Primary Outcomes of a Randomized Controlled Crossover Trial to Explore the Effects of a High Chlorophyll Dietary Intervention to Reduce Colon Cancer Risk in Adults: The Meat and Three Greens (M3G) Feasibility Trial
Abstract
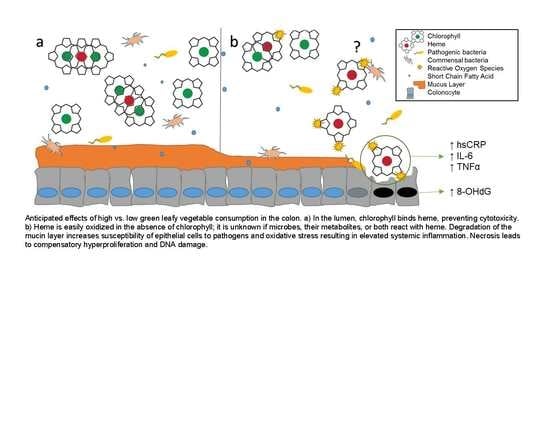
1. Introduction
2. Materials and Methods
2.1. Study Design and Aims
2.1.1. Primary Aim
2.1.2. Secondary Aims
2.2. Participant Recruitment and Informed Consent
2.2.1. Recruitment
2.2.2. Eligibility
2.3. Baseline
2.4. Randomization
2.5. Interventions
2.6. Measures/Time Points Data Collection
2.7. Hypothesis/Power/Statistical Analysis
3. Results
3.1. Feasibility Outcomes
3.2. Acceptability Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hughes, R.; Cross, A.; Pollock, J.; Bingham, S. Dose-dependent effect of dietary meat on endogenous colonic N-nitrosation. Carcinogenesis 2001, 22, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Ollberding, N.J.; Wilkens, L.R.; Henderson, B.E.; Kolonel, L.N.; Le Marchand, L. Meat consumption, heterocyclic amines and colorectal cancer risk: The Multiethnic Cohort Study. Int. J. Cancer 2012, 131, E1125–E1133. [Google Scholar] [CrossRef]
- Turesky, R.J.; Vouros, P. Formation and analysis of heterocyclic aromatic amine–DNA adducts in vitro and in vivo. J. Chromatogr. B 2004, 802, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Mantey, J.A.; Rekhadevi, P.V.; Diggs, D.L.; Ramesh, A. Metabolism of benzo (a) pyrene by subcellular fractions of gastrointestinal (GI) tract and liver in Apc Min mouse model of colon cancer. Tumor Boil. 2014, 35, 4929–4935. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.J.; Sinha, R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ. Mol. Mutagen. 2004, 44, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Sesink, A.L.; Termont, D.S.; Kleibeuker, J.H.; Van Der Meer, R. Red meat and colon cancer: Dietary haem-induced colonic cytotoxicity and epithelial hyperproliferation are inhibited by calcium. Carcinogenesis 2001, 22, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- de Vogel, J.; van-Eck, W.B.; Sesink, A.L.; Jonker-Termont, D.S.; Kleibeuker, J.; van der Meer, R. Dietary heme injures surface epithelium resulting in hyperproliferation, inhibition of apoptosis and crypt hyperplasia in rat colon. Carcinogenesis 2008, 29, 398–403. [Google Scholar] [CrossRef] [PubMed]
- de Vogel, J.; Jonker-Termont, D.S.; van Lieshout, E.M.; Katan, M.B.; van der Meer, R. Green vegetables, red meat and colon cancer: Chlorophyll prevents the cytotoxic and hyperproliferative effects of haem in rat colon. Carcinogenesis 2005, 26, 387–393. [Google Scholar] [CrossRef]
- Balder, H.F.; Vogel, J.; Jansen, M.C.; Weijenberg, M.P.; Westenbrink, S.; Van Der Meer, R.; Goldbohm, R.A.; van den Brandt, P.A. Heme and Chlorophyll Intake and Risk of Colorectal Cancer in the Netherlands Cohort Study. Cancer Epidemiol. Biomark. Prev. 2006, 15, 717–725. [Google Scholar] [CrossRef]
- IJssennagger, N.; Derrien, M.; van Doorn, G.M.; Rijnierse, A.; van den Bogert, B.; Müller, M.; Dekker, J.; Kleerebezem, M.; van der Meer, R. Dietary heme alters microbiota and mucosa of mouse colon without functional changes in host-microbe cross-talk. PLoS ONE 2012, 7, e49868. [Google Scholar] [CrossRef]
- Martin, O.C.; Lin, C.; Naud, N.; Tache, S.; Raymond-Letron, I.; Corpet, D.E.; Pierre, F.H. Antibiotic suppression of intestinal microbiota reduces heme-induced lipoperoxidation associated with colon carcinogenesis in rats. Nutr Cancer 2015, 67, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Ijssennagger, N.; Belzer, C.; Hooiveld, G.J.; Dekker, J.; Van Mil, S.W.C.; Muller, M.; Kleerebezem, M.; Van Der Meer, R. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. Proc. Natl. Acad. Sci. USA 2015, 112, 10038–10043. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Rundquist, S.; Greene, M.; Frugé, A. Development of the Dietary Habits and Colon Cancer Beliefs Survey (DHCCBS): An Instrument Assessing Health Beliefs Related to Red Meat and Green Leafy Vegetable Consumption. J. Acad. Nutr. Diet. 2018, 118, 151. [Google Scholar] [CrossRef]
- Barnard, N.D.; Gloede, L.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Green, A.A.; Ferdowsian, H. A low-fat vegan diet elicits greater macronutrient changes, but is comparable in adherence and acceptability, compared with a more conventional diabetes diet among individuals with type 2 diabetes. J. Am. Diet. Assoc. 2009, 109, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.J.; McGrievy, M.E.; Turner-McGrievy, G.M. Dietary adherence and acceptability of five different diets, including vegan and vegetarian diets, for weight loss: The New DIETs study. Eat. Behav. 2015, 19, 33–38. [Google Scholar] [CrossRef]
- Subar, A.F.; Thompson, F.E.; Kipnis, V.; Midthune, D.; Hurwitz, P.; McNutt, S.; McIntosh, A.; Rosenfeld, S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: The Eating at America’s Table Study. Am. J. Epidemiol. 2001, 154, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Langdon, A.; Crook, N.; Dantas, G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016, 8, 39. [Google Scholar] [CrossRef]
- Haro, C.; Rangel-Zúñiga, O.A.; Alcala-Diaz, J.F.; Gomez-Delgado, F.; Perez-Martinez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Landa, B.B.; Navas-Cortés, J.A.; Tena-Sempere, M.; et al. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS ONE 2016, 11, e0154090. [Google Scholar] [CrossRef]
- Urbaniak, G.C.; Plous, S. Research Randomizer. Available online: https://www.randomizer.org/ (accessed on 5 June 2019).
- Püssa, T. Nutritional and Toxicological Aspects of the Chemical Changes of Food Components and Nutrients during Freezing. In Handbook of Food Chemistry; Cheung, P.C.K., Mehta, B.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–23. [Google Scholar]
- Miglio, C.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effects of Different Cooking Methods on Nutritional and Physicochemical Characteristics of Selected Vegetables. J. Agric. Food Chem. 2008, 56, 139–147. [Google Scholar] [CrossRef]
- Bandura, A. Social Foundations of Thought and Action: A Social Cognitive Theory; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 1986. [Google Scholar]
- Subar, A.F.; Kirkpatrick, S.I.; Mittl, B.; Zimmerman, T.P.; Thompson, F.E.; Bingley, C.; Willis, G.; Islam, N.G.; Baranowski, T.; McNutt, S.; et al. The Automated Self-Administered 24-hour dietary recall (ASA24): A resource for researchers, clinicians, and educators from the National Cancer Institute. J. Acad. Nutr. Diet. 2012, 112, 1134–1137. [Google Scholar] [CrossRef]
- Woods, J.A.; Allen, J.; Miller, M.B.; White, B.; Gaskins, H.; Nehra, V. Exercise alters the gut microbiome and microbial metabolites: Implications for colorectal cancer and inflammatory bowel disease. Brain Behav. Immun. 2015, 49, e7. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Barnard, N.; Scialli, A.R.; Bertron, P.; Hurlock, D.; Edmonds, K. Acceptability of a Therapeutic Low-Fat, Vegan Diet in Premenopausal Women. J. Nutr. Educ. Behav. 2000, 32, 314–319. [Google Scholar] [CrossRef]
- Smith, K.S.; Raney, S.V.; Greene, M.W.; Frugé, A.D. Development and Validation of the Dietary Habits and Colon Cancer Beliefs Survey (DHCCBS): An Instrument Assessing Health Beliefs Related to Red Meat and Green Leafy Vegetable Consumption. J. Oncol. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.; Rubin, D.B. R equivalent: A simple effect size indicator. Psychol. Methods 2003, 8, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Buchs, N.C.; Ris, F. Risk of colorectal cancer in patients with diverticular disease. World J. Clin. Oncol. 2018, 9, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Lemstra, M.; Bird, Y.; Nwankwo, C.; Rogers, M.; Moraros, J. Weight loss intervention adherence and factors promoting adherence: A meta-analysis. Patient Preference Adherence 2016, 10, 1547–1559. [Google Scholar]
- Adams, R.N.; Mosher, C.E.; Blair, C.K.; Snyder, D.C.; Sloane, R.; Demark-Wahnefried, W. Cancer survivors’ uptake and adherence in diet and exercise intervention trials: An integrative data analysis. Cancer 2015, 121, 77–83. [Google Scholar] [CrossRef]
- Makarem, N.; Lin, Y.; Bandera, E.V.; Jacques, P.F.; Parekh, N. Concordance with World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) guidelines for cancer prevention and obesity-related cancer risk in the Framingham Offspring cohort (1991-2008). Cancer Causes Control 2015, 26, 277–286. [Google Scholar] [CrossRef]
- Pan, P.; Skaer, C.; Yu, J.; Zhao, H.; Ren, H.; Oshima, K.; Wang, L.-S. Berries and other natural products in the pancreatic cancer chemoprevention in human clinical trials. J. Berry Res. 2017, 7, 147–161. [Google Scholar] [CrossRef]
- Lampe, J.W.; Peterson, S. Brassica, biotransformation and cancer risk: Genetic polymorphisms alter the preventive effects of cruciferous vegetables. J. Nutr. 2002, 132, 2991–2994. [Google Scholar] [CrossRef] [PubMed]
- Gianfredi, V.; Salvatori, T.; Villarini, M.; Moretti, M.; Nucci, D.; Realdon, S. Is dietary fibre truly protective against colon cancer? A systematic review and meta-analysis. Int. J. Food Sci. Nutr. 2018, 69, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Crowder, S.L.; Frugé, A.D.; Douglas, K.G.; Chen, Y.T.; Moody, L.; Delk-Licata, A.; Erdman, J.W.; Black, M.; Carroll, W.R.; Spencer, S.A.; et al. Feasibility Outcomes of a Pilot Randomized Clinical Trial to Increase Cruciferous and Green Leafy Vegetable Intake in Posttreatment Head and Neck Cancer Survivors. J. Acad. Nutr. Diet. 2019, 119, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.P.; Natarajan, L.; Caan, B.J.; Parker, B.A.; Greenberg, E.R.; Flatt, S.W.; Rock, C.L.; Kealey, S.; Al-Delaimy, W.K.; Bardwell, W.A.; et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: The Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA 2007, 298, 289–298. [Google Scholar] [CrossRef]
- Satia, J.A.; Campbell, M.K.; Galanko, A.J.; James, A.; Carr, C.; Sandler, R.S. Longitudinal changes in lifestyle behaviors and health status in colon cancer survivors. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1022–1031. [Google Scholar]
- Baker, A.N.; Parsons, M.; Donnelly, S.M.; Johnson, L.; Day, J.; Mervis, A.; James, B.; Burt, R.; Magill, M.K. Improving colon cancer screening rates in primary care: A pilot study emphasising the role of the medical assistant. Qual. Saf. Health Care 2009, 18, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.S.; Oman, M.; Patel, A.M.; Vega, K.J. Health disparities in colorectal cancer among racial and ethnic minorities in the United States. J. Gastrointest. Oncol. 2016, 7 (Suppl. S1), S32–S43. [Google Scholar]



| Base | 4 Weeks | 8 Weeks | 12 Weeks | |
|---|---|---|---|---|
| Primary Outcome: | ||||
| Feasibility—accrual, adherence, retention | X | X | X | X |
| Secondary Outcomes: | ||||
| 24-h dietary recalls—diet composition | X | X | X | X |
| International Physical Activity Questionnaire | X | X | X | X |
| Anthropometrics—height (only at baseline), weight, body mass index (BMI), waist and hip measurements | X | X | X | X |
| Fecal samples—gut microbiome, oxidized guanine species | X | X | X | X |
| Blood samples—serum inflammatory cytokines, Plasma Vitamin K, oxidized guanine species | X | X | X | X |
| Acceptability—Food Acceptability Questionnaire | X | X | ||
| Demographics | X | |||
| Dietary Habits and Colon Cancer Beliefs Survey | X | X |
| Participants | Non-Participants | ||
|---|---|---|---|
| (n = 50) | (n = 128) | ||
| ------- Mean 1 (SD) ------ | p | ||
| RM servings per week | 10.3 (5.1) | 8.1 (7.2) | 0.050 |
| GLV servings per week | 0.21 (0.25) | 0.28 (0.45) | 0.215 |
| Age (years) | 48 (13) | 45 (12) | 0.100 |
| Body Mass Index (kg/m2) | 36.2 (4.9) | 31.2 (9.0) | <0.0001 |
| -------- N 2 (%) -------- | p | ||
| Gender | 0.211 | ||
| Male | 19 (38%) | 36 (28.1%) | |
| Female | 31 (62%) | 92 (71.9%) | |
| Race | 0.293 | ||
| Asian | 0 (0%) | 4 (3.1%) | |
| African-American | 10 (20%) | 25 (19.5%) | |
| White | 40 (80%) | 94 (73.4%) | |
| More than one race | 0 (0%) | 5 (3.9%) | |
| Education | 0.060 | ||
| Less than bachelor’s degree | 7 (14%) | 39 (30.5%) | |
| Bachelor’s degree | 19 (38%) | 28 (21.9%) | |
| Master’s degree | 13 (26%) | 31 (24.2%) | |
| Doctoral/Professional degree | 11 (22%) | 30 (23.4%) | |
| Marital Status | 0.998 | ||
| Single | 12 (24%) | 31 (24.2%) | |
| Married | 28 (56%) | 71 (55.5%) | |
| Widowed/Divorced/Separated | 10 (20%) | 26 (20.3%) | |
| T0 | T4 | Change (T4–T0) | T8 | T12 | Change (T12–T8) | |||
|---|---|---|---|---|---|---|---|---|
| Immediate Group | ------- Mean (SD) ------ | p1 | ------- Mean (SD) ------ | p1 | ||||
| Vitamin K (mcg) | 195.5 (264.1) | 703.6 (752.5) | 508.1 (854.5) | 0.009 | 221.2 (203.5) | 252.9 (238.6) | 31.7 (319.7) | 0.639 |
| Dark Green Vegetables (cup eq.) | 0.27 (0.36) | 0.86 (0.92) | 0.58 (1.02) | 0.011 | 0.35 (0.41) | 0.48 (0.50) | 0.13 (0.69) | 0.391 |
| Red Meat (28 g eq.) | 1.80 (1.87) | 2.50 (2.45) | 0.70 (2.92) | 0.262 | 1.38 (1.21) | 1.72 (1.83) | 0.34 (2.01) | 0.418 |
| Delayed Group | ||||||||
| Vitamin K (mcg) | 189.1 (162.9) | 294.3 (395.5) | 105.2 (389.3) | 0.208 | 247.6 (380.6) | 537.3 (471.8) | 289.7 (373.5) | 0.001 |
| Dark Green Vegetables (cup eq.) | 0.30 (0.37) | 0.38 (0.47) | 0.08 (0.63) | 0.554 | 0.37 (0.567) | 0.85 (0.79) | 0.48 (0.76) | 0.006 |
| Red Meat (28 g eq.) | 1.81 (1.82) | 1.55 (1.45) | −0.26 (2.07) | 0.548 | 0.977 (1.057) | 2.14 (1.96) | 1.17 (2.34) | 0.026 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frugé, A.D.; Smith, K.S.; Riviere, A.J.; Demark-Wahnefried, W.; Arthur, A.E.; Murrah, W.M.; Morrow, C.D.; Arnold, R.D.; Braxton-Lloyd, K. Primary Outcomes of a Randomized Controlled Crossover Trial to Explore the Effects of a High Chlorophyll Dietary Intervention to Reduce Colon Cancer Risk in Adults: The Meat and Three Greens (M3G) Feasibility Trial. Nutrients 2019, 11, 2349. https://doi.org/10.3390/nu11102349
Frugé AD, Smith KS, Riviere AJ, Demark-Wahnefried W, Arthur AE, Murrah WM, Morrow CD, Arnold RD, Braxton-Lloyd K. Primary Outcomes of a Randomized Controlled Crossover Trial to Explore the Effects of a High Chlorophyll Dietary Intervention to Reduce Colon Cancer Risk in Adults: The Meat and Three Greens (M3G) Feasibility Trial. Nutrients. 2019; 11(10):2349. https://doi.org/10.3390/nu11102349
Chicago/Turabian StyleFrugé, Andrew D., Kristen S. Smith, Aaron J. Riviere, Wendy Demark-Wahnefried, Anna E. Arthur, William M. Murrah, Casey D. Morrow, Robert D. Arnold, and Kimberly Braxton-Lloyd. 2019. "Primary Outcomes of a Randomized Controlled Crossover Trial to Explore the Effects of a High Chlorophyll Dietary Intervention to Reduce Colon Cancer Risk in Adults: The Meat and Three Greens (M3G) Feasibility Trial" Nutrients 11, no. 10: 2349. https://doi.org/10.3390/nu11102349
APA StyleFrugé, A. D., Smith, K. S., Riviere, A. J., Demark-Wahnefried, W., Arthur, A. E., Murrah, W. M., Morrow, C. D., Arnold, R. D., & Braxton-Lloyd, K. (2019). Primary Outcomes of a Randomized Controlled Crossover Trial to Explore the Effects of a High Chlorophyll Dietary Intervention to Reduce Colon Cancer Risk in Adults: The Meat and Three Greens (M3G) Feasibility Trial. Nutrients, 11(10), 2349. https://doi.org/10.3390/nu11102349