1. Introduction
Vitamin B12 (B12, cobalamin) is an essential water-soluble molecule, primarily present in foods of animal origin [
1]. B12 deficiency is characterized by a decrease in total plasma B12, including the plasma B12 bound to its transport protein transcobalamin (holotranscobalamin, holoTC), which signifies the fraction of total plasma B12 readily available for tissue uptake [
1]. B12 deficiency results in increased concentrations of methylmalonic acid (MMA) and homocysteine (Hcy), due to decreased activity of methylmalonyl-coenzyme A mutase and methionine synthase, respectively [
1,
2,
3,
4,
5]. While severe deficiency can cause megaloblastic anemia and permanent neurological damage, early physiological manifestations are generally subtle [
1,
6].
Retrospective assessments of dietary rations have shown that an improvement in B12 status highly correlates with ingestion of dairy products, whereas its relations to intake of meat, poultry, eggs, or fish are weaker [
7]. Intervention studies have also revealed that the B12 status of vegetarians is positively associated with their intake of dairy products [
8], suggesting that milk and milk products are efficient carriers of highly bioavailable B12. Cow and buffalo milk contain approximately the same amount of B12, 2–7 µg/L [
9]. While cow milk is the dominant milk form consumed in Western countries, buffalo milk is widely consumed in India. The form of B12 present in both types of milk is mainly hydroxo-B12 (HO-B12) [
10], which is different from cyano-B12 (CN-B12), a synthetic form of the vitamin typically present in vitamin pills and food fortification.
In humans, oral uptakes of CN-B12 and HO-B12 are similar [
11]. Employing an animal model, we have previously demonstrated that, though the two forms of B12 are absorbed alike, they distribute differently within the body [
12,
13]. Thus, CN-B12 had a tendency to accumulate in the blood and the kidneys, whereas HO-B12 targeted mainly to the liver. These findings question whether the two vitamin forms are of equal value for improvement of B12 status.
The present study was undertaken to compare biomarkers of B12 status during four weeks of supplementation with equivalent amounts of B12, administered in either vitamin capsules (CN-B12), cow milk (dietary HO-B12), or buffalo milk (dietary HO-B12), in Indian individuals with a low B12 status.
2. Materials and Methods
2.1. Study Design and Supplementation Products
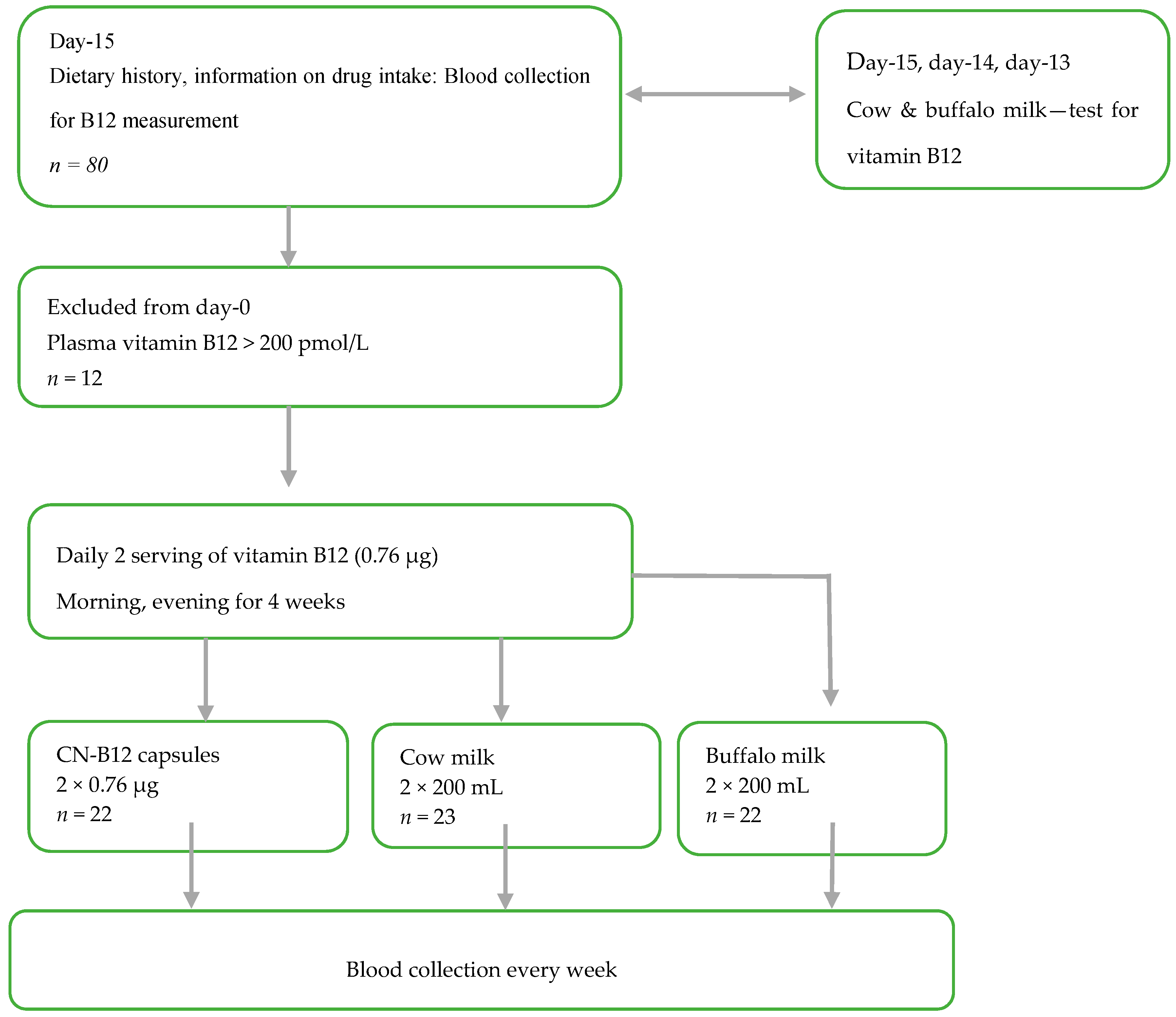
This randomized intervention trial (
Figure 1 and
Supplementary Table S1) was designed to investigate the B12 status in lactovegetarian Indian individuals, supplemented for four weeks with two doses of B12 (morning and evening), as either vitamin capsules with CN-B12 = 0.76 µg (CN group) 200 mL of cow milk (cow milk group), or 200 mL of buffalo milk (buffalo milk group), with both milk types containing B12 ≈ 0.78 µg in 200 mL (see measurements in
Supplementary Table S2, as well as further details in ref. [
8]). CN-B12 capsules were prepared in Aarhus, Denmark as previously described [
14,
15]. The compliance of the milk/capsule intake was monitored by the Whatsapp online program. Each participant was contacted to confirm capsule/milk intake every day, in the morning and evening, through Whatsapp. The whole exercise was free of cost. Deenanath Mangeshkar Hospital and M/S Chitale Bandhu dairy firm entered into a memorandum of agreement stating that Chitale Bandhu would deliver (2 × 200 mL × 25) cow milk for 25 participants and 2 × 200 mL × 25) of buffalo milk for another 25 participants) to Deenanath Mangeshkar Hospital and Research Center, Pune, India. Fifty milk packets each were delivered every morning. Each participant was given two packets to be consumed, one at night and the other next day morning. They poured the milk in a vessel and heated it until it was boiling and then cooled immediately and drank. This procedure continued for 4 weeks.
2.2. Participants
Participants (
n = 68; 30 males and 38 females) between the age of 18–50 years were recruited from the Pune area in India, and the study was carried out at Deenanath Mangeshkar Hospital and Research Center, Pune, India, from April 2017 to June 2017. Inclusion criterion was a lactovegetarian diet. Exclusion criteria were the use of vitamin pills containing > 1 µg B12 within the last two weeks, drugs (methotrexate, antacids, and metformin) known to influence B12 absorption/biomarkers, and known chronic systemic disease. Based on our knowledge from a previous B12 intervention study in a comparable Indian population [
15], the number of participants included in each intervention group was calculations using a statistical power of 80% and a two-sided significance level of 5%. Anticipating a mean plasma B12 increase of 20% for the participants receiving intervention with two daily doses of 0.76 µg CN-B12 for four weeks, the sample size calculations suggested
n = 19 in each group. In order to account for possible dropouts, 68 participants were divided evenly and randomly into three groups: capsule group, cow milk group, and buffalo milk group (1 female excluded post-trial due to a baseline value for holoTC above the upper limit for the reference interval), see result section. The participants were allowed to continue on their regular lactovegetarian diet throughout the study. The participants received no compensation for participation in the study.
Folate and vitamin B12 deficiency were defined as plasma concentrations <4.54 nmol/L and <150 pmol/L, respectively [
8]. Anemia was defined as a hemoglobin concentration <7.5 nmol/L (120 g/L) in females and <8.0 nmol/L (130 g/L) in males [
8]. Macrocytosis was stipulated as MCV >100 fl [
8].
The investigation was performed within the confines of the Helsinki Declaration II, and the study was approved by the Institutional Ethics Committee of Deenanath Mangeshkar Hospital and Research Center (project No. 2015_APR_SN_167). All individuals gave their informed consent before inclusion in the study. The study is registered with Clinical Trial Registry of India, bearing the indicator CTRI/2017/08/009342.
2.3. Blood Sampling and Biochemical Methods
At baseline and each week throughout the study, non-fasting blood samples were drawn into EDTA and plain vacutainers and kept in an ice-cold box. Hemoglobin and mean cell volume were determined on the XN 3000 Hematology Analyzer (Sysmex, Japan) [
8]. Blood samples were centrifuged (10 min at 2300 g) within two hours after withdrawal and serum/plasma was stored at −56 °C until analysis/shipment of samples. Plasma creatinine (only baseline), cholesterol, triglycerides, and HDL-cholesterol were measured in the samples collected at the baseline and at the end of the study (4 weeks) using ISO (International Organization for Statndardization) certified enzymatic methods on the RX Imola (Randox Laboratories, London, UK) [
8]. Plasma B12 (cobalamin, B12), holotranscobalamin (holoTC), folate, and total homocysteine (Hcy) concentrations were measured using ARCHITECT (Abbott, Columbus, OH, USA) every week in batches, each batch having internal quality control samples [
8]. Plasma aliquots were shipped to Aarhus University Hospital, Aarhus, Denmark, on dry ice for analysis of MMA, measured in one run for all participants. MMA was quantified by liquid chromatography-tandem mass spectrometry on the AB SCIEX Tripel Quad 5500 System (AB SCIEX, IL, USA) [
15]. Intra-batch coefficients of variation (in percent) for vitamin B12, holoTC, Hcy, folate and MMA were 4.5%, 6.3%, 4.2%, 5.2% and 5.4% and inter-batch coefficients were 4.8%, 6.9%, 4.5% and 5.8%, respectively.
2.4. Combined Analysis of Markers
The four-component combined indicator of B12 status 4cB12 (or its three-component variant 3cB12 at occasional missed analysis, or 2cB12 at separate analysis of B12-related vs metabolite-related markers), was calculated from the measurements of serum B12, holoTC, MMA, and Hcy using the formula presented by Fedosov et al. [
16]. The effect of plasma folate on the combined B12 index was examined by entering its values into the calculation spreadsheets [
16].
2.5. Statistical Procedures and Software
Examination of the overall identity of baselines between the supplementation groups (for each given marker) was conducted by a non-parametric Kruskal-Wallis test. The pairwise comparisons between the groups were performed by a Steel-Dwass test (multiple comparisons without assumption of normality). The final outcome of the treatment (baseline vs. endpoint) was assessed by a Wilcoxon signed rank test for paired groups (without assumption of normality) [
17]. The shift of scaled responses (
Section 2.6) from the baseline at each time point was assessed by the parametric paired
t-test (to maintain consistency with the parametric method of least squares also used in these charts). The overall identity of all scaled responses at each time point was examined by single factor ANOVA, while assessment of their pairwise identity was done by a Tukey-Kramer test (with correction for multiple comparisons).
Statistical analysis and fitting of the data (
Section 2.7) employed KyPlot 5.0 software (free software from Kyenslab Inc, Tokyo, Japan).
2.6. Correction for Baselines and Smoothing of Data
All responses were corrected for the baselines. Time-dependent changes (Δ
y) in plasma B12 and holoTC (
y) from their respective baselines (
y0) were calculated for each participant as Δ
y =
y −
y0. This scaling was expected to suppress the excessive dispersion caused by different starting points. Changes in MMA and Hcy over time were presented as ratios (R(
y) =
y/
y0) between the concentration at a given time point (
y) and the concentration at the baseline (
y0). Such normalization partially compensated for larger (smaller) responses at higher (lower) initial metabolites, usually observed at identical changes in the B12 statuses, see also ref. [
15].
All adjusted responses were plotted over time and smoothed. The latter procedure was intended to compensate for a relatively high noise to signal ratio and expose the shape of dependency to simplify selection of the fitting equation. Smoothing was done individually for each participant and involved a two-dimensional averaging of two adjacent points (for example, for weeks 1 and 2) rendered as Δy1,2 = (Δyweek 1 + Δyweek 2)/2 on the marker axis and as x1,2 = (1 + 2)/2 = 1.5 (i.e., week 1.5) on the time axis. As a result, the original data at weeks 1, 2, 3, and 4 were transformed to their average values at weeks 1.5, 2.5, and 3.5, respectively.
2.7. Approximating Models and Comparison of Supplements
The response of a marker over time was approximated by an arbitrary function (from the set of equations below), capable of reproducing the shape of dependency, but claiming no physiological meaning:
Here
y stands for the relevant scaled marker (Δ
y or R(
y));
P1 is a baseline value of
y (fixed at 0 or 1, depending on the context);
P2 and
P3 represent the floating parameters assessed by fitting;
x is the time of treatment. The value of
x received a negligible increment of 10
−20 in Equation (2) to avoid
x = 0, forbidden in a power function. The equations were chosen to guarantee a reasonably accurate shape of the curve, while avoiding “superfluous” floating parameters, frequently tending to infinity and/or exhibiting a very high likelihood for error. The shapes of the functions obeyed: (i) a linear start followed by a bending for Equation (1); (ii) an initial bending followed by a linear continuation for Equation (2); (iii) a quasi-linear start tending to a plateau for Equation (3). Equations (1, 2, or 3) were applied in accordance with the detected shapes of dependencies.
Only one function (e.g., Equation (2) was used to approximate the datasets for each given marker (e.g., ΔB12) to simplify the parametric comparison of fits. Parameters of this fitting function (e.g., P1, P2, P3) and their errors (e.g., SE1, SE2, SE3) for each supplementation were estimated by the method of least squares. The “baseline parameter” P1 was fixed at either 0 (for ΔB12, ΔholoTC) or 1 (for R(MMA), R(Hcy)). The fixed coefficient P1 was retained in the equation (as well as in the covariance table) to account for its error, affecting the two floating parameters P2 and P3.
Presence or absence of a response was assessed by t-test, comparing each fitting parameter to its reference value (0 or 1 depending on the context). The probability (p) of a “zero function” (with all parameters equal to their reference values, 0 or 1) was calculated as the probability of several simultaneous events, e.g., p = p1·p2·p3, with p1 = 1 for the fixed parameter P1.
The same approach was used to assess, whether the two supplementations (e.g., “A” and “B”) gave the identical time-dependent curves (e.g., “curve A” = “curve B”). The respective sets of fitting parameters (from the same function) were examined on their equality in pairs (
P1A =
P1B,
P2A =
P2B,
P3A =
P3B) by
t-test, essentially as discussed in ref [
18], with
tiAB = (
PiA −
PiB)/
SEiAB,
SEiAB2 =
SEiA2 +
SEiB2 and degrees of freedom
df =
nA +
nB − 2
m (
n = total number of points in sets “A” and “B”,
m = the number of parameters, i.e., 3 in the current case). The probabilities of identity (
p1,
p2,
p3) for the individual parameter pairs were calculated, finally giving the overall identity between the two curves (
p =
p1·
p2·
p3). Multiple (three) comparisons (“A” = “B”, “B” = “C” and “A” = “C”) increased the likelihood of a “false discovery” (type I error), and this issue was addressed by applying a Holm-Bonferroni correction to the critical
p-values, changed from e.g.,
p = 0.05 to 0.05/3 (for the lowest
p from three comparisons), followed by 0.05/2 and 0.05/1 (for the sequentially higher
p).
4. Discussion
We reported changes in biomarkers related to vitamin B12 status in a lactovegetarian Indian population during four weeks of daily supplementation with ≈ 1.5 µg of B12, administered as two oral doses of CN-B12 (vitamin capsules) or 2 × 200 mL of cow or buffalo milk, containing the natural forms of B12 (mainly HO-B12).
A weakness of this study concerns differences in baselines for holoTC and Hcy among the three intervention groups, despite similar levels of the circulating total B12 (
Table 1). The dispersion of baselines within each supplementation group was also high, indicating that the selection criterion of total plasma B12 < 200 pM was not sufficiently strict. We attempted to counteract the variations of baselines by recording the difference between actual and baseline values (for total B12 and holoTC) or the ratio between actual and baseline values (for Hcy and MMA). Another weakness was the lack of information on dietary intake during the study period. Theoretically, groups offered supplementation with milk could have decreased the intake of other food items, thereby possibly underestimating the benefit of the milk intervention.
The recommended daily dose of B12 is 2.4 µg, based on Western standards [
19], or 1 µg, based on Indian standards [
20]. It has been estimated that the daily intake by Indian lactovegetarians is at the magnitude of B12 ≈ 1.65 µg/day [
8], implying a proportionally lower total body store of B12, in comparison to a Western population with Σ B12 ≈ 3 mg [
19] and references thereof). An increment of ≈1.5 µg/day increased the intake of B12 by our cohort to a dose slightly exceeding the Western recommended allowance [
19]. Thereby we addressed the issue of whether such a dose can improve the biochemical markers of B12 status within a four week trial, though the complete replenishment of B12 body stores was out of reach after the overall intake of Σ B12 ≈ 90 µg. We also anticipated that any major benefit of milk supplementation, as compared to CN-B12 capsules, would be more transparent at low doses (i.e., not saturating the transport system). For convenience, the milk servings were administrated in the mornings and evenings. The same pattern was followed with the vitamin capsules, even though they are generally ingested only once a day. Our choice was also driven by the fact that more B12 is believed to be absorbed from a divided dose than from a single dose [
21].
Supplementation with CN-B12 capsules or one or the other form of milk provided small but detectable differences “within the group” in most of the B12 biomarkers. Differences between the groups were also observed in a few instances (e.g., for changes in total B12,
Figure 2a). Differences between the groups can be ascribed to (i) the particular form of B12 administered (synthetic CN-B12 in capsules or the natural vitamin forms (e.g., HO-B12) in milk), and/or (ii) the matrix of the food supplements.
It should be taken into account that all pasteurized milk (cow and buffalo) in India is boiled before ingestion. Accordingly, a considerable portion of B12 is released from the endogenous milk carriers, haptocorrin (HC), and/or transcobalamin (TC). This paves the way for B12 binding to casein, the main protein fraction of ruminant milk, which, besides being heat stable, also has the capacity to bind large amounts of HO-B12 [
22]. Therefore, a possible matrix effect is likely to be similar for both types of milk, irrespective of their specific binders, generally destroyed by boiling. Intake of milk, as well as other food items, stimulates both gastric acid production and the secretion of intrinsic factor [
1,
23,
24]. The combination of these effects is expected to promote a more efficient uptake of B12 (in comparison to a vitamin capsule ingested without any bulk filling). Casein has long been known to stretch the time of intestinal transit, thereby increasing the duration of B12 uptake and reducing the saturation degree of its absorption system. Finally, some effects of bioactive peptides in milk may be at play.
When interpreting our results concerning the increase in total B12 and holoTC, the dynamics of plasma B12 also has to be considered. B12 in blood circulates in two pools: one is bound to heptocorrin (HC) and the other to transcobalamin (TC). HC is almost fully saturated with B12 (at least in B12 replete individuals), it has a slow turnover rate and shows marginal importance for B12 tissue delivery [
1,
24]. TC circulates mainly unsaturated, it has a fast turnover rate and plays a major role in B12 tissue delivery [
1,
24]. Newly absorbed B12 preferentially binds to the surplus TC, producing a TC-B12 complex (holoTC) taken up by the cells. The excessive or unprocessed cellular B12 is released back to the blood with a repetition of the aforementioned cycles, leading to a gradual accumulation of the unprocessed vitamin within the slow-exchanging HC pool. All in all, the total plasma B12 can be interpreted as the “inert” HC complex (carrying most of the circulating B12), whereas holoTC reflects the fast-exchanging “active” component of the vitamin pool, that in a blood sample usually accounts for 1/8–1/4 of the total B12 content [
25].
We observed higher levels of total B12 after ingestion of CN-B12, compared to ingestion of the natural B12 forms (mainly HO-B12) present in milk, which was in accord with our previous data for supplementation of CN-B12 vs. HO-B12 in capsules [
13,
15]. The increase in total B12 after administration of CN-B12 was almost exclusively driven by accumulation of HC-bound B12, as follows from a high proportion of total ΔB12: ΔholoTC ≈ 6–10 (
Figure 2a vs.
Figure 2b, ○-red symbols). In contrast, a much lower accumulation of B12 on HC was observed after administration of milk (
Figure 2a vs.
Figure 2b, ▲-green and ■-blue symbols). We hypothesize that a high level of total B12 in the blood after administration of CN-B12 (in comparison to HO-B12 from milk) originated from accumulation of CN-B12 on HC, because more processing cycles are seemingly required to remove the protective CN-group in CN-B12. The turnover rates of holoTC were apparently similar for all supplementations, with a marginally better response in the milk-groups (
Figure 2b). Comparable changes in holoTC were observed in our earlier works upon administration of CN-B12 vs. HO-B12, where an insignificantly higher increase was observed for the CN-B12 supplementation [
13,
15].
The changes in the metabolic markers MMA and Hcy were rather small (
Figure 3 and
Table 2). As discussed above, a low impact on metabolites may reflect a marginal alteration in the body B12 store and thereby cellular content of B12. The effect on Hcy (
Figure 3a) was more pronounced and similar in all groups (
Figure 3b, see also
Table 2). Regarding MMA, we assumed comparably low responses irrespective of supplementation. A more explicit change in Hcy was in accord with our previous data, indicating Hcy as the metabolic marker responding first to an increased intake of B12 [
8].
We used the newly introduced combined indicator of B12 status (cB12) to compare the three supplementations and assess synchronization between the “pure B12” index (including B12 and holoTC) and the “metabolic index” (including MMA and Hcy). Regardless of supplementation, the “metabolic index” showed a lower response at week 1.5. We believe this represents the fact that B12 and holoTC have to increase in plasma prior to entering the cells and benefiting intracellular B12 metabolism. The observation is in agreement with our previous findings indicating that a daily oral supplementation of 3-µg CN-B12 or 3-µg HO-B12 hardly changes the metabolic markers MMA and Hcy in lactovegetarian Indians [
13,
15]. Uniting all our measures into the 4cB12 index by the end of the study, we found comparable values for the three intervention groups, suggesting that CN-B12, cow milk, and buffalo milk have comparable effects on the measured markers of B12 status in the Indian cohort. This effect was judged to be by far insufficient to reach a fully replete B12 status that would require a 4cB12 value of 0 ± 0.5 or more [
16].