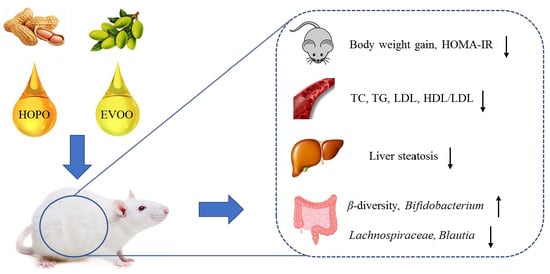
High Oleic Acid Peanut Oil and Extra Virgin Olive Oil Supplementation Attenuate Metabolic Syndrome in Rats by Modulating the Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Treatment
2.3. Insulin Resistance Assessment
2.4. Serum Biochemical Analysis
2.5. Histopathological Examination and TG Level in Liver Tissue
2.6. Gut Microbiota Analysis
2.7. Statistical Analysis
3. Results
3.1. Body Weight, Body Weight Gain, Energy Intake and Energy Efficiency
3.2. Histopathological Examination and TG Level in Liver Tissue
3.3. Biomarkers of Blood Glucose Level and Insulin Resistance
3.4. Biomarkers of Metabolic Syndrome in Serum
3.5. Overall Structural Changes of Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A consensus statement from the international diabetes federation. Diabetic Med. 2010, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, D.; Reynolds, K.; Wu, X.; Chen, J.; Duan, X.; Reynolds, R.F.; Whelton, P.K.; He, J. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet 2005, 365, 1398–1405. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Yu, D.; Wang, Z.; Ding, G. Metabolic syndrome prevalence and its risk factors among adults in China: A nationally representative cross-sectional study. PLoS ONE 2018, 13, e0199293. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef] [Green Version]
- Portune, K.J.; Benítez-Páez, A.; Del Pulgar, E.M.; Cerrudo, V.; Sanz, Y. Gut microbiota, diet and obesity-related disorders—the good, the bad and the future challenges. Mol. Nutr. Food Res. 2017, 61, 1600252. [Google Scholar] [CrossRef] [Green Version]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Tang, H.; Zhang, C.; Zhao, Y.; Derrien, M.; Rocher, E.; Van-Hylckama Vlieg, J.E.; Strissel, K.; Zhao, L.; Obin, M. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015, 9, 1. [Google Scholar] [CrossRef]
- Martínez, I.; Perdicaro, D.J.; Brown, A.W.; Hammons, S.; Carden, T.J.; Carr, T.P.; Eskridge, K.M.; Walter, J. Diet-induced alterations of host cholesterol metabolism are likely to affect the gut microbiota composition in hamsters. Appl. Environ. Microb. 2013, 79, 516–524. [Google Scholar] [CrossRef] [Green Version]
- Ziętak, M.; Kovatcheva-Datchary, P.; Markiewicz, L.; Ståhlman, M.; Kozak, L.; Bäckhed, F. Altered microbiota contributes to reduced diet-induced obesity upon cold exposure. Cell Metab. 2016, 23, 1216–1223. [Google Scholar] [CrossRef] [Green Version]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. New Engl. J. Med. 2016, 375, 2369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segarra, A.; Ruiz-Sanz, J.; Ruiz-Larrea, M.; Ramirez-Sanchez, M.; De Gasparo, M.; Banegas, I.; Martinez-Canamero, M.; Vives, F.; Prieto, I. The profile of fatty acids in frontal cortex of rats depends on the type of fat used in the diet and correlates with neuropeptidase activities. Horm. Metab. Res. 2011, 43, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodas, M.C.; Valenzuela, R.; Echeverría, F.; Rincón-Cervera, M.Á.; Espinosa, A.; Illesca, P.; Muñoz, P.; Corbari, A.; Romero, N.; Gonzalez-Mañan, D. Supplementation with docosahexaenoic acid and extra virgin olive oil prevents liver steatosis induced by a high-fat diet in mice through PPAR-α and Nrf2 upregulation with concomitant SREBP-1c and NF-kB downregulation. Mol. Nutr. Food Res. 2017, 61, 1700479. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef]
- Ley, R.E.; Fredrik, B.C.; Peter, T.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [Green Version]
- Indiani, C.M.D.S.P.; Rizzardi, K.F.; Castelo, P.M.; Ferraz, L.F.C.; Darrieux, M.; Parisotto, T.M. Childhood obesity and Firmicutes/Bacteroidetes ratio in the gut microbiota: A systematic review. Child. Obes. 2018, 14, 501–509. [Google Scholar] [CrossRef]
- De, W.N.; Derrien, M.; Bosch-Vermeulen, H.; Oosterink, E.; Keshtkar, S.; Duval, C.; De Vogel-van den Bosch, J.; Kleerebezem, M.; Müller, M.; Van der Meer, R. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G589. [Google Scholar]
- Bray, G.A. Fructose and risk of cardiometabolic disease. Curr. Atheroscler. Rep. 2012, 14, 570–578. [Google Scholar] [CrossRef]
- Johnson, R.J.; Segal, M.S.; Yuri, S.; Takahiko, N.; Feig, D.I.; Duk-Hee, K.; Gersch, M.S.; Steven, B.; Sánchez-Lozada, L.G. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr. 2007, 86, 899. [Google Scholar]
- Bray, G.A.; Popkin, B.M. Calorie-sweetened beverages and fructose: What have we learned 10 years later. Pediatr. Obes. 2013, 8, 242–248. [Google Scholar] [CrossRef]
- Kelishadi, R.; Mansourian, M.; Heidari-Beni, M. Association of fructose consumption and components of metabolic syndrome in human studies: A systematic review and meta-analysis. Nutrition 2014, 30, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Mortera, R.; Bains, Y.; Gugliucci, A. Fructose at the crossroads of the metabolic syndrome and obesity epidemics. Front. Biosci. 2019, 24, 186–211. [Google Scholar]
- Martínez-González, M.Á.; Martín-Calvo, N. The major European dietary patterns and metabolic syndrome. Rev. Endocr. Metab. Dis. 2013, 14, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Grossi, C.; Rigacci, S.; Ambrosini, S.; Dami, T.E.; Luccarini, I.; Traini, C.; Stefani, M. The polyphenol oleuropein aglycone protects TgCRND8 mice against Aβ plaque pathology. PLoS ONE 2013, 8, e71702. [Google Scholar] [CrossRef] [PubMed]
- Prieto, I.; Hidalgo, M.; Segarra, A.B.; Martãnez-Rodrãguez, A.M.; Cobo, A.; Ramãrez, M.; Abriouel, H.; Gálvez, A.; Martínez-Cañamero, M. Influence of a diet enriched with virgin olive oil or butter on mouse gut microbiota and its correlation to physiological and biochemical parameters related to metabolic syndrome. PLoS ONE 2018, 13, e0190368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Clifton, P. Metabolic Syndrome-Role of Dietary Fat Type and Quantity. Nutrients 2019, 11, 1438. [Google Scholar] [CrossRef] [Green Version]
- Sales-Campos, H.; Souza, P.R.; Peghini, B.C.; Da Silva, J.S.; Cardoso, C.R. An overview of the modulatory effects of oleic acid in health and disease. Mini-Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar]
- Wang, Q. Peanut Processing Characteristics and Quality Evaluation; Springer: Singapore, 2018. [Google Scholar]
- Clemente, J.C.; Ursell, L.K.; Laura Wegener, P.; Rob, K. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- Nehra, V.; Allen, J.M.; Mailing, L.J.; Kashyap, P.C.; Woods, J.A. Gut microbiota: Modulation of host physiology in obesity. Physiology 2016, 31, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Marcinkevicius, E.; Shirasu-Hiza, M. Message in a biota: Gut microbes signal to the circadian clock. Cell Host Microbe 2015, 17, 541–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalliomaki, M.; Collado, M.; Salminen, S.; Isolauri, E. Early differences in fecal microbiota composition in children may predict overweight. Am. J. Clin. Nutr. 2008, 87, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Keishi, K.; Kikuji, I. Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes Environ. 2014, 29, 427–430. [Google Scholar]
- Maitreyi, R.; Iftikhar, A.; Gillevet, P.M.; Probert, C.S.; Ratcliffe, N.M.; Steve, S.; Rosemary, G.; Masoumeh, S.; Victor, L.; Pam, C. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2013, 11, 868. [Google Scholar]
- Zhang, X.; Shen, D.; Fang, Z.; Jie, Z.; Qiu, X.; Zhang, C.; Chen, Y.; Ji, L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE 2013, 8, e71108. [Google Scholar] [CrossRef]
- Kashtanova, D.; Tkacheva, O.; Doudinskaya, E.; Strazhesko, I.; Kotovskaya, Y.; Popenko, A.; Tyakht, A.; Alexeev, D. Gut microbiota in patients with different metabolic statuses: Moscow study. Microorganisms 2018, 6, 98. [Google Scholar] [CrossRef] [Green Version]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Vincent, M.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Nadja, L.; Vogensen, F.K.; Berg, F.W.J.; Van Den Dennis Sandris, N.; Anne Sofie, A.; Pedersen, B.K.; Waleed Abu, A.S.; Sørensen, S.R.J.; Hansen, L.H.; Mogens, J. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar]
- Jean-Pierre, F.; Ling-Chun, K.; Julien, T.; Christine, P.; Arnaud, B.; Jean-Luc, B.; Denis, M.; Gérard, C.; JoëL, D.; Corneliu, H. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes 2010, 59, 3049–3057. [Google Scholar]
- Duncan, S.H.; Lobley, G.E.; Holtrop, G.; Ince, J.; Johnstone, A.M.; Louis, P.; Flint, H.J. Human colonic microbiota associated with diet, obesity and weight loss. Int. J. Obes. 2008, 32, 1720–1724. [Google Scholar] [CrossRef] [Green Version]
- Amandine, E.; Clara, B.; Lucie, G.; Ouwerkerk, J.P.; Céline, D.; Bindels, L.B.; Yves, G.; Muriel, D.; Muccioli, G.G.; Delzenne, N.M. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar]




| NC | M | HOPO | EVOO | |
|---|---|---|---|---|
| FBG (mmol/L) | 4.38 ± 0.42 a | 6.08 ± 0.76 bc | 6.53 ± 0.65 c | 5.79 ± 0.56 b |
| OGTT AUC | ||||
| 0 weeks | 842.75 ± 77.82 a | 879.81 ± 70.11 a | 874.69 ± 46.69 a | 862.56 ± 73.47 a |
| 4 weeks | 951.63 ± 80.58 a | 1070.88 ± 109.16 b | 1072.50 ± 33.43 b | 1052.81 ± 98.91 b |
| 8 weeks | 914.06 ± 62.62 a | 983.63 ± 108.81 ab | 957.50 ± 59.60 ab | 1005.56 ± 121.55 b |
| 12 weeks | 892.19 ± 110.37 a | 972.13 ± 96.69 a | 972.56 ± 82.52 a | 959.75 ± 100.30 a |
| ITT AUC | ||||
| 0 weeks | 408.69 ± 32.14 a | 414.56 ± 38.97 a | 433.69 ± 30.55 a | 402.94 ± 51.52 a |
| 4 weeks | 417.69 ± 98.06 a | 524.13 ± 61.95 b | 544.19 ± 80.49 b | 506.00 ± 80.16 b |
| 8 weeks | 389.31 ± 64.06 a | 532.31 ± 69.35 c | 470.81 ± 44.06 b | 479.94 ± 55.97 b |
| 12 weeks | 393.56 ± 71.98 a | 523.19 ± 83.27 b | 425.56 ± 87.93 a | 407.56 ± 67.30 a |
| Insulin (ug/L) | 0.89 ± 0.12 a | 1.70 ± 0.33 c | 1.19 ± 0.21 b | 1.36 ± 0.26 b |
| HOMA-IR | 4.15 ± 0.73 a | 10.88 ± 1.71 c | 8.67 ± 2.02 b | 8.43 ± 1.86 b |
| NC | M | HOPO | EVOO | |
|---|---|---|---|---|
| TC (mmol/L) | 3.00 ± 0.79 a | 3.62 ± 0.50 b | 3.06 ± 0.65 a | 3.18 ± 0.40 ab |
| TG (mmol/L) | 1.95 ± 0.42 a | 5.99 ± 2.09 c | 4.23 ± 1.45 b | 5.79 ± 2.22 c |
| HDL (mmol/L) | 0.84 ± 0.15 a | 0.71 ± 0.25 a | 0.69 ± 0.23 a | 0.76 ± 0.25 a |
| LDL (mmol/L) | 0.40 ± 0.14 a | 0.56 ± 0.21 b | 0.38 ± 0.13 a | 0.50 ± 0.18 ab |
| HDL/LDL | 2.10 ± 0.25 c | 1.27 ± 0.12 a | 1.82 ± 0.18 b | 1.52 ± 0.21 b |
| FFA (umol/L) | 459.96 ± 38.76 a | 555.80 ± 55.34 b | 526.80 ± 60.18 b | 512.06 ± 47.15 b |
| TNF-a (ug/mL) | 196.11 ± 34.67 a | 257.74 ± 75.73 b | 208.16 ± 58.73 ab | 220.35 ± 63.03 ab |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Shi, A.; Wang, Q.; Zhou, J. High Oleic Acid Peanut Oil and Extra Virgin Olive Oil Supplementation Attenuate Metabolic Syndrome in Rats by Modulating the Gut Microbiota. Nutrients 2019, 11, 3005. https://doi.org/10.3390/nu11123005
Zhao Z, Shi A, Wang Q, Zhou J. High Oleic Acid Peanut Oil and Extra Virgin Olive Oil Supplementation Attenuate Metabolic Syndrome in Rats by Modulating the Gut Microbiota. Nutrients. 2019; 11(12):3005. https://doi.org/10.3390/nu11123005
Chicago/Turabian StyleZhao, Zhihao, Aimin Shi, Qiang Wang, and Jinrong Zhou. 2019. "High Oleic Acid Peanut Oil and Extra Virgin Olive Oil Supplementation Attenuate Metabolic Syndrome in Rats by Modulating the Gut Microbiota" Nutrients 11, no. 12: 3005. https://doi.org/10.3390/nu11123005
APA StyleZhao, Z., Shi, A., Wang, Q., & Zhou, J. (2019). High Oleic Acid Peanut Oil and Extra Virgin Olive Oil Supplementation Attenuate Metabolic Syndrome in Rats by Modulating the Gut Microbiota. Nutrients, 11(12), 3005. https://doi.org/10.3390/nu11123005