Hypertension Programmed by Perinatal High-Fat Diet: Effect of Maternal Gut Microbiota-Targeted Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Gas Chromatography-Flame Ionization Detector (GC-FID)
2.3. Liquid Chromatography–Mass Spectrometry (LC–MS/MS) Analysis
2.4. Analysis of Gut-Microbiota Composition
2.5. Western Blot
2.6. Quantitative Real-Time Polymerase Chain Reaction (PCR)
2.7. Statistical Analysis
3. Results
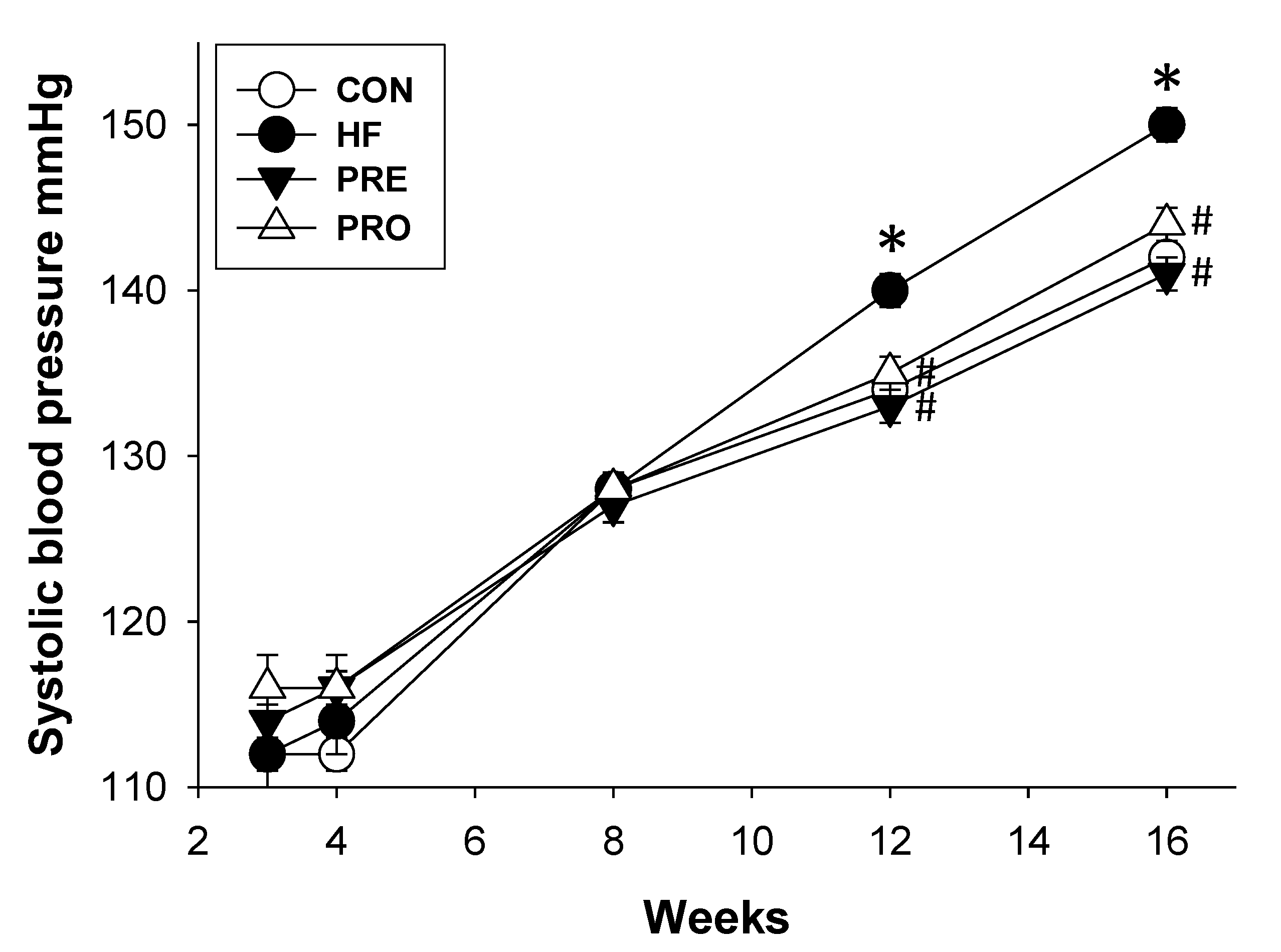
3.1. Effect of HF, Probiotic, and Prebiotic on Morphological Features and Blood Pressures
3.2. Effect of HF, Probiotic, and Prebiotic on Gut Microbiota-Derived Metabolites
3.3. Effect of HF, Probiotic, and Prebiotic on the RAS
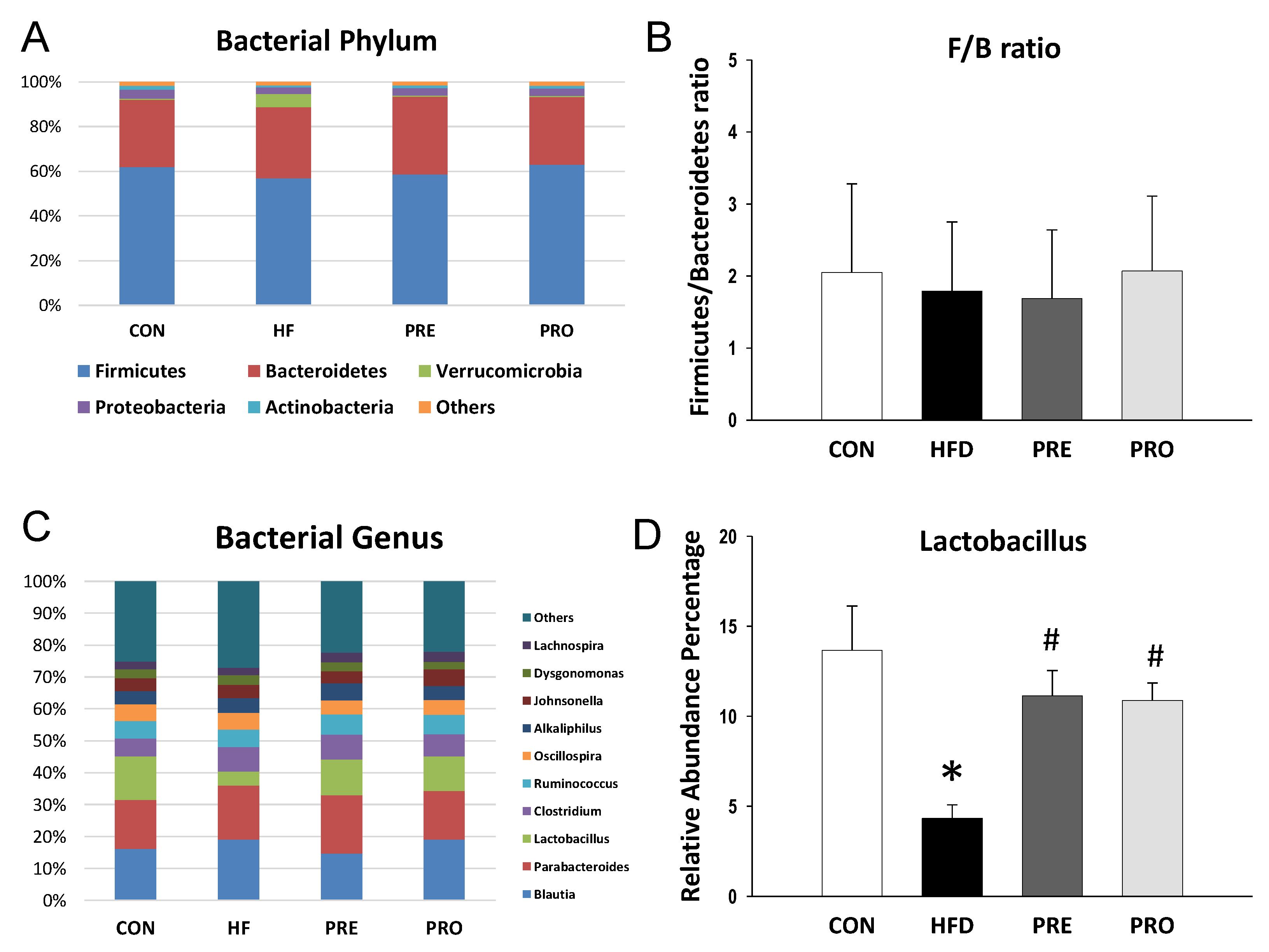
3.4. Effect of HF, Probiotic, and Prebiotic on Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hsu, C.N.; Tain, Y.L. The Good, the Bad, and the Ugly of Pregnancy Nutrients and Developmental Programming of Adult Disease. Nutrients 2019, 11, 894. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A. Programming by early nutrition: An experimental approach. J. Nutr. 1998, 128, 401S–406S. [Google Scholar] [CrossRef] [PubMed]
- Haugen, A.C.; Schug, T.T.; Collman, G.; Heindel, J.J. Evolution of DOHaD: The impact of environmental health sciences. J. Dev. Orig. Health Dis. 2015, 6, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef]
- Williams, L.; Seki, Y.; Vuguin, P.M.; Charron, M.J. Animal models of in utero exposure to a high fat diet: A review. Biochim. Biophys. Acta 2014, 1842, 507–519. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lin, Y.J.; Sheen, J.M.; Yu, H.R.; Tiao, M.M.; Chen, C.C.; Tsai, C.C.; Huang, L.T.; Hsu, C.N. High Fat Diets Sex-Specifically Affect the Renal Transcriptome and Program Obesity, Kidney Injury, and Hypertension in the Offspring. Nutrients 2017, 9, 357. [Google Scholar] [CrossRef]
- Tain, Y.L.; Joles, J.A. Reprogramming: A preventive strategy in hypertension focusing on the kidney. Int. J. Mol. Sci. 2015, 17, 23. [Google Scholar] [CrossRef]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut dysbiosis is linked to hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef]
- Al Khodor, S.; Reichert, B.; Shatat, I.F. The Microbiome and Blood Pressure: Can Microbes Regulate Our Blood Pressure? Front. Pediatr. 2017, 5, 138. [Google Scholar] [CrossRef]
- Tang, W.H.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.X.; Rey, F.; Wang, T.; et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef] [PubMed]
- Pluznick, J.L. Microbial Short-Chain Fatty Acids and Blood Pressure Regulation. Curr. Hypertens. Rep. 2017, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Te Riet, L.; van Esch, J.H.; Roks, A.J.; van den Meiracker, A.H.; Danser, A.H. Hypertension: Renin-angiotensin-aldosterone system alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef] [PubMed]
- Bogdarina, I.; Welham, S.; King, P.J.; Burns, S.P.; Clark, A.J. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ. Res. 2007, 100, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Chappell, M.C.; Marshall, A.C.; Alzayadneh, E.M.; Shaltout, H.A.; Diz, D.I. Update on the Angiotensin converting enzyme 2-Angiotensin (1–7)-MAS receptor axis: Fetal programing, sex differences, and intracellular pathways. Front. Endocrinol. 2014, 4, 201. [Google Scholar] [CrossRef]
- Jaworska, K.; Hering, D.; Mosieniak, G.; Bielak-Zmijewska, A.; Pilz, M.; Konwerski, M.; Gasecka, A.; Kapłon-Cieślicka, A.; Filipiak, K.; Sikora, E.; et al. TMA, A Forgotten Uremic Toxin, but Not TMAO, Is Involved in Cardiovascular Pathology. Toxins 2019, 11, 490. [Google Scholar] [CrossRef]
- Ge, X.; Zheng, L.; Zhuang, R.; Yu, P.; Xu, Z.; Liu, G.; Xi, X.; Zhou, X.; Fan, H. The Gut Microbial Metabolite Trimethylamine N-Oxide and Hypertension Risk: A Systematic Review and Dose-Response Meta-analysis. Adv. Nutr. 2019. [Google Scholar] [CrossRef]
- Ufnal, M.; Jazwiec, R.; Dadlez, M.; Drapala, A.; Sikora, M.; Skrzypecki, J. Trimethylamine-N-oxide: A carnitine-derived metabolite that prolongs the hypertensive effect of angiotensin II in rats. Can. J. Cardiol. 2014, 30, 1700–1705. [Google Scholar] [CrossRef]
- Liu, S. The Development of Our Organ of Other Kinds—The Gut Microbiota. Front. Microbiol. 2016, 7, 2107. [Google Scholar] [CrossRef]
- Wankhade, U.D.; Zhong, Y.; Kang, P.; Alfaro, M.; Chintapalli, S.V.; Piccolo, B.D.; Mercer, K.E.; Andres, A.; Thakali, K.M.; Shankar, K. Maternal High-Fat Diet Programs Offspring Liver Steatosis in a Sexually Dimorphic Manner in Association with Changes in Gut Microbial Ecology in Mice. Sci. Rep. 2018, 8, 16502. [Google Scholar] [CrossRef]
- Ducatelle, R.; Eeckhaut, V.; Haesebrouck, F.; Van Immerseel, F. A review on prebiotics and probiotics for the control of dysbiosis: Present status and future perspectives. Animal 2015, 9, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Lin, Y.J.; Hou, C.Y.; Tain, Y.L. Maternal Administration of Probiotic or Prebiotic Prevents Male Adult Rat Offspring against Developmental Programming of Hypertension Induced by High Fructose Consumption in Pregnancy and Lactation. Nutrients 2018, 10, 1229. [Google Scholar] [CrossRef] [PubMed]
- Karimi, G.; Sabran, M.R.; Jamaluddin, R.; Parvaneh, K.; Mohtarrudin, N.; Ahmad, Z.; Khazaai, H.; Khodavandi, A. The anti-obesity effects of Lactobacillus casei strain Shirota versus Orlistat on high fat diet-induced obese rats. Food Nutr. Res. 2015, 59, 29273. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F. Gender differences in the regulation of blood pressure. Hypertension 2001, 37, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsieh, C.S.; Lin, I.C.; Chen, C.C.; Sheen, J.M.; Huang, L.T. Effects of maternal L-citrulline supplementation on renal function and blood pressure in offspring exposed to maternal caloric restriction: The impact of nitric oxide pathway. Nitric Oxide 2010, 23, 34–41. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, W.C.; Wu, K.L.H.; Leu, S.; Chan, J.Y.H. Resveratrol Prevents the Development of Hypertension Programmed by Maternal Plus Post-Weaning High-Fructose Consumption through Modulation of Oxidative Stress, Nutrient-Sensing Signals, and Gut Microbiota. Mol. Nutr. Food Res. 2018, 62, 1800066. [Google Scholar] [CrossRef]
- Hsu, C.N.; Lu, P.C.; Lo, M.H.; Lin, I.C.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Gut Microbiota-Dependent Trimethylamine N-Oxide Pathway Associated with Cardiovascular Risk in Children with Early-Stage Chronic Kidney Disease. Int. J. Mol. Sci. 2018, 19, 3699. [Google Scholar] [CrossRef]
- Torrens, C.; Ethirajan, P.; Bruce, K.D.; Cagampang, F.R.; Siow, R.C.; Hanson, M.A.; Byrne, C.D.; Mann, G.E.; Clough, G.F. Interaction between maternal and offspring diet to impair vascular function and oxidative balance in high fat fed male mice. PLoS ONE 2012, 7, e50671. [Google Scholar] [CrossRef]
- Resende, A.C.; Emiliano, A.F.; Cordeiro, V.S.; de Bem, G.F.; de Cavalho, L.C.; de Oliveira, P.R.; Neto, M.L.; Costa, C.A.; Boaventura, G.T.; de Moura, R.S. Grape skin extract protects against programmed changes in the adult rat offspring caused by maternal high-fat diet during lactation. J. Nutr. Biochem. 2013, 24, 2119–2126. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Lee, C.T.; Chan, J.Y.H.; Tain, Y.L. The Interplay between Maternal and Post-Weaning High-Fat Diet and Gut Microbiota in the Developmental Programming of Hypertension. Nutrients 2019, 11, 1982. [Google Scholar] [CrossRef]
- Kang, Y.; Cai, Y. Gut microbiota and hypertension: From pathogenesis to new therapeutic strategies. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Yan, Z.; Zhao, X.; Gang, X.; He, G.; Sun, L.; Li, Z.; Wang, G. The effects of gut microbiota on metabolic outcomes in pregnant women and their offspring. Food Funct. 2018, 9, 4537–4547. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.M.; Meyer, K.M.; Prince, A.L.; Aagaard, K.M. Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microbes 2016, 7, 459–470. [Google Scholar] [CrossRef] [Green Version]
- Mokkala, K.; Houttu, N.; Cansev, T.; Laitinen, K. Interactions of dietary fat with the gut microbiota: Evaluation of mechanisms and metabolic consequences. Clin. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Prince, A.L.; Bader, D.; Hu, M.; Ganu, R.; Baquero, K.; Blundell, P.; Alan Harris, R.; Frias, A.E.; Grove, K.L.; et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat. Commun. 2014, 5, 3889. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Sun, Y.; Wu, J.; Huang, S.; Jin, G.; Guo, Z.; Zhang, Y.; Liu, T.; Liu, X.; Cao, X.; et al. Maternal High Fat Diet Alters Gut Microbiota of Offspring and Exacerbates DSS-Induced Colitis in Adulthood. Front. Immunol. 2018, 9, 2608. [Google Scholar] [CrossRef]
- Jose, P.A.; Raj, D. Gut microbiota in hypertension. Curr. Opin. Nephrol. Hypertens. 2015, 24, 403–409. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Lin, S.; Vanhoutte, P.M.; Woo, C.W.; Xu, A. Akkermansia Muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe−/− mice. Circulation 2016, 133, 2434–2446. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; de Vos, W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef]
- DiRienzo, D.B. Effect of probiotics on biomarkers of cardiovascular disease: Implications for heart-healthy diets. Nutr. Rev. 2014, 72, 18–29. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Liu, H.; Tang, Y.; Zhan, Q.; Lai, W.; Ao, L.; Meng, X.; Ren, H.; Xu, D.; et al. The intestinal microbiota associated with cardiac valve calcification differs from that of coronary artery disease. Atherosclerosis 2019, 284, 121–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsimichas, T.; Antonopoulos, A.S.; Katsimichas, A.; Ohtani, T.; Sakata, Y.; Tousoulis, D. The intestinal microbiota and cardiovascular disease. Cardiovasc. Res. 2019, 115, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- Damjanovic, M.; Barton, M. Fat intake and cardiovascular response. Curr. Hypertens. Rep. 2008, 10, 25–31. [Google Scholar] [CrossRef]
- Summer, A.; Formaggioni, P.; Franceschi, P.; Di Frangia, F.; Righi, F.; Malacarne, M. Cheese as Functional Food: The Example of Parmigiano Reggiano and Grana Padano. Food Technol. Biotechnol. 2017, 55, 277–289. [Google Scholar] [CrossRef]
- Yadav, H.; Lee, J.H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef] [Green Version]
- Pichette, J.; Fynn-Sackey, N.; Gagnon, J. Hydrogen Sulfide and Sulfate Prebiotic Stimulates the Secretion of GLP-1 and Improves Glycemia in Male Mice. Endocrinology 2017, 158, 3416–3425. [Google Scholar] [CrossRef]
- Reimer, R.A.; Willis, H.J.; Tunnicliffe, J.M.; Park, H.; Madsen, K.L.; Soto-Vaca, A. Inulin-type fructans and whey protein both modulate appetite but only fructans alter gut microbiota in adults with overweight/obesity: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Kim, Y.A.; Keogh, J.B.; Clifton, P.M. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr. Res. Rev. 2018, 31, 35–51. [Google Scholar] [CrossRef]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef]



| Gene | Forward | Reverse |
|---|---|---|
| Ren | 5 aacattaccagggcaactttcact 3 | 5 acccccttcatggtgatctg 3 |
| Agt | 5 gcccaggtcgcgatgat 3 | 5 tgtacaagatgctgagtgaggcaa 3 |
| Ace | 5 caccggcaaggtctgctt 3 | 5 cttggcatagtttcgtgaggaa 3 |
| Rn18s | 5 gccgcggtaattccagctcca 3 | 5 cccgcccgctcccaagatc 3 |
| Groups | CON | HF | PRE | PRO |
|---|---|---|---|---|
| BW (g) | 576 ± 7 | 567 ± 8 | 595 ± 13 | 544 ± 15 |
| Left kidney weight (g) | 2.41 ± 0.05 | 2.21 ± 0.06 | 2.28 ± 0.05 | 2.22 ± 0.1 |
| Left kidney weight/100 g BW | 0.42 ± 0.01 | 0.39 ± 0.01 | 0.38 ± 0.01 | 0.41 ± 0.01 |
| Systolic blood pressure (mm Hg) | 142 ± 1 | 150 ± 1 * | 141 ± 1 # | 144 ± 1 # |
| Diastolic blood pressure (mm Hg) | 65 ± 2 | 78 ± 3 * | 66 ± 4 # | 68 ± 2 |
| Mean arterial pressure (mm Hg) | 91 ± 1 | 102 ± 2 * | 91 ± 3 # | 93 ± 1 # |
| Creatinine (μM) | 14.5 ± 0.9 | 16.2± 1.3 * | 13.5 ± 1.6 # | 14.6 ± 1 |
| Group | CON | HF | PRE | PRO |
|---|---|---|---|---|
| 3 weeks | ||||
| Acetate, mM/g feces | 4.46 ± 0.47 | 5.12 ± 0.33 | 4.72 ± 0.37 | 5.72 ± 0.86 |
| Propionate, mM/g feces | 1.33 ± 0.38 | 0.49 ± 0.05 * | 0.24 ± 0.04 * # | 0.47 ± 0.08 * † |
| Butyrate, mM/g feces | 0.45 ± 0.07 | 0.47 ± 0.04 | 0.4 ± 0.04 | 0.4 ± 0.07 |
| 16 weeks | ||||
| Acetate, mM/g feces | 3.71 ± 0.14 | 3.66 ± 0.24 | 3.08 ± 0.21 * | 3.89 ± 0.27 † |
| Propionate, mM/g feces | 0.88 ± 0.05 | 0.76 ± 0.05 | 0.72 ± 0.06 | 0.78 ± 0.03 |
| Butyrate, mM/g feces | 1.67 ± 0.23 | 1.5 ± 0.31 | 1.14 ± 0.24 | 2.03 ± 0.31 † |
| Group | CON | HF | PRE | PRO |
|---|---|---|---|---|
| TMAO, ng/mL | 326 ± 18 | 328 ± 22 | 233 ± 15 *# | 204 ± 7 * # |
| TMA, ng/mL | 269 ± 15 | 363 ± 30 * | 356 ± 31 * | 395 ± 21 * |
| DMA, ng/mL | 149 ± 4 | 127 ± 4 * | 139 ± 6 | 115 ± 3 * # † |
| TMAO-to-TMA ratio | 1.24 ± 0.11 | 0.93 ± 0.06 * | 0.69 ± 0.08 *# | 0.53 ± 0.03 * # |
| DMA-to-TMAO ratio | 0.47 ± 0.03 | 0.4 ± 0.02 | 0.62 ± 0.06 *# | 0.57 ± 0.03 * # |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-N.; Hou, C.-Y.; Chan, J.Y.H.; Lee, C.-T.; Tain, Y.-L. Hypertension Programmed by Perinatal High-Fat Diet: Effect of Maternal Gut Microbiota-Targeted Therapy. Nutrients 2019, 11, 2908. https://doi.org/10.3390/nu11122908
Hsu C-N, Hou C-Y, Chan JYH, Lee C-T, Tain Y-L. Hypertension Programmed by Perinatal High-Fat Diet: Effect of Maternal Gut Microbiota-Targeted Therapy. Nutrients. 2019; 11(12):2908. https://doi.org/10.3390/nu11122908
Chicago/Turabian StyleHsu, Chien-Ning, Chih-Yao Hou, Julie Y.H. Chan, Chien-Te Lee, and You-Lin Tain. 2019. "Hypertension Programmed by Perinatal High-Fat Diet: Effect of Maternal Gut Microbiota-Targeted Therapy" Nutrients 11, no. 12: 2908. https://doi.org/10.3390/nu11122908
APA StyleHsu, C.-N., Hou, C.-Y., Chan, J. Y. H., Lee, C.-T., & Tain, Y.-L. (2019). Hypertension Programmed by Perinatal High-Fat Diet: Effect of Maternal Gut Microbiota-Targeted Therapy. Nutrients, 11(12), 2908. https://doi.org/10.3390/nu11122908








