Dietary Intake of Green Nut Oil or DHA Ameliorates DHA Distribution in the Brain of a Mouse Model of Dementia Accompanied by Memory Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal
2.2. Chemicals and Reagents
2.3. Group Size and Samples
2.4. Tissue Preparation
2.5. DESI-IMS
2.6. DESI-IMS Data Analysis
2.7. Mouse Behavior
2.8. Statistical Analysis
3. Results
3.1. Detection of Target Peaks (m/z) from DESI-IMS Spectra
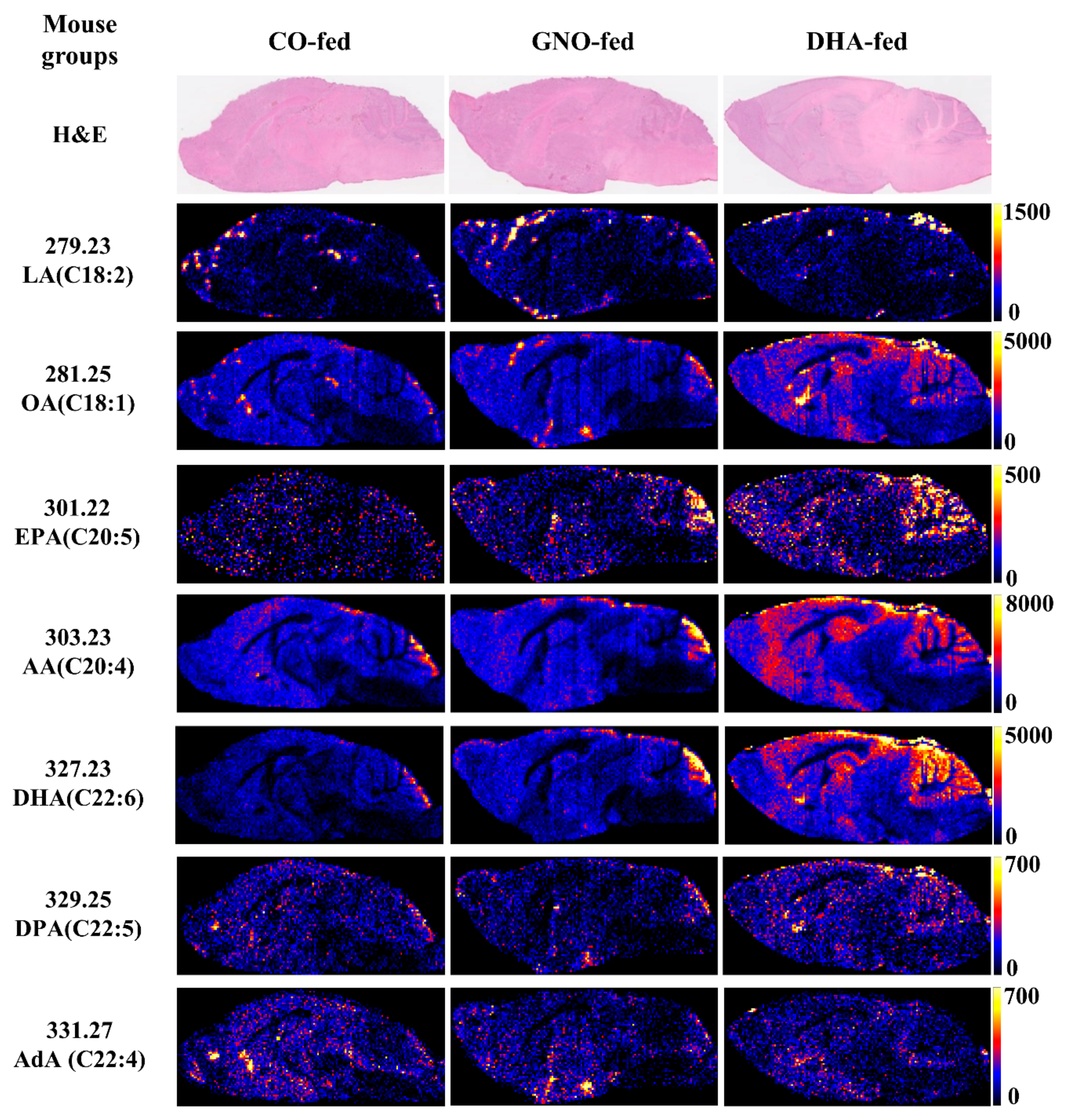
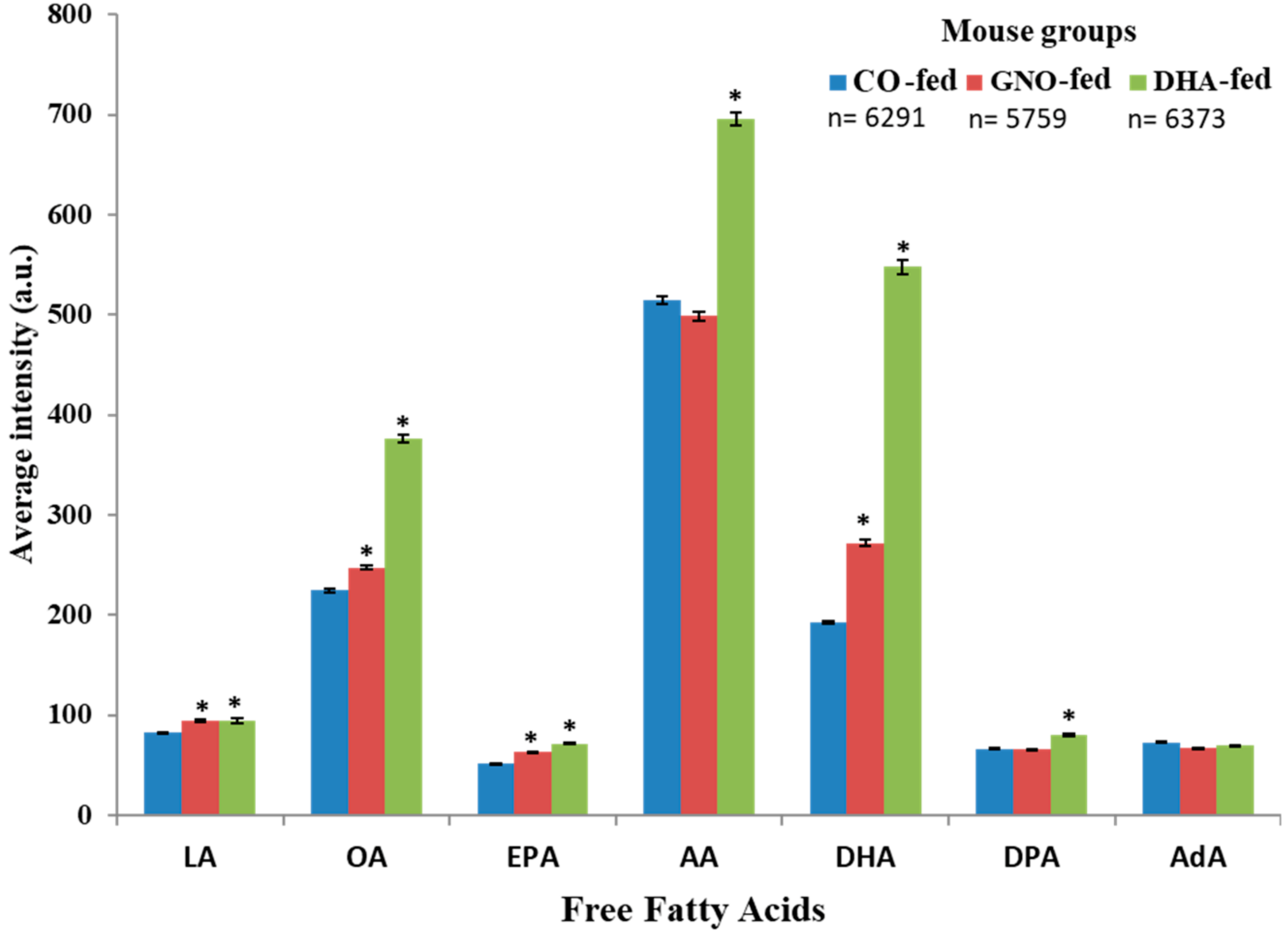
3.2. Effects of Dietary Intake of GNO or DHA on Brain Distribution of FFAs
3.3. Effects of Dietary Intake of GNO or DHA on Brain Distribution of DHA
3.4. Effects of GNO or DHA on Memory Efficiency of SAMP8 Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harsløf, L.B.S.; Ciappolino, V.; Agostoni, C. DHA Effects in Brain Development and Function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef]
- Lenihan-Geels, G.; Bishop, K.S.; Ferguson, L.R. Alternative Sources of Omega-3 Fats: Can We Find a Sustainable Substitute for Fish? Nutrients 2013, 5, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Bishop, K.S.; Erdrich, S.; Karunasinghe, N.; Han, D.Y.; Zhu, S.; Jesuthasan, A.; Ferguson, L.R. An Investigation into the Association between DNA Damage and Dietary Fatty Acid in Men with Prostate Cancer. Nutrients 2015, 7, 405–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiser, M.J.; Butt, C.M.; Mohajeri, M.H. Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrients 2016, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Vasconcelos, R.O.; Gomes, R.N.; Calviello, G. Protective Effects of ω-3 PUFA in Anthracycline-Induced Cardiotoxicity: A Critical Review. Int. J. Mol. Sci. 2017, 18, 2689. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2018, 14, 367–429. [Google Scholar]
- Schaeffer, E.L.; Figueiró, M.; Gattaz, W.F. Insights into Alzheimer disease pathogenesis from studies in transgenic animal models. Clinics 2011, 66, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martikainen, I.; Kemppainen, N.; Johansson, J.; Teuho, J.; Helin, S.; Liu, Y.; Helisalmi, S.; Soininen, H.; Parkkola, R.; Ngandu, T. Brain β-amyloid and atrophy in individuals at increased risk of cognitive decline. Am. J. Neur. 2019, 40, 80–85. [Google Scholar] [CrossRef]
- Snowden, S.G.; Ebshiana, A.A.; Hye, A.; An, Y.; Pletnikova, O.; O’Brien, R.; Troncoso, J.; Legido-Quigley, C.; Thambisetty, M. Association between fatty acid metabolism in the brain and Alzheimer disease neuropathology and cognitive performance: A nontargeted metabolomic study. PLoS Med. 2017, 14, e1002266. [Google Scholar] [CrossRef]
- Yuki, D.; Sugiura, Y.; Zaima, N.; Akatsu, H.; Takei, S.; Yao, I.; Maesako, M.; Kinoshita, A.; Yamamoto, T.; Kon, R.; et al. DHA-PC and PSD-95 decrease after loss of synaptophysin and before neuronal loss in patients with Alzheimer’s disease. Sci. Rep. 2014, 4, 7130. [Google Scholar] [CrossRef] [PubMed]
- Green, K.N.; Martinez-Coria, H.; Khashwji, H.; Hall, E.B.; Yurko-Mauro, K.A.; Ellis, L.; LaFerla, F.M. Dietary Docosahexaenoic Acid and Docosapentaenoic Acid Ameliorate Amyloid-β and Tau Pathology via a Mechanism Involving Presenilin 1 Levels. J. Neurosci. 2007, 27, 4385–4395. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Choy, K.H.; Marriott, P.J.; Chai, S.Y.; Scanlon, M.J.; Porter, C.J.; Short, J.L.; Nicolazzo, J.A. Reduced blood-brain barrier expression of fatty acid-binding protein 5 is associated with increased vulnerability of APP/PS1 mice to cognitive deficits from low omega-3 fatty acid diets. J. Neurochem. 2018, 144, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Florent, S.; Youssef, I.; Kriem, B.; Koziel, V.; Escanyé, M.-C.; Fifre, A.; Sponne, I.; Olivier, J.-L.; Pillot, T.; Oster, T.; et al. Docosahexaenoic acid prevents neuronal apoptosis induced by soluble amyloid-β oligomers. J. Neurochem. 2006, 96, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Yip, P.K.; Bowes, A.L.; Hall, J.C.; Burguillos, M.A.; Ip, T.; Baskerville, T.; Liu, Z.-H.; Mohamed, M.A.; Getachew, F.; Lindsay, A.D. Docosahexaenoic acid reduces microglia phagocytic activity via miR-124 and induces neuroprotection in rodent models of spinal cord contusion injury. Hum. Mol. Gene 2019, 28, 2427–2448. [Google Scholar] [CrossRef]
- Takeda, T.; Hosokawa, M.; Higuchi, K. The Senescence-Accelerated Mouse (SAM) Achievements and Future Directions; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Akiguchi, I.; Pallàs, M.; Budka, H.; Akiyama, H.; Ueno, M.; Han, J.; Yagi, H.; Nishikawa, T.; Chiba, Y.; Sugiyama, H.; et al. SAMP8 mice as a neuropathological model of accelerated brain aging and dementia: Toshio Takeda’s legacy and future directions. Neuropathology 2017, 37, 293–305. [Google Scholar] [CrossRef]
- Bondioli, P.; Bella, L.D.; RETrKE, P. Alpha linolenic acid rich oils. Composition of Plukenetia volubilis (Sacha Inchi) oil from Peru. Riv. Ital. Delle Sostanze Grasse 2006, 83, 120. [Google Scholar]
- Takeyama, E.; Fukushima, M. Physicochemical Properties of Plukenetia volubilis L. Seeds and Oxidative Stability of Cold-pressed Oil (Green Nut Oil). Food Sci. Technol. Res. 2013, 19, 875–882. [Google Scholar] [CrossRef] [Green Version]
- Dulvy, N.K.; Sadovy, Y.; Reynolds, J.D. Extinction vulnerability in marine populations. Fish Fish. 2003, 4, 25–64. [Google Scholar] [CrossRef]
- Zaima, N.; Hayasaka, T.; Goto-Inoue, N.; Setou, M. Matrix-Assisted Laser Desorption/Ionization Imaging Mass Spectrometry. Int. J. Mol. Sci. 2010, 11, 5040–5055. [Google Scholar] [CrossRef] [Green Version]
- Shintani-Domoto, Y.; Hayasaka, T.; Maeda, D.; Masaki, N.; Ito, T.K.; Sakuma, K.; Tanaka, M.; Kabashima, K.; Takei, S.; Setou, M. Different desmin peptides are distinctly deposited in cytoplasmic aggregations and cytoplasm of desmin-related cardiomyopathy patients. Biochim. Biophys. Acta BBA Proteins Proteom. 2017, 1865, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, Y.; Masaki, N.; Takei, S.; Horikawa, M.; Matsushita, S.; Sugiyama, E.; Ogura, H.; Shiiya, N.; Setou, M. Recurrent triple-negative breast cancer (TNBC) tissues contain a higher amount of phosphatidylcholine (32:1) than non-recurrent TNBC tissues. PLoS ONE 2017, 12, e0183724. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, S.; Masaki, N.; Sato, K.; Hayasaka, T.; Sugiyama, E.; Hui, S.-P.; Chiba, H.; Mase, N.; Setou, M. Selective improvement of peptides imaging on tissue by supercritical fluid wash of lipids for matrix-assisted laser desorption/ionization mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Horikawa, M.; Sato, S.; Miyake, H.; Setou, M. Discovery of lipid biomarkers correlated with disease progression in clear cell renal cell carcinoma using desorption electrospray ionization imaging mass spectrometry. Oncotarget 2019, 10, 1688–1703. [Google Scholar] [CrossRef] [Green Version]
- Mihara, Y.; Horikawa, M.; Sato, S.; Eto, F.; Hanada, M.; Banno, T.; Arima, H.; Ushirozako, H.; Yamada, T.; Xu, D.; et al. Lysophosphatidic acid precursor levels decrease and an arachidonic acid-containing phosphatidylcholine level increases in the dorsal root ganglion of mice after peripheral nerve injury. Neurosci. Lett. 2019, 698, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Girod, M.; Shi, Y.; Cheng, J.-X.; Cooks, R.G. Desorption electrospray ionization imaging mass spectrometry of lipids in rat spinal cord. J. Am. Soc. Mass Spectrom. 2010, 21, 1177–1189. [Google Scholar] [CrossRef] [Green Version]
- Tillner, J.; Wu, V.; Jones, E.A.; Pringle, S.D.; Karancsi, T.; Dannhorn, A.; Veselkov, K.; McKenzie, J.S.; Takats, Z. Faster, More Reproducible DESI-MS for Biological Tissue Imaging. J. Am. Soc. Mass Spectrom. 2017, 28, 2090–2098. [Google Scholar] [CrossRef] [Green Version]
- Takats, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Mass Spectrometry Sampling Under Ambient Conditions with Desorption Electrospray Ionization. Science 2004, 306, 471–473. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H.; Suzuki, H.; Suda, K.; Yamazaki, Y.; Takino, A.; Kim, Y.-I.; Goto, T.; Iijima, Y.; Aoki, K.; Shibata, D.; et al. Long-Chain Free Fatty Acid Profiling Analysis by Liquid Chromatography–Mass Spectrometry in Mouse Treated with Peroxisome Proliferator-Activated Receptor α Agonist. Biosci. Biotechnol. Biochem. 2013, 77, 2288–2293. [Google Scholar] [CrossRef]
- Yamada, K.; Komori, Y.; Tanaka, T.; Senzaki, K.; Nikai, T.; Sugihara, H.; Kameyama, T.; Nabeshima, T. Brain dysfunction associated with an induction of nitric oxide synthase following an intracerebral injection of lipopolysaccharide in rats. Neuroscience 1999, 88, 281–294. [Google Scholar] [CrossRef]
- Hong, L.; Zahradka, P.; Cordero-Monroy, L.; Wright, B.; Taylor, C.G. Dietary Docosahexaenoic Acid (DHA) and Eicosapentaenoic Acid (EPA) Operate by Different Mechanisms to Modulate Hepatic Steatosis and Hyperinsulemia in fa/fa Zucker Rats. Nutrients 2019, 11, 917. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Schneider, J.A.; Tangney, C.; Tremblay-Mercier, J.; Fortier, M.; Bennett, D.A.; Morris, M.C. Plasma and Brain Fatty Acid Profiles in Mild Cognitive Impairment and Alzheimer’s Disease. J. Alzheimer’s Dis. 2012, 29, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Kalish, B.T.; Fallon, E.M.; Puder, M. A tutorial on fatty acid biology. J. Parent. Enter. Nutr. 2012, 36, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Barbagallo, M. Nutritional prevention of cognitive decline and dementia. Acta Bio Med. Atenei Parm. 2018, 89, 276–290. [Google Scholar] [PubMed]
- Bentsen, H. Dietary polyunsaturated fatty acids, brain function and mental health. Microb. Ecol. Heal. Dis. 2017, 28, 1281916. [Google Scholar] [CrossRef]
- Metherel, A.H.; Chouinard-Watkins, R.; Trépanier, M.-O.; Lacombe, R.J.S.; Bazinet, R.P. Retroconversion is a minor contributor to increases in eicosapentaenoic acid following docosahexaenoic acid feeding as determined by compound specific isotope analysis in rat liver. Nutr. Metab. 2017, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Darios, F.; Davletov, B. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature 2006, 440, 813–817. [Google Scholar] [CrossRef]
- Jackson, T.C.; Foster, T.C. Regional Health and Function in the hippocampus: Evolutionary compromises for a critical brain region. Biosci. Hypotheses 2009, 2, 245–251. [Google Scholar] [CrossRef]
- Arruda-Carvalho, M.; Restivo, L.; Guskjolen, A.; Epp, J.R.; Elgersma, Y.; Josselyn, S.A.; Frankland, P.W. Conditional Deletion of α-CaMKII Impairs Integration of Adult-Generated Granule Cells into Dentate Gyrus Circuits and Hippocampus-Dependent Learning. J. Neurosci. 2014, 34, 11919–11928. [Google Scholar] [CrossRef]
- Petursdottir, A.; Farr, S.; Morley, J.; Banks, W.; Skuladottir, G. Lipid peroxidation in brain during aging in the senescence-accelerated mouse (SAM). Neurobiol. Aging 2007, 28, 1170–1178. [Google Scholar] [CrossRef]
- Pan, Y.; Short, J.L.; Choy, K.H.C.; Zeng, A.X.; Marriott, P.J.; Owada, Y.; Scanlon, M.J.; Porter, C.J.H.; Nicolazzo, J.A. Fatty Acid-Binding Protein 5 at the Blood–Brain Barrier Regulates Endogenous Brain Docosahexaenoic Acid Levels and Cognitive Function. J. Neurosci. 2016, 36, 11755–11767. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C. The role of omega-3 fatty acids in adult hippocampal neurogenesis. Oléagineux Corps Gras Lipides 2011, 18, 242–245. [Google Scholar] [CrossRef]
- Lledo, P.-M.; Valley, M. Adult olfactory bulb neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, a018945. [Google Scholar] [CrossRef] [PubMed]
- Neulinger, K.; Oram, J.; Tinson, H.; O’Gorman, J.; Shum, D.H. Prospective memory and frontal lobe function. Aging Neuropsychol. Cogn. 2016, 23, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Burguière, E.; Arabo, A.; Jarlier, F.; De Zeeuw, C.I.; Rondi-Reig, L. Role of the Cerebellar Cortex in Conditioned Goal-Directed Behavior. J. Neurosci. 2010, 30, 13265–13271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manto, M.; Bower, J.M.; Conforto, A.B.; Delgado-García, J.M.; Da Guarda, S.N.F.; Gerwig, M.; Habas, C.; Hagura, N.; Ivry, R.B.; Mariën, P.; et al. Consensus paper: roles of the cerebellum in motor control--the diversity of ideas on cerebellar involvement in movement. Cerebellum 2012, 11, 457–487. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Ojemann, G.A. The Role of the Human Thalamus in Language and Memory: Evidence from Electrophysiological Studies. Brain Cogn. 2000, 42, 218–230. [Google Scholar] [CrossRef] [Green Version]
- Saper, C.B.; Lowell, B.B. The hypothalamus. Curr. Biol. 2014, 24, R1111–R1116. [Google Scholar] [CrossRef] [Green Version]
- Arsenault, D.; Julien, C.; Tremblay, C.; Calon, F. DHA Improves Cognition and Prevents Dysfunction of Entorhinal Cortex Neurons in 3xTg-AD Mice. PLoS ONE 2011, 6, e17397. [Google Scholar] [CrossRef]
- Quinn, J.F.; Raman, R.; Thomas, R.G.; Yurko-Mauro, K.; Nelson, E.B.; Van Dyck, C.; Galvin, J.E.; Emond, J.; Jack, C.R.; Weiner, M.; et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA 2010, 304, 1903–1911. [Google Scholar] [CrossRef]
- Yurko-Mauro, K.; Alexander, D.D.; Van Elswyk, M.E. Docosahexaenoic Acid and Adult Memory: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, 0120391. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. In Pre-Clinical Models; Springer: Berlin, Germany, 2019; pp. 105–111. [Google Scholar]


| DESI Ion Source Parameters | Capillary Voltage | −4 kV |
|---|---|---|
| Source Temperature | 100 °C | |
| Spray Impact angle | 80° | |
| Solvent | 98% Methanol (v/v) | |
| Solvent Flow Rate | 2 µL/min | |
| Nebulizing N2 Gas Pressure | 0.4 MPa | |
| DESI stage parameters | Pixel size | 100 µM |
| Scanning speed (X axis) | 200 µM/sec | |
| Data acquisition parameters | Polarity | −Ve |
| m/z range | 100 to 1200 Da | |
| Resolution | 20000 | |
| Mass window | 0.02 Da | |
| Acquisition rate | 1 spectrum sec-1 | |
| Inlet voltage | 4000 V |
| Free Fatty Acids | Theoretical m/z | Observed m/z | Mass Accuracy (ppm) |
|---|---|---|---|
| Linoleic acid (LA) | 279.2330 | 279.2331 | 0.36 |
| Oleic acid (OA) | 281.2486 | 281.2487 | 0.36 |
| Eicosapentaenoic acid (EPA) | 301.2173 | 301.2170 | 1.00 |
| Docosapentaenoic acid (DPA) | 329.2486 | 329.2451 | 10.63 |
| Adrenic acid (AdA) | 331.2643 | 331.2639 | 1.21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeyama, E.; Islam, A.; Watanabe, N.; Tsubaki, H.; Fukushima, M.; Mamun, M.A.; Sato, S.; Sato, T.; Eto, F.; Yao, I.; et al. Dietary Intake of Green Nut Oil or DHA Ameliorates DHA Distribution in the Brain of a Mouse Model of Dementia Accompanied by Memory Recovery. Nutrients 2019, 11, 2371. https://doi.org/10.3390/nu11102371
Takeyama E, Islam A, Watanabe N, Tsubaki H, Fukushima M, Mamun MA, Sato S, Sato T, Eto F, Yao I, et al. Dietary Intake of Green Nut Oil or DHA Ameliorates DHA Distribution in the Brain of a Mouse Model of Dementia Accompanied by Memory Recovery. Nutrients. 2019; 11(10):2371. https://doi.org/10.3390/nu11102371
Chicago/Turabian StyleTakeyama, Emiko, Ariful Islam, Nakamichi Watanabe, Hiroe Tsubaki, Masako Fukushima, Md. Al Mamun, Shumpei Sato, Tomohito Sato, Fumihiro Eto, Ikuko Yao, and et al. 2019. "Dietary Intake of Green Nut Oil or DHA Ameliorates DHA Distribution in the Brain of a Mouse Model of Dementia Accompanied by Memory Recovery" Nutrients 11, no. 10: 2371. https://doi.org/10.3390/nu11102371







