The Vitamin E Derivative Gamma Tocotrienol Promotes Anti-Tumor Effects in Acute Myeloid Leukemia Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Isolation and Culture of Mesenschnymal Stem Cells (MSCs) from Rat Bone Marrow
2.3. Drug Preparation
2.4. Cell Viability Assay
2.5. Cell Cycle Analysis
2.6. Apoptosis Detection
2.7. DNA Fragemnetation using Cell Death ELISA
2.8. Western Blotting
2.9. Detecteion of Reacrtive Oxygen Species
2.10. Statistical Analysis
3. Results
3.1. Effect of γ-Tocotrienol on the Proliferation of AML Cell Lines
3.2. Effect of γ-Tocotrienol on the Proliferation of Mesenchymal Stem Cells
3.3. Effect of γ-Tocotrienol on the Cell Cycle Progression of AML Cell Lines
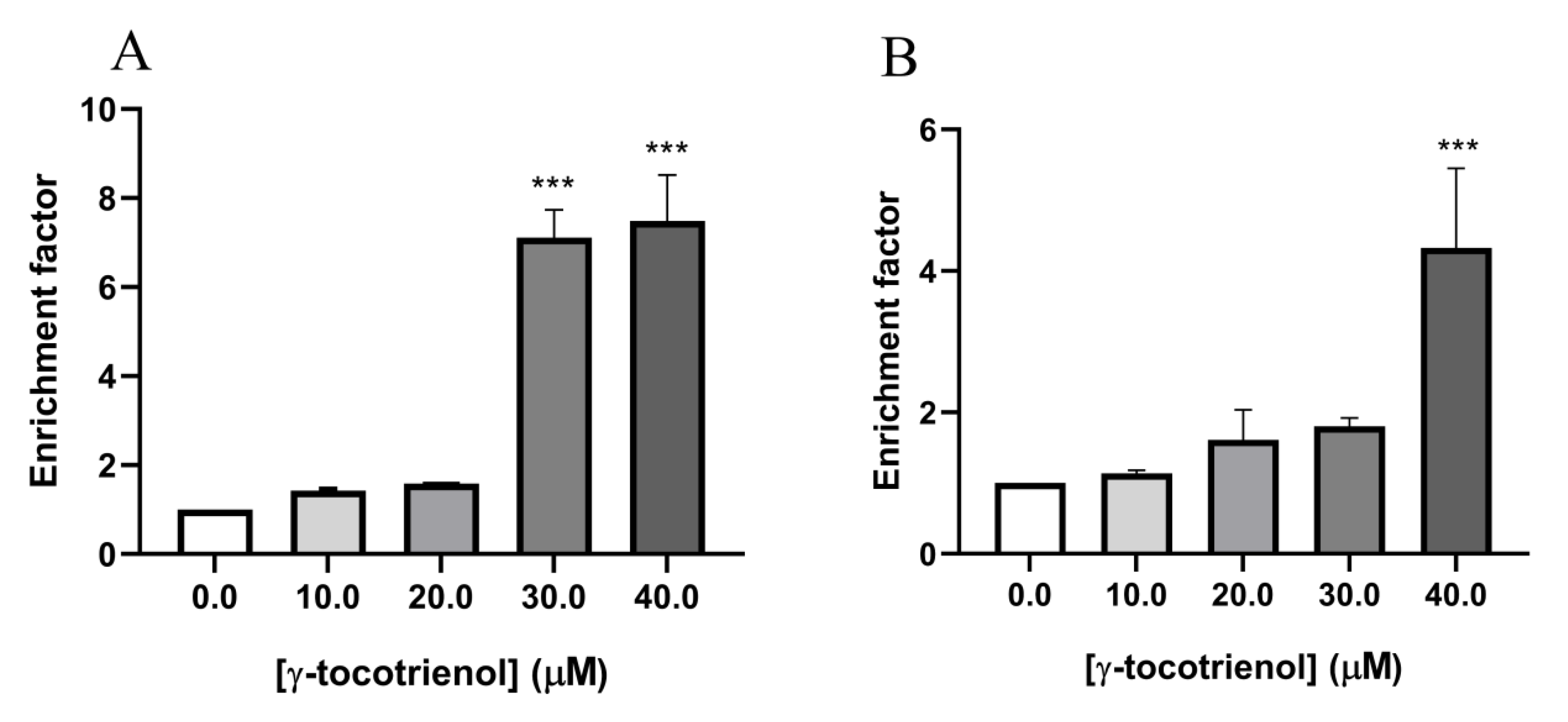
3.4. Effect of γ-Tocotrienol on Apoptosis in AML Cell Lines
3.5. Effect of γ-Tocotrienol on DNA Fragmentation in AML Cell Lines
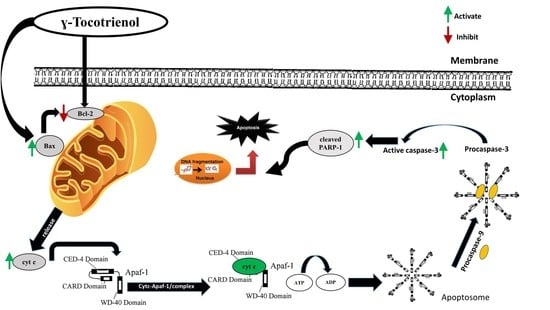
3.6. Effect of γ-Tocotrienol on the Expression of Proteins involved in Pro-Apoptotic and Anti-Proliferative Pathways
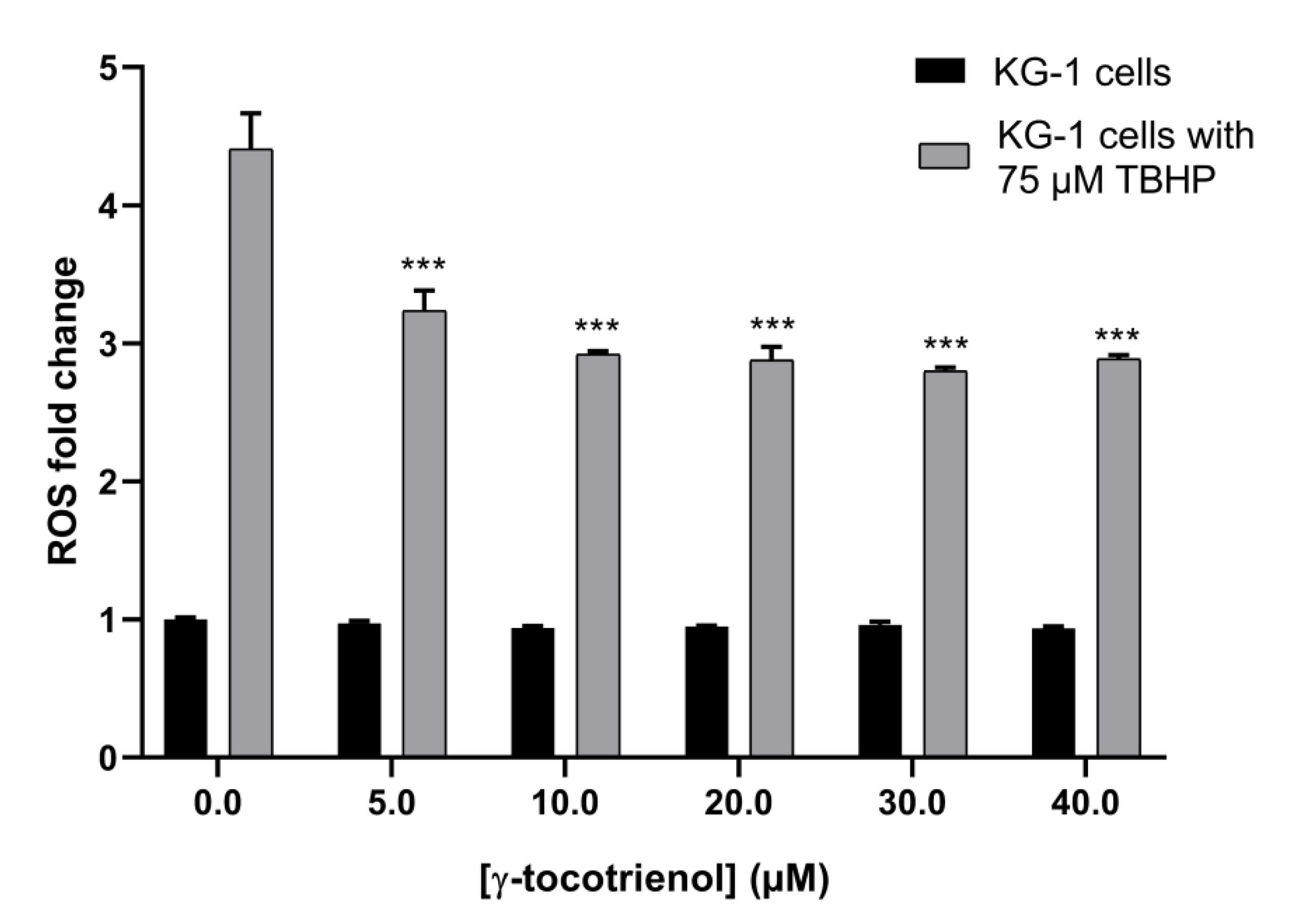
3.7. Effect of γ-Tocotrienol on ROS Production in KG-1 Cell Line
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siveen, K.S.; Uddin, S.; Mohammad, R.M. Targeting acute myeloid leukemia stem cell signaling by natural products. Mol. Cancer 2017, 16, 13. [Google Scholar] [CrossRef]
- Jain, A.; Tiwari, A.; Verma, A.; Jain, S.K. Vitamins for Cancer Prevention and Treatment: An Insight. Curr. Mol. Med. 2017, 17, 321–340. [Google Scholar] [CrossRef]
- Ng, K.L.; Radhakrishnan, A.K.; Selvaduray, K.R. Gamma-Tocotrienol Inhibits Proliferation of Human Chronic Myeloid Leukemic Cells via Activation of Extrinsic and Intrinsic Apoptotic Pathways. J. Bld. Dis. Ther. 2016, 1, 102. [Google Scholar]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006, 78, 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Manor, D. Vitamin E. Adv. Nutr. 2012, 3, 330–331. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, C.; Hyatt, J.A.; Vraka, P.S.; Papas, A.; Papas, K.A.; Neophytou, C.; Hadjivassiliou, V.; Constantinou, A.I. Induction of caspase-independent programmed cell death by vitamin E natural homologs and synthetic derivatives. Nutr. Cancer 2009, 61, 864–874. [Google Scholar] [CrossRef]
- Rasool, A.H.; Yuen, K.H.; Yusoff, K.; Wong, A.R.; Rahman, A.R. Dose dependent elevation of plasma tocotrienol levels and its effect on arterial compliance, plasma total antioxidant status, and lipid profile in healthy humans supplemented with tocotrienol rich vitamin E. J. Nutr. Sci. Vitaminol. (Tokyo) 2006, 52, 473–478. [Google Scholar] [CrossRef]
- Aggarwal, V.; Kashyap, D.; Sak, K.; Tuli, H.S.; Jain, A.; Chaudhary, A.; Garg, V.K.; Sethi, G.; Yerer, M.B. Molecular Mechanisms of Action of Tocotrienols in Cancer: Recent Trends and Advancements. Int. J. Mol. Sci. 2019, 20, 656. [Google Scholar] [CrossRef]
- Schauss, A. Tocotrienols: A review. In Tocotrienols: Vitamin E Beyond Tocopherols; Watson, R.R., Preedy, V.R., Eds.; AOCS Press: Urbana, IL, USA; Taylor & Francis/CRC Press: Boca Raton, FL, USA, 2009; pp. 1–12. [Google Scholar]
- Singh, V.K.; Beattie, L.A.; Seed, T.M. Vitamin E: Tocopherols and tocotrienols as potential radiation countermeasures. J. Radiat. Res. 2013, 54, 973–988. [Google Scholar] [CrossRef]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tocotrienols: A review. Nutr. Metab. (Lond) 2014, 11, 52. [Google Scholar] [CrossRef]
- Park, S.K.; Sanders, B.G.; Kline, K. Tocotrienols induce apoptosis in breast cancer cell lines via an endoplasmic reticulum stress-dependent increase in extrinsic death receptor signaling. Breast Cancer Res. Treat. 2010, 124, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-S.; Li, D.-M.; Ma, Y.; He, N.; Gu, Q.; Wang, F.-S.; Jiang, S.-Q.; Chen, B.-Q.; Liu, J.-R. γ-Tocotrienol Induces Paraptosis-Like Cell Death in Human Colon Carcinoma SW620 Cells. PLoS ONE 2013, 8, e57779. [Google Scholar] [CrossRef]
- Luk, S.U.; Yap, W.N.; Chiu, Y.T.; Lee, D.T.; Ma, S.; Lee, T.K.; Vasireddy, R.S.; Wong, Y.C.; Ching, Y.P.; Nelson, C.; et al. Gamma-tocotrienol as an effective agent in targeting prostate cancer stem cell-like population. Int. J. Cancer 2011, 128, 2182–2191. [Google Scholar] [CrossRef]
- Kannappan, R.; Yadav, V.R.; Aggarwal, B.B. gamma-Tocotrienol but not gamma-tocopherol blocks STAT3 cell signaling pathway through induction of protein-tyrosine phosphatase SHP-1 and sensitizes tumor cells to chemotherapeutic agents. J. Biol. Chem. 2010, 285, 33520–33528. [Google Scholar] [CrossRef] [PubMed]
- Malaviya, A.; Sylvester, P.W. Mechanisms Mediating the Effects of γ-Tocotrienol When Used in Combination with PPARγ Agonists or Antagonists on MCF-7 and MDA-MB-231 Breast Cancer Cells. Int. J. Breast Cancer 2013, 2013, 17. [Google Scholar] [CrossRef]
- Soleimani, M.; Nadri, S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat. Protoc. 2009, 4, 102–106. [Google Scholar] [CrossRef]
- Xu, W.L.; Liu, J.R.; Liu, H.K.; Qi, G.Y.; Sun, X.R.; Sun, W.G.; Chen, B.Q. Inhibition of proliferation and induction of apoptosis by gamma-tocotrienol in human colon carcinoma HT-29 cells. Nutrition 2009, 25, 555–566. [Google Scholar] [CrossRef]
- Yap, W.N.; Chang, P.N.; Han, H.Y.; Lee, D.T.W.; Ling, M.T.; Wong, Y.C.; Yap, Y.L. Gamma-tocotrienol suppresses prostate cancer cell proliferation and invasion through multiple-signalling pathways. Br. J. Cancer 2008, 99, 1832–1841. [Google Scholar] [CrossRef]
- Bachawal, S.V.; Wali, V.B.; Sylvester, P.W. Enhanced antiproliferative and apoptotic response to combined treatment of γ-tocotrienol with erlotinib or gefitinib in mammary tumor cells. BMC Cancer 2010, 10, 84. [Google Scholar] [CrossRef]
- Sakai, M.; Okabe, M.; Yamasaki, M.; Tachibana, H.; Yamada, K. Induction of apoptosis by tocotrienol in rat hepatoma dRLh-84 cells. Anticancer Res. 2004, 24, 1683–1688. [Google Scholar]
- Wilankar, C.; Khan, N.M.; Checker, R.; Sharma, D.; Patwardhan, R.; Gota, V.; Sandur, S.K.; Devasagayam, T.P. gamma-Tocotrienol induces apoptosis in human T cell lymphoma through activation of both intrinsic and extrinsic pathways. Curr. Pharm. Des. 2011, 17, 2176–2189. [Google Scholar] [CrossRef]
- Rickmann, M.; Vaquero, E.C.; Malagelada, J.R.; Molero, X. Tocotrienols induce apoptosis and autophagy in rat pancreatic stellate cells through the mitochondrial death pathway. Gastroenterology 2007, 132, 2518–2532. [Google Scholar] [CrossRef]
- Sun, W.; Xu, W.; Liu, H.; Liu, J.; Wang, Q.; Zhou, J.; Dong, F.; Chen, B. gamma-Tocotrienol induces mitochondria-mediated apoptosis in human gastric adenocarcinoma SGC-7901 cells. J. Nutr. Biochem. 2009, 20, 276–284. [Google Scholar] [CrossRef]
- Hafid, S.R.; Iran, N. Tocotrienols and Acute Myeloid Leukaemia. Palm Oil Dev. 2018, 68, 33–37. [Google Scholar]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghanem, P.; Zouein, A.; Mohamad, M.; Hodroj, M.H.; Haykal, T.; Abou Najem, S.; Naim, H.Y.; Rizk, S. The Vitamin E Derivative Gamma Tocotrienol Promotes Anti-Tumor Effects in Acute Myeloid Leukemia Cell Lines. Nutrients 2019, 11, 2808. https://doi.org/10.3390/nu11112808
Ghanem P, Zouein A, Mohamad M, Hodroj MH, Haykal T, Abou Najem S, Naim HY, Rizk S. The Vitamin E Derivative Gamma Tocotrienol Promotes Anti-Tumor Effects in Acute Myeloid Leukemia Cell Lines. Nutrients. 2019; 11(11):2808. https://doi.org/10.3390/nu11112808
Chicago/Turabian StyleGhanem, Paola, Annalise Zouein, Maya Mohamad, Mohammad H. Hodroj, Tony Haykal, Sonia Abou Najem, Hassan Y. Naim, and Sandra Rizk. 2019. "The Vitamin E Derivative Gamma Tocotrienol Promotes Anti-Tumor Effects in Acute Myeloid Leukemia Cell Lines" Nutrients 11, no. 11: 2808. https://doi.org/10.3390/nu11112808
APA StyleGhanem, P., Zouein, A., Mohamad, M., Hodroj, M. H., Haykal, T., Abou Najem, S., Naim, H. Y., & Rizk, S. (2019). The Vitamin E Derivative Gamma Tocotrienol Promotes Anti-Tumor Effects in Acute Myeloid Leukemia Cell Lines. Nutrients, 11(11), 2808. https://doi.org/10.3390/nu11112808