1349–1353. [Google Scholar] [CrossRef]
- Kim, E.Y.; Moudgil, K.D. Regulation of autoimmune inflammation by pro-inflammatory cytokines. Immunol. Lett. 2008, 120, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, N.; Lacroix, S.; Rivest, S. An Essential Role of Interleukin-1β in Mediating NF-kB Activity and COX-2 Transcription in Cells of the Blood–Brain Barrier in Response to a Systemic and Localized Inflammation But Not During Endotoxemia. J. Neurosci. 1999, 19, 10923–10930. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Torres, R. Role of interleukin-1beta during pain and inflammation. Brain Res. Rev. 2009, 60, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Lupinacci, E.; Meijerink, J.; Vincken, J.P.; Gabriele, B.; Gruppen, H.; Witkamp, R.F. Xanthohumol from Hop (Humulus lupulus L.) Is an Efficient Inhibitor of Monocyte Chemoattractant Protein-1 and Tumor Necrosis Factor-alpha Release in LPS-Stimulated RAW 264.7 Mouse Macrophages and U937 Human Monocytes. J. Agric. Food Chem. 2009, 57, 7274–7281. [Google Scholar] [CrossRef] [PubMed]
- Burkovská, A.; Čikoš, Š.; Juhás, Š.; Il’Ková, G.; Rehák, P.; Koppel, J. Effects of a combination of thyme and oregano essential oils on TNBS-induced colitis in mice. Mediat. Inflamm. 2007, 23296. [Google Scholar] [CrossRef]
- Hotta, M.; Nakata, R.; Katsukawa, M.; Hori, K.; Takahashi, S.; Inoue, H. Carvacrol, a component of thyme oil, activates PPARα and γ and suppresses COX-2 expression. J. Lipid Res. 2010, 51, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.J.; Moon, J.Y.; Song, G.; Lee, Y.K.; Han, M.S.; Lee, J.S.; Ihm, B.S.; Lee, W.J.; Lee, N.H.; Hyun, C.G. Artemisia fukudo essential oil attenuates LPS-induced inflammation by suppressing NF-κB and MAPK activation in RAW264.7 macrophages. Food Chem. Toxicol. 2010, 48, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-N.; Ko, Y.-J.; Yang, H.-M.; Ham, Y.-M.; Roh, S.W.; Jeon, Y.-J.; Ahn, G.; Kang, M.-C.; Yoon, W.-J.; Kim, D.; et al. Anti-inflammatory effect of essential oil and its constituents from fingered citron (Citrus medica L. var. sarcodactylis) through blocking JNK, ERK and NF-kB signaling pathways in LPS-activated RAW 264.7 cells. Food Chem. Toxicol. 2013, 57, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G. Antioxidant and Anti-Inflammatory Activities of Essential Oils: A Short Review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.L.; Simas, D.L.R.; Pinheiro, M.M.G.; Moreno, D.S.A.; Alviano, C.S.; da Silva, A.J.R.; Dias Fernandes, P. Anti-Inflammatory Properties and Chemical Characterization of the Essential Oils of Four Citrus Species. PLoS ONE 2016, 11, e0153643. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-Y.; Jiang, J.-G.; Zhu, W.; Ou-Yang, Q. Anti-inflammatory Effect of Essential Oil from Citrus aurantium L. var. amara Engl. J. Agric. Food Chem. 2017, 65, 8586–8594. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Surveswaran, S.; Cai, Y.-Z.; Corke, H.; Sun, M. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chem. 2007, 102, 938–953. [Google Scholar] [CrossRef]
- Fazio, A.; Plastina, P.; Meijerink, J.; Witkamp, R.F.; Gabriele, B. Comparative analyses of seeds of wild fruits of Rubus and Sambucus species from Southern Italy: Fatty acid composition of the oil, total phenolic content, antioxidant and anti-inflammatory properties of the methanolic extracts. Food Chem. 2013, 140, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-A.; Jeon, S.-K.; Lee, E.-J.; Im, N.-K.; Jhee, K.-H.; Lee, S.-P.; Lee, I.-S. Radical Scavenging Activity of the Essential Oil of Silver Fir (Abies alba). J. Clin. Biochem. Nutr. 2009, 44, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.-M.; Lin, J.-Y. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013, 141, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Thompson, P.A.; Hakim, I.A.; Chow, H.H.S.; Thomson, C.A. d-Limonene: A bioactive food component from citrus and evidence for a potential role in breast cancer prevention and treatment. Oncol. Rev. 2011, 5, 31–42. [Google Scholar] [CrossRef]
- Yang, S.-A.; Jeon, S.-K.; Lee, E.-J.; Shim, C.-H.; Lee, I.-S. Comparative study of the chemical composition and antioxidant activity of six essential oils and their components. Nat. Prod. Res. 2010, 24, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Peana, A.T.; D’Aquila, P.S.; Panin, F.; Serra, G.; Pippia, P.; Moretti, M.D.L. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine 2002, 9, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Yamazaki, M.; Katagata, Y. Kuromoji (Lindera umbellata) Essential Oil Inhibits LPS-Induced Inflammation in RAW 264.7 Cells. Biosci. Biotechnol. Biochem. 2013, 77, 482–486. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Ramalho, T.R.; Filgueiras, L.R.; de Oliveira, M.T.P.; de Araujo Lima, A.L.; Bezerra-Santos, C.R.; Jancar, S.; Piuvezam, M.R. Gamma-Terpinene Modulation of LPS-Stimulated Macrophages is Dependent on the PGE2/IL-10 Axis. Planta Med. 2016, 82, 1341–1345. [Google Scholar] [CrossRef]



| No. | Constituent | CAS nr | Peak Area (%) a | ||
|---|---|---|---|---|---|
| CEO1 | CEO2 | CEO3 | |||
| 1 | β-Pinene | 127-91-3 | <0.1 a | 0.5 b | 0.5 b |
| 2 | Limonene | 5989-27-5 | 26.9 a | 54.3 b | 48.7 b |
| 3 | Sabinene | 3387-41-5 | 0.2 a,b | 0.1 a | 0.3 b |
| 4 | Myrcene | 123-35-3 | 0.1 a | 0.6 b | 1.2 c |
| 5 | γ-Terpinene | 99-85-4 | <0.1 a | 3.7 b | 0.4 c |
| 7 | Linalool | 78-70-6 | 19.6 a | 12.1 b | 32.4 c |
| 8 | Neral | 5392-40-5 | 0.2 a | 0.3 a,b | 0.5 b |
| 9 | Linalyl acetate | 115-95-7 | 47.5 a | 22.9 b | 12.0 c |
| 10 | Geranial | 5392-40-5 | 0.9 a | 0.5 b | 0.8 a |
| 11 | Geranyl acetate | 105-87-3 | 1.0 a | 0.3 b | 0.2 b |
| 12 | β-Caryophyllene | 87-44-5 | 0.3 a,b | 0.4 b | 0.1 c |
| Total identified | 96.7 | 95.9 | 97.5 | ||
| CEO | Ripening Stage | Scavenging Activity a | |
|---|---|---|---|
| DPPH• | ABTS•+ | ||
| CEO1 | Green | 6.1 ± 0.8 a | 11.1 ± 0.1 a |
| CEO2 | Half ripe | 7.8 ± 0.2 b | 10.8 ± 0.3 a |
| CEO3 | Ripe | 8.1 ± 0.4 b | 9.4 ± 0.1 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plastina, P.; Apriantini, A.; Meijerink, J.; Witkamp, R.; Gabriele, B.; Fazio, A. In Vitro Anti-Inflammatory and Radical Scavenging Properties of Chinotto (Citrus myrtifolia Raf.) Essential Oils. Nutrients 2018, 10, 783. https://doi.org/10.3390/nu10060783
Plastina P, Apriantini A, Meijerink J, Witkamp R, Gabriele B, Fazio A. In Vitro Anti-Inflammatory and Radical Scavenging Properties of Chinotto (Citrus myrtifolia Raf.) Essential Oils. Nutrients. 2018; 10(6):783. https://doi.org/10.3390/nu10060783
Chicago/Turabian StylePlastina, Pierluigi, Astari Apriantini, Jocelijn Meijerink, Renger Witkamp, Bartolo Gabriele, and Alessia Fazio. 2018. "In Vitro Anti-Inflammatory and Radical Scavenging Properties of Chinotto (Citrus myrtifolia Raf.) Essential Oils" Nutrients 10, no. 6: 783. https://doi.org/10.3390/nu10060783
APA StylePlastina, P., Apriantini, A., Meijerink, J., Witkamp, R., Gabriele, B., & Fazio, A. (2018). In Vitro Anti-Inflammatory and Radical Scavenging Properties of Chinotto (Citrus myrtifolia Raf.) Essential Oils. Nutrients, 10(6), 783. https://doi.org/10.3390/nu10060783