The Effect of Mushroom Extracts on Human Platelet and Blood Coagulation: In vitro Screening of Eight Edible Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mushroom Cultivation
2.2. Extract Preparation
2.3. In vitro Studies
2.3.1. Blood Collection
2.3.2. Aggregation Assay
2.3.3. Coagulation Assay
2.3.4. Cytotoxicity Assay
2.3.5. Erythrocyte Sedimentation Rate Assay
2.4. Extract Characterization
2.4.1. Total Antioxidant Capacity
2.4.2. DPPH Scavenging Activity
2.4.3. Total Phenolic Content
2.4.4. Phenolic Compounds and Organic Acid Concentration
2.4.5. Separation and Compositional Analysis of Polysaccharides
2.4.6. Organic Acid Concentration
2.4.7. Ergosterol Concentration
2.4.8. Macro and Trace Element Concentration
2.5. Statistical Analyses
3. Results
3.1. In vitro Studies
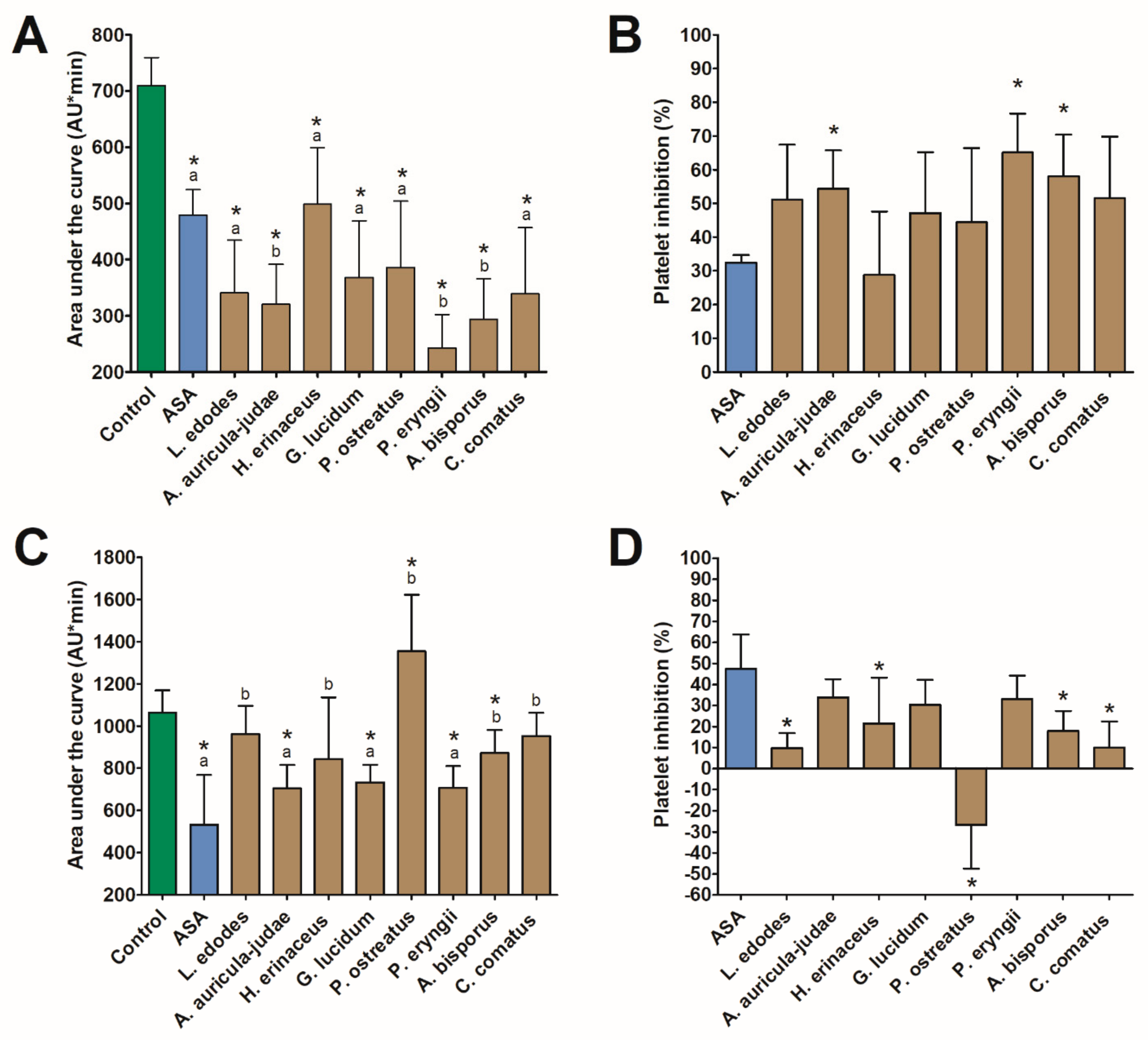
3.1.1. Aggregation Assays
3.1.2. Coagulation Assay
3.1.3. Cytotoxicity and ESR Assay
3.2. Extract Characteristics
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- European Heart Network: European Cardiovascular Disease Statistics. 2017. Available online: http://www.ehnheart.org/cvd-statistics.html (accessed on 27 August 2017).
- Atlas Writing Group; Timmis, A.; Townsend, N.; Gale, C.; Grobbee, R.; Maniadakis, N.; Flather, M.; Wilkins, E.; Wright, L.; Vos, R.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur. Heart J. 2018, 39, 508–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigotti, N.A.; Clair, C. Managing tobacco use: The neglected cardiovascular disease risk factor. Eur. Heart J. 2013, 34, 3259–3267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, A.J.; Viana, J.L.; Cavalcante, S.L.; Oliveira, N.L.; Duarte, J.A.; Mota, J.; Oliveira, J.; Ribeiro, F. Physical activity in primary and secondary prevention of cardiovascular disease: Overview updated. World J. Cardiol. 2016, 8, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Malik, V.S.; Hu, F.B. Cardiovascular disease prevention by diet modification: JACC health promotion series. J. Am. Coll. Cardiol. 2018, 72, 914–926. [Google Scholar] [CrossRef]
- Malek, L.A.; Spiewak, M.; Filipiak, K.J.; Grabowski, M.; Szpotanska, M.; Rosiak, M.; Glowczynska, R.; Imiela, T.; Huczek, Z.; Opolski, G. Persistent platelet activation is related to very early cardiovascular events in patients with acute coronary syndromes. Kardiol. Pol. 2007, 65, 40–45. [Google Scholar]
- Puurunen, M.K.; Hwang, S.; Larson, M.G.; Vasan, R.S.; O’Donnell, C.J.; Tofler, G.; Johnson, A.D. ADP platelet hyperreactivity predicts cardiovascular disease in the FHS (Framingham Heart Study). J. Am. Heart Assoc. 2018, 7, e008522. [Google Scholar] [CrossRef]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redón, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. Task Force Members. ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2013, 31, 1281–1357. [Google Scholar] [CrossRef] [Green Version]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar]
- Krasinska, B.; Paluszkiewicz, L.; Miciak-Lawicka, E.; Krasinski, M.; Rzymski, P.; Tykarski, A.; Krasiński, Z. The effect of acetylsalicylic acid dosed at bedtime on the anti-aggregation effect in patients with coronary heart disease and arterial hypertension: A randomized, controlled trial. Cardiol. J. 2018. [Google Scholar] [CrossRef] [Green Version]
- Krasińska, B.; Osińska, A.; Osińska, M.; Krasińska, A.; Rzymski, P.; Tykarski, A.; Krasiński, Z. Standardised tomato extract as an alternative to acetylsalicylic acid in patients with primary hypertension and high cardiovascular risk—A randomised, controlled trial. Arch. Med. Sci. 2018, 14, 773–780. [Google Scholar] [CrossRef]
- Krasińska, B.; Osińska, A.; Krasińska, A.; Osiński, M.; Rzymski, P.; Tykarski, A.; Krasiński, Z. Favourable hypotensive effect after standardised tomato extract treatment in hypertensive subjects at high cardiovascular risk: A randomised controlled trial. Kardiol. Pol. 2018, 76, 388–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, P.C. Mushrooms as Functional Foods; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- De Silva, D.D.; Rapior, S.; Sudarman, E.; Stadler, M.; Xu, J.C.; Alias, S.A.; Hyde, K.D. Bioactive metabolites from macrofungi: Ethnopharmacology, biological activities and chemistry. Fungal Divers. 2013, 62, 1–40. [Google Scholar] [CrossRef]
- Mori, K.; Kikuchi, H.; Obara, Y.; Iwashita, M.; Azumi, Y.; Kinugasa, S.; Inatomi, S.; Oshima, Y.; Nakahata, N. Inhibitory effect of hericenone B from Hericium erinaceus on collagen-induced platelet aggregation. Phytomedicine 2010, 17, 1082–1085. [Google Scholar] [CrossRef] [PubMed]
- Kamruzzaman, S.M.; Endale, M.; Oh, W.J.; Park, S.C.; Kim, T.H.; Lee, I.K.; Cho, J.Y.; Park, H.J.; Kim, S.K.; Yun, B.S.; et al. Antiplatelet activity of Phellinus baumii methanol extract is mediated by cyclic AMP elevation and inhibition of collagen-activated integrin-alpha (IIb) beta and MAP kinase. Phytother. Res. 2011, 25, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Jose, N.; Ajith, T.A.; Janardhanan, K.K. Methanol extract of the oyster mushroom, Pleurotus florida, inhibits inflammation and platelet aggregation. Phytother. Res. 2004, 18, 43–46. [Google Scholar] [CrossRef]
- Doljak, B.; Cateni, F.; Anderluh, M.; Procida, G.; Zilic, J.; Zacchigna, M. Glycerolipids as selective thrombin inhibitors from the fungus Stereum hirsutum. Drug Dev. Ind. Pharm. 2006, 32, 635–643. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Mleczek, M.; Niedzielski, P.; Siwulski, M.; Gąsecka, M.; Kozak, L.; Komosa, A.; Rzymski, P. Bio-enriched Pleurotus mushrooms for deficiency control and improved antioxidative protection of human platelets? Eur. Food Res. Technol. 2017, 243, 2187–2198. [Google Scholar] [CrossRef]
- Komosa, A.; Rzymski, P.; Perek, B.; Ropacka-Lesiak, M.; Lesiak, M.; Siller-Matula, J.M.; Poniedziałek, B. Platelets redox balance assessment: Current evidence and methodological considerations. Vasc. Pharmacol. 2017, 93–95, 6–13. [Google Scholar] [CrossRef]
- Kalač, P. Chemical composition and nutritional value of European species of wild growing mushrooms: A review. Food Chem. 2009, 113, 9–16. [Google Scholar] [CrossRef]
- Loll, P.J.; Picot, D.; Garavito, R.M. The structural basis of aspirin activity inferred from the crystal structure of inactivated prostaglandin H2 synthase. Nat. Struct. Biol. 1995, 2, 637–643. [Google Scholar] [CrossRef]
- Kakutani, M.; Masaki, T.; Sawamura, T. A platelet-endothelium interaction mediated by lectin-like oxidized low-density lipoprotein receptor-1. Proc. Natl. Acad. Sci. USA 2000, 97, 360–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rzymski, P.; Mleczek, M.; Niedzielski, P.; Siwulski, M.; Gąsecka, M.A. Cultivation of Agaricus bisporus enriched with selenium, zinc and copper. J. Sci. Food Agric. 2017, 97, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Niedzielski, P.; Siwulski, M.; Mleczek, M.; Budzyńska, S.; Gąsecka, M.; Poniedziałek, B.B. Lithium biofortification of medicinal mushrooms Agrocybe cylindracea and Hericium erinaceus. J. Food Sci. Technol. 2017, 54, 2387–2393. [Google Scholar] [CrossRef] [PubMed]
- Mleczek, M.; Siwulski, M.; Rzymski, P.; Budzyńska, S.; Gąsecka, M.; Kalac, P.; Niedzielski, P. Cultivation of mushrooms for production of food biofortified with lithium. Eur. Food Res. Technol. 2017, 243, 1097–1104. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, A.F.; Neubauer, H.; Franken, C.C.; Kruger, J.C.; Mugge, A.; Meves, S.H. Which is the best anticoagulant for whole blood aggregometry platelet function testing? Comparison of six anticoagulants and diverse storage conditions. Platelets 2012, 23, 359–367. [Google Scholar] [CrossRef]
- O’Kennedy, N.; Crosbie, L.; van Lieshout, M.; Broom, J.I.; Broom, J.I.; Webb, D.J.; Duttaroy, A.K. Effects of antiplatelet components of tomato extract on platelet function in vitro and ex vivo: A time-course cannulation study in healthy humans. Am. J. Clin. Nutr. 2006, 84, 570–579. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lee, J.; Kang, S.; Moon, H.; Chung, K.H.; Kim, K.R. Antiplatelet and Antithrombotic Effects of the Extract of Lindera obtusiloba Leaves. Biomol. Ther. 2016, 24, 659–664. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Lee, W.; Bae, J.-S.; Ma, E. Synthesis and in vitro and in vivo Anticoagulant and Antiplatelet Activities of Amidino- and Non-Amidinobenzamides. Molecules 2016, 21, 676. [Google Scholar] [CrossRef]
- Yuan, S.; Ferrell, C.; Chandler, W.L. Comparing the prothrombin time INR versus the APTT to evaluate the coagulopathy of acute trauma. Thromb. Res. 2007, 120, 29–37. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.J. Total antioxidant status in plasma and body fluids. Method Enzym. 1994, 234, 279–293. [Google Scholar]
- Dong, J.; Zhang, M.; Lu, L.; Sun, L.; Xu, M. Nitric oxide fumigation stimulates flavonoid and phenolic accumulation and enhances antioxidant activity of mushroom. Food Chem. 2012, 135, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C. Antioxidant properties and phenolic profile of the most widely appreciated cultivated mushrooms: A comparative study between in vivo and in vitro samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Method Enzym. 1999, 299, 152–178. [Google Scholar]
- Gąsecka, M.; Rzymski, P.; Mleczek, M.; Siwulski, M.; Budzyńska, S.; Magdziak, Z.; Niedzielski, P.; Sobieralski, K. The relationship between metal composition, phenolic acid and flavonoid content in Imleria badia from non-polluted and polluted areas. J. Environ. Sci. Health B 2017, 52, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Gąsecka, M.; Magdziak, Z.; Siwulski, M.; Mleczek, M. Profile of phenolic and organic acids, antioxidant properties and ergosterol content in cultivated and wild growing species of Agaricus. Eur. Food Res. Technol. 2018, 244, 259–268. [Google Scholar] [CrossRef]
- Sawardeker, J.S.; Sloneker, J.H.; Jeanes, A. Quantitative determination of monosaccharides as their alditol acetates by gas liquid chromatography. Anal. Chem. 1965, 37, 1602–1604. [Google Scholar] [CrossRef]
- Ciukanu, I.; Kerek, F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 1984, 131, 209–217. [Google Scholar] [CrossRef]
- Mleczek, M.; Magdziak, Z.; Gąsecka, M.; Niedzielski, P.; Kalač, P.; Siwulski, M.; Rzymski, P.; Zalicka, S.; Sobieralski, K. Content of selected elements and low molecular weight organic acids in fruiting bodies of edible mushroom Boletus badius (Fr.) Fr. from unpolluted and polluted areas. Environ. Sci Pollut. R 2016, 23, 20609–20618. [Google Scholar] [CrossRef] [Green Version]
- Siwulski, M.; Budzyńska, S.; Rzymski, P.; Gąsecka, M.; Niedzielski, P.; Kalac, P.; Mleczek, M. The effects of germanium and selenium on growth, metalloid accumulation and ergosterol content in mushrooms: Experimental study in Pleurotus ostreatus and Ganoderma lucidum. Eur. Food Res. Technol. 2019, 245, 1799–1810. [Google Scholar] [CrossRef] [Green Version]
- Rzymski, P.; Budzulak, J.; Niedzielski, P.; Klimaszyk, P.; Proch, J.; Kozak, L.; Poniedziałek, B. Essential and toxic elements in commercial microalgal food supplements. J. Appl. Phycol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Niedzielski, P.; Mleczek, M.; Siwulski, M.; Rzymski, P.; Gąsecka, M.; Kozak, L. Supplementation of cultivated mushroom species with selenium: Bioaccumulation and speciation study. Eur. Food Res. Technol. 2015, 241, 419–426. [Google Scholar] [CrossRef]
- VanderMolen, K.M.; Little, J.G.; Sica, V.P.; El-Elimat, T.; Raja, H.A.; Oberlies, N.H.; Baker, T.R.; Mahony, C. Safety assessment of mushrooms in dietary supplements by combining analytical data with in silico toxicology evaluation. Food Chem. Toxicol. 2017, 103, 133–147. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. 2018. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 21 September 2018).
- Friedman, M. Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans. Foods 2016, 5, 80. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.J.; Yu, M.A.; Pyun, Y.R.; Hwang, J.K.; Chu, D.C.; Juneja, L.R.; Mourão, P.A. The nontoxic mushroom Auricularia auricula contains a polysaccharides with anticoagulant activity mediated by antithrombin. Thromb. Res. 2003, 112, 151–158. [Google Scholar] [CrossRef]
- Pawlaczyk, I.; Czerchawski, L.; Pilecki, W.; Lamer-Zarawska, E.; Gancarz, R. Polyphenolic–polysaccharide compounds from selected medicinal plants of Asteraceae and Rosaceae families: Chemical characterization and blood anticoagulant activity. Carbohydr. Polym. 2009, 77, 568–575. [Google Scholar] [CrossRef]
- Yamanaka, K. Mushroom cultivation in Japan. World Soc. Mushroom Biol. Mushroom Prod. Bull. 2011, 4, 1–10. [Google Scholar]
- Siwulski, M.; Mleczek, M.; Rzymski, P.; Budka, A.; Jasińska, A.; Niedzielski, P.; Kalac, P.; Gąsecka, M.; Budzyńska, S.; Mikołajczak, P. Screening the multi-element content of Pleurotus mushroom species using inductively coupled plasma optical emission spectrometer (ICP-OES). Food Anal. Methods 2017, 10, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Matetzky, S.; Shenkman, B.; Guetta, V.; Shechter, M.; Beinart, R.; Goldenberg, I.; Novikov, I.; Pres, H.; Savion, N.; Varon, D.; et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 2004, 109, 3171–3175. [Google Scholar] [CrossRef]
- Gasparyan, A.Y.; Watson, T.; Lip, G.Y. The role of aspirin in cardiovascular prevention: Implications of aspirin resistance. J. Am. Coll. Cardiol. 2008, 51, 1829–1843. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, K.C.; Russo, F.X.; Parks, R.E., Jr. Inhbition of human and rat platelet aggregation by extracts of Mo-er (Auricularia auricula). Thromb. Haemost. 1982, 48, 162–165. [Google Scholar] [PubMed]
- Rzymski, P.; Mleczek, M.; Niedzielski, P.; Siwulski, M.; Gąsecka, M. Potential of cultivated Ganoderma lucidum mushrooms for the production of supplements enriched with essential elements. J. Food Sci. 2016, 81, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.F. Ganoderma lucidum: Persuasive biologically active constituents and their health endorsement. Biomed. Pharm. 2018, 107, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Wachtel-Galor, S.; Yuen, J.; Buswell, J.A. Ganoderma lucidum (Lingzhi or Reishi): A Medicinal Mushroom. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; Chapter 9. [Google Scholar]
- Xu, Z.; Chen, X.; Zhong, Z.; Chen, L.; Wang, Y. Ganoderma lucidum polysaccharides: Immunomodulation and potential anti-tumor activities. Am. J. Chin. Med. 2011, 39, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.S.; Ho, S.Y.; Nan, F.H.; Chen, S.N. Ganoderma lucidum beta 1,3/1,6 glucan as an immunomodulator in inflammation induced by a high-cholesterol diet. BMC Complement. Altern. Med. 2016, 16, 500. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.R.; Yue, Q.X.; Wu, Z.Y.; Song, X.Y.; Tao, S.J.; Wu, X.H.; Xu, P.P.; Liu, X.; Guan, S.H.; Guo, D.A. Cytotoxic triterpenoids from Ganoderma lucidum. Phytochemistry 2010, 71, 1579–1585. [Google Scholar] [CrossRef]
- Li, P.; Deng, Y.P.; Wei, X.X.; Xu, J.H. Triterpenoids from Ganoderma lucidum and their cytotoxic activities. Nat. Prod. Res. 2013, 27, 17–22. [Google Scholar] [CrossRef]
- Zheng, S.L.; Roddick, A.J. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: A systematic review and meta-analysis. JAMA 2019, 321, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Palacios, I.; Lozano, M.; Moro, C.; D’Arrigo, M.; Rostagno, M.A.; Martinez, J.A.; Garcia-Lafuente, A.; Guillamon, E.; Villares, A. Antioxidant properties of phenolic compounds occurring in edible mushroom. Food Chem. 2011, 128, 674–678. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Zhang, J.; Hu, C.; Che, G.; Zhou, M.; Jia, L. Antioxidant and hepatoprotective activities of intracellular polysaccharide from Pleurotus eryngii SI-04. Int. J. Biol. Macromolec. 2016, 91, 568–577. [Google Scholar] [CrossRef]
- Sobotková, A.; Mášová-Chrastinová, L.; Suttnar, J.; Štikarová, J.; Májek, P.; Reicheltová, Z.; Kotlín, R.; Weisel, J.W.; Malý, M.; Dyr, J.E. Antioxidants change platelet responses to various stimulating events. Free Radic. Biol. Med. 2009, 47, 1707–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hyppönen, E.; Berry, J.; Vieth, R.; et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sławińska, A.; Fornal, E.; Radzki, W.; Skrzypczak, K.; Zalewska-Korona, M.; Michalak-Majewska, M.; Parfieniuk, E.; Stachniuk, A. Study on vitamin D2 stability in dried mushrooms during drying and storage. Food Chem. 2016, 199, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.; Twito, O.; Tohami, T.; Ramati, E.; Neumark, E.; Rashid, G. Vitamin D diminishes the high platelet aggregation of type 2 diabetes mellitus patients. Platelets 2019, 30, 120–125. [Google Scholar] [CrossRef]
- Feng, L.; Hou, J.; Cheng, S. Anti-platelet Aggregation Activity of Ergosterol in vitro. Herb. Med. 2017, 36, 1116–1119. [Google Scholar]
- Zhang, Y.; Mills, G.L.; Nair, M.G. Cyclooxygenase inhibitory and antioxidant compounds from the mycelia of the edible mushroom Grifola frondosa. J. Agric. Food Chem. 2002, 50, 7581–7585. [Google Scholar] [CrossRef]
- Kalantzi, K.I.; Tsoumani, M.E.; Goudevenos, I.A.; Tselepis, A.D. Pharmacodynamic properties of antiplatelet agents: Current knowledge and future perspectives. Expert Rev. Clin. Pharm. 2012, 5, 319–336. [Google Scholar] [CrossRef]
- Marx, G.; Krugliak, J.; Shaklai, M. Nutritional zinc increases platelet reactivity. Am. J. Hematol. 1991, 38, 161–165. [Google Scholar] [CrossRef]
- Watson, B.R.; White, N.A.; Taylor, K.A.; Howes, J.M.; Malcor, J.D.; Bihan, D.; Sage, S.O.; Farndale, R.W.; Pugh, N. Zinc is a transmembrane agonist that induces platelet activation in a tyrosine phosphorylation-dependent manner. Metallomics 2016, 8, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Thetsrimuang, C.; Khammuang, S.; Sarnthima, R. Antioxidant activity of crude polysaccharides from edible fresh and dry mushroom fruiting bodies of Lentinus sp. strain RJ-2. Int. J. Pharm. 2011, 7, 58–65. [Google Scholar]




| Parameter | Unit | L. edodes | A. auricula-judae | H. erinaceus | G. lucidum | P. ostreatus | P. eryngii | A. bisporus | C. comatus | |
|---|---|---|---|---|---|---|---|---|---|---|
| TAC | mM Trolox equivalent | 0.36 | 0.49 | 0.43 | 0.47 | 0.42 | 0.52 | 0.49 | 0.18 | |
| DPPH inhibition | % | 7.0 | 19.0 | 7.0 | 5.0 | 4.0 | 12.0 | 16.0 | 0.0 | |
| TPC | µg/mL | n.d. | 10.0 | 2.1 | 0.4 | 4.0 | 0.0 | 4.0 | 0.4 | |
| Organic acids | acetic | ng/mL | 156.7 | 8.9 | 5380.9 | 104.9 | n.d. | n.d. | 8.8 | 39.3 |
| citric | n.d. | 3.6 | 30.4 | n.d. | n.d. | n.d. | n.d. | 6.3 | ||
| fumaric | 7.0 | n.d. | 6745.1 | 2.4 | n.d. | n.d. | 1.8 | 0.5 | ||
| lactic | n.d. | n.d. | n.d. | 184.2 | n.d. | n.d. | n.d. | 15.2 | ||
| malic | n.d. | 24.0 | 1588.0 | 190.3 | n.d. | n.d. | n.d. | 0.7 | ||
| malonic | 1.6 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ||
| succinic | n.d. | n.d. | n.d. | 21.2 | n.d. | n.d. | 14.9 | n.d. | ||
| quinic | 5.6 | n.d. | n.d. | 17.5 | n.d. | n.d. | 4.4 | n.d. | ||
| Ergosterol | ng/mL | 119.8 | 25.3 | 18.6 | 14.6 | 27.9 | 195.8 | 38.4 | 72.9 | |
| Total polysaccharides | mg/mL | 0.92 | 1.41 | 1.38 | 0.79 | 0.67 | 2.30 | 0.78 | 2.50 | |
| Sugar Component | L. edodes | A. auricula-judae | H. erinaceus | G. lucidum | P. ostreatus | P. eryngii | A. bisporus | C. comatus |
|---|---|---|---|---|---|---|---|---|
| Ribose | - | trace | - | - | - | trace | 3.3 | - |
| (Rib) | - | trace | - | - | - | trace | (1.8) | - |
| 3-OMe-hexose | - | - | - | - | - | - | 12.3 | - |
| (3-OMe-Hex) | - | - | - | - | - | - | (6.9) | - |
| 6-deoxy-hexose | trace | 7.7 | 7.3 | 19.0 | 8.57 | trace | 3.4 | trace |
| (6-D-Hex) | trace | (2.3) | (3.5) | (2.4) | (2.8) | trace | (1.9) | trace |
| Xylose | trace | trace | - | - | - | trace | 7.0 | - |
| (Xyl) | trace | trace | - | - | - | trace | (3.9) | - |
| Mannose | 107.7 | 145.6 | 12.7 | 138.1 | 32.7 | 29.1 | 17.1 | trace |
| (Man) | (12.5) | (42.8) | (6.0) | (17.3) | (10.7) | (9.7) | (9.6) | trace |
| Glucose | 409.9 | 146.4 | 106.5 | 403.5 | 211.7 | 177.4 | 86.0 | 359.7 |
| (Glc) | (47.5) | (43.0) | (50.7) | (50.7) | (68.7) | (59.1) | (48.1) | (83.7) |
| Galactose | 344.8 | 40.4 | 76.1 | 212.7 | 54.7 | 93.5 | 49.4 | 70.2 |
| (Gal) | (40.0) | (11.9) | (36.3) | (26.7) | (17.8) | (31.2) | (27.7) | (16.3) |
| Glucosamine | - | - | 7.3 | 22.8 | - | trace | trace | - |
| (GlcN) | - | - | (3.5) | (2.9) | - | trace | trace | - |
| Component | Retention Time [min] | L. edodes | A. auricula-judae | H. erinaceus | G. lucidum | P. ostreatus | P. eryngii | A. bisporus | C. comatus |
|---|---|---|---|---|---|---|---|---|---|
| Terminal pentose | 9.24 | n.d. | trace | n.d. | n.d. | n.d. | 0.52 | 1.57 | n.d. |
| Terminal 6-deoxy-hexose | 9.90 | 0.33 | 0.44 | 4.73 | 1.94 | trace | 0.67 | 3.27 | 1.12 |
| →2)-linked pentose | 11.47 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1.37 | n.d. |
| Terminal hexose I (Man) | 12.30 | 4.31 | 27.57 | 5.89 | 2.23 | 9.17 | 9.27 | 7.42 | n.d. |
| Terminal hexose II (Glc) | 12.37 | 5.16 | n.d. | 5.94 | 14.33 | 10.81 | 9.52 | 3.72 | 10.42 |
| Terminal hexose III (Gal) | 12.79 | 0.52 | 1.93 | 2.06 | 1.50 | trace | 0.48 | 2.97 | 1.12 |
| →2,4)-linked pentose | 14.25 | n.d. | n.d. | n.d. | 11.80 | n.d. | 3.23 | 2.70 | n.d. |
| →3)-linked hexose | 14.35 | 30.84 | 22.86 | 10.17 | 3.00 | 8.88 | 2.73 | 1.55 | 5.59 |
| →4)-linked hexose | 14.70 | 16.46 | 8.86 | 10.92 | 20.54 | 37.58 | 53.96 | 14.72 | 57.65 |
| →2)-linked hexose | 14.73 | 0.52 | trace | 0.54 | trace | 3.55 | n.d. | n.d. | n.d. |
| →6)-linked hexose | 14.94 | 3.51 | 1.47 | 11.45 | 5.37 | 9.20 | 1.21 | 13.74 | 1.78 |
| →3,4)-linked hexose | 15.63 | 12.33 | 2.62 | 33.61 | 28.10 | 0.56 | 8.46 | 38.05 | 12.22 |
| →4,6)-linked hexose | 16.75 | 1.37 | 1.47 | n.d. | 2.70 | 5.49 | 3.94 | 2.06 | 5.75 |
| →3,6)-linked hexose | 16.90 | 12.68 | 28.45 | n.d. | 1.93 | 2.17 | 0.89 | 1.36 | n.d. |
| →2,4,6)-linked hexose | 17.38 | 9.51 | 0.91 | 13.68 | 6.03 | 11.67 | 5.12 | 4.90 | 4.35 |
| →3,4,6)-linked hexose | 17.85 | n.d. | n.d. | n.d. | n.d. | 0.92 | n.d. | 0.61 | n.d. |
| →2,3,6)-linked hexose | 18.67 | 1.46 | 3.42 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| →2,3,4,6)-linked hexose | 19.35 | 0.36 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| →3)-linked hexosamine | 19.95 | 0.64 | n.d. | 1.01 | 0.53 | n.d. | n.d. | n.d. | n.d. |
| Element | L. edodes | A. auricula-judae | H. erinaceus | G. lucidum | P. ostreatus | P. eryngii | A. bisporus | C. comatus |
|---|---|---|---|---|---|---|---|---|
| Macro Elements | ||||||||
| Ca | 2.9 | 7.9 | 1.0 | 2.1 | 0.60 | 0.20 | 1.1 | 0.70 |
| K | 91.0 | 19.7 | 241 | 67.9 | 112 | 125 | 175 | 171 |
| Mg | 5.1 | 3.1 | 5.4 | 3.5 | 4.1 | 3.9 | 3.9 | 4.1 |
| Na | 2.3 | 2.3 | 0.64 | 0.31 | 1.2 | 0.30 | 2.6 | 3.8 |
| P | 14.5 | 4.6 | 39.3 | 28.2 | 24.8 | 15.2 | 27.5 | 25.5 |
| Trace Elements | ||||||||
| Cr | 0.073 | 0.018 | 0.018 | 0.014 | 0.014 | 0.069 | 0.009 | 0.009 |
| Cu | 0.013 | 0.017 | 0.14 | 0.002 | 0.019 | 0.044 | 0.074 | 0.15 |
| Fe | 0.77 | 0.26 | 0.67 | 0.083 | 0.20 | 0.22 | 0.10 | 0.24 |
| Mn | 0.11 | 0.017 | 0.11 | 0.029 | 0.040 | 0.030 | 0.029 | 0.036 |
| Se | 0.044 | 0.012 | 0.12 | 0.049 | 0.038 | 0.069 | 0.068 | 0.072 |
| Zn | 0.083 | 0.096 | 0.22 | 0.040 | 0.23 | 0.003 | 0.031 | 0.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poniedziałek, B.; Siwulski, M.; Wiater, A.; Komaniecka, I.; Komosa, A.; Gąsecka, M.; Magdziak, Z.; Mleczek, M.; Niedzielski, P.; Proch, J.; et al. The Effect of Mushroom Extracts on Human Platelet and Blood Coagulation: In vitro Screening of Eight Edible Species. Nutrients 2019, 11, 3040. https://doi.org/10.3390/nu11123040
Poniedziałek B, Siwulski M, Wiater A, Komaniecka I, Komosa A, Gąsecka M, Magdziak Z, Mleczek M, Niedzielski P, Proch J, et al. The Effect of Mushroom Extracts on Human Platelet and Blood Coagulation: In vitro Screening of Eight Edible Species. Nutrients. 2019; 11(12):3040. https://doi.org/10.3390/nu11123040
Chicago/Turabian StylePoniedziałek, Barbara, Marek Siwulski, Adrian Wiater, Iwona Komaniecka, Anna Komosa, Monika Gąsecka, Zuzanna Magdziak, Mirosław Mleczek, Przemysław Niedzielski, Jędrzej Proch, and et al. 2019. "The Effect of Mushroom Extracts on Human Platelet and Blood Coagulation: In vitro Screening of Eight Edible Species" Nutrients 11, no. 12: 3040. https://doi.org/10.3390/nu11123040
APA StylePoniedziałek, B., Siwulski, M., Wiater, A., Komaniecka, I., Komosa, A., Gąsecka, M., Magdziak, Z., Mleczek, M., Niedzielski, P., Proch, J., Ropacka-Lesiak, M., Lesiak, M., Henao, E., & Rzymski, P. (2019). The Effect of Mushroom Extracts on Human Platelet and Blood Coagulation: In vitro Screening of Eight Edible Species. Nutrients, 11(12), 3040. https://doi.org/10.3390/nu11123040












