Multiple Micronutrient Supplementation Using Spirulina platensis during the First 1000 Days is Positively Associated with Development in Children under Five Years: A Follow up of A Randomized Trial in Zambia
Abstract
1. Introduction
2. Materials and Methods
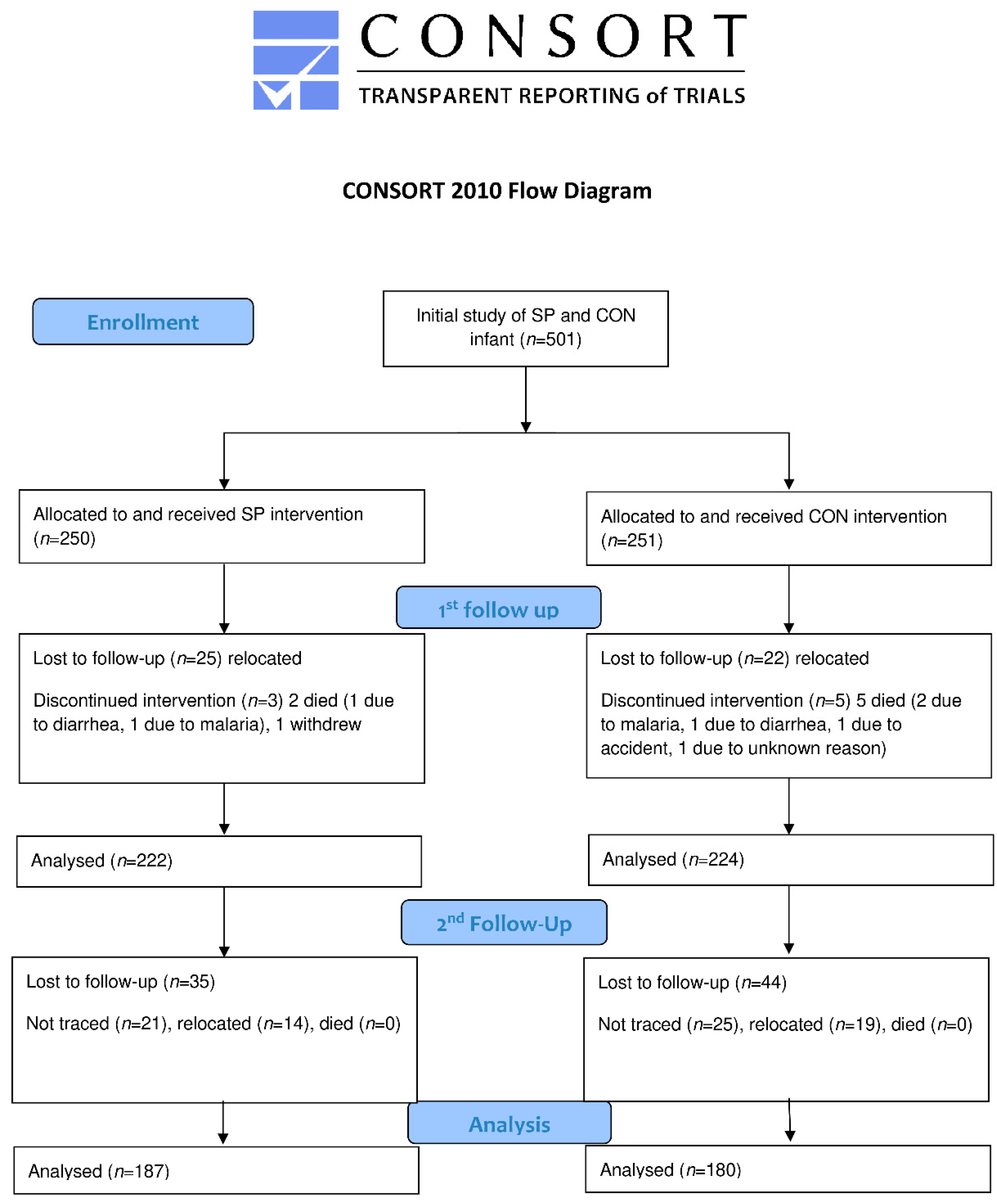
2.1. Study Design
2.2. Measurement
2.3. Ethical Statement
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Black, M.M.; Walker, S.P.; Fernald, L.C.; Andersen, C.T.; DiGirolamo, A.M.; Lu, C.; McCoy, D.C.; Fink, G.; Shawar, Y.R.; Shiffman, J.; et al. Lancet Early Childhood Development Series Steering Committee. Early childhood development coming of age: science through the life course. Lancet 2017, 389, 77–90. [Google Scholar] [CrossRef]
- Heckman, J.J. Skill formation and the economics of investing in disadvantaged children. Science 2006, 312, 1900–1902. [Google Scholar] [CrossRef] [PubMed]
- Grantham-McGregor, S.; Cheung, Y.B.; Cueto, S.; Glewwe, P.; Richter, L.; Strupp, B. International Child Development Steering Group. Developmental potential in the first 5 years for children in developing countries. Lancet 2007, 369, 60–70. [Google Scholar] [CrossRef]
- Sánchez, M.; Bernal-Castillo, J.; Rozo, C.; Rodríguez, I. Spirulina (Arthrospira): An edible microorganism: A review. Univ. Sci. (Bogota) 2003, 1, 7–24. [Google Scholar]
- Khan, Z.; Bhadouria, P.; Bisen, P.S. Nutritional and therapeutic potential of spirulina. Curr. Pharm. Biotechnol. 2005, 5, 373–379. [Google Scholar] [CrossRef]
- Habib, M.A.; Parvin, M.; Huntington, T.C.; Hasan, M.R. A review on culture, production and use of spirulina as food for humans and feeds for domestic animals and fish. FAO Fish. Aquac. Circ. 2008, 1034, 33. [Google Scholar]
- Ravi, M.; De, S.L.; Azharuddin, S.; Paul, S.F. The beneficial effects of Spirulina focusing on its immunomodulatory and antioxidant properties. Nutr. Diet Suppl. 2010, 2, 73–83. [Google Scholar]
- Campanella, L.; Russo, M.V.; Avino, P. Free and total amino acid composition in blue-green algae. Ann. Chim. 2002, 4, 343–352. [Google Scholar]
- Siva Kiran, R.R.; Madhu, G.M.; Satyanarayana, S.V. Spirulina in combating protein energy malnutrition (pem) and protein energy wasting (PEW)—A review. J. Nutr. Res. 2015, 1, 62–79. [Google Scholar]
- Ötleş, S.; Pire, R. Fatty acid composition of Chlorella and spirulina microalgae species. J. AOAC Int. 2001, 6, 1708–1714. [Google Scholar]
- Sinha, S.; Patro, N.; Patro, I.K. Maternal protein malnutrition: Current and future perspectives of spirulina supplementation in neuroprotection. Front. Neurosci. 2018, 12, 966. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Inoue, Y.; Inoue, R.; Nakamura, A.; Chitundu, M.; Murakami, J.; Ota, Y.; Matsugami, J. Spirulina effectiveness study on child malnutrition in Zambia. IDS Res. 2014, 49–56, Special Collection. [Google Scholar]
- Simpore, J.; Kabore, F.; Zongo, F.; Dansou, D.; Bere, A.; Pignatelli, S.; Biondi, D.M.; Ruberto, G.; Musumeci, S. Nutrition rehabilitation of undernourished children utilizing spiruline and misola. Nutr. J. 2006, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Simpore, J.; Zongo, F.; Kabore, F.; Dansou, D.; Bere, A.; Nikiema, J.B.; Pignatelli, S.; Biondi, D.M.; Ruberto, G.; Musumeci, S. Nutrition rehabilitation of HIV-infected and HIV-negative undernourished children utilizing spirulina. Ann. Nutr. Metab. 2005, 6, 373–380. [Google Scholar] [CrossRef]
- Abed, E.; Ihab, A.N.; Suliman, E.; Mahmoud, A. Impact of spirulina on nutritional status, haematological profile and anaemia status in malnourished children in the Gaza Strip: randomized clinical trial. Matern. Pediatr. Nutr. 2016, 110, 2. [Google Scholar]
- Masuda, K.; Chitundu, M. Multiple micronutrient supplementation using spirulina platensis and infant growth, morbidity, and motor development: evidence from a randomized trial in Zambia. PLoS ONE 2019, 14, e0211693. [Google Scholar] [CrossRef]
- Masuda, K.; Chitundu, M. Post Intervention Morbidity and Growth Among Zambian Children Who Received Multiple Micronutrient Supplementation Using Spirulina Platensis: Evidence From A Randomized Trial in Zambia. Available online: http://cei.ier.hit-u.ac.jp/Japanese/WP2018-21.pdf (accessed on 16 February 2019).
- Gladstone, M.; Lancaster, G.A.; Umar, E.; Nyirenda, M.; Kayira, E.; van den Broek, N.R.; Smyth, R.L. The Malawi Developmental Assessment Tool (MDAT): The creation, validation, and reliability of a tool to assess child development in rural African settings. PLoS Med. 2010, 7, e1000273. [Google Scholar] [CrossRef]
- WHO Multicentre Growth Reference Study Group; de Onis, M. WHO Child Growth Standards based on length/height, weight and age. Acta. Paediatr. 2006, 95, 76–85. [Google Scholar]
- Larson, L.M.; Young, M.F.; Bauer, P.J.; Mehta, R.; Girard, A.W.; Ramakrishnan, U.; Verma, P.; Chaudhuri, I.; Srikantiah, S.; Martorell, R. Effectiveness of a home fortification programme with multiple micronutrients on infant and young child development: A cluster-randomised trial in rural Bihar, India. Br. J. Nutr. 2018, 120, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Matias, S.L.; Mridha, M.K.; Tofail, F.; Arnold, C.D.; Khan, M.S.A.; Siddiqui, Z.; Ullah, M.B.; Dewey, K.G. Home fortification during the first 1000 d improves child development in Bangladesh: A cluster-randomized effectiveness trial–3. Am. J. Clin. Nutr. 2017, 105, 958–969. [Google Scholar] [CrossRef]
- Prado, E.L.; Abbeddou, S.; Yakes Jimenez, E.; Somé, J.W.; Ouédraogo, Z.P.; Vosti, S.A.; Dewey, K.G.; Brown, K.H.; Hess, S.Y.; Ouédraogo, J.B. Lipid- based nutrient supplements plus malaria and diarrhea treatment increase infant development scores in a cluster-randomized trial in Burkina Faso–3. J. Nutr. 2015, 146, 814–822. [Google Scholar] [CrossRef]
- Yousafzai, A.K.; Rasheed, M.A.; Rizvi, A.; Armstrong, R.; Bhutta, Z.A. Effect of integrated responsive stimulation and nutrition interventions in the Lady Health Worker programme in Pakistan on child development, growth, and health outcomes: A cluster-randomised factorial effectiveness trial. Lancet 2014, 384, 1282–1293. [Google Scholar] [CrossRef]
- Prado, E.L.; Alcock, K.J.; Muadz, H.; Ullman, M.T.; Shankar, A.H. Maternal multiple micronutrient supplements and child cognition: a randomized trial in Indonesia. Pediatrics 2012, 130, e536–e546. [Google Scholar] [CrossRef]
- Lozoff, B.; Castillo, M.; Clark, K.M.; Smith, J.B. Iron-fortified vs low-iron infant formula: Developmental outcome at 10 years. Arch. Pediatr. Adolesc. Med. 2012, 166, 208–215. [Google Scholar] [CrossRef]
- Moramarco, S.; Amerio, G.; Gozza Maradini, G.; Garuti, E. The Rainbow Project: A Model to Fight Child Malnutrition in Zambia. IDS Res. 2014, 57–61, Special Collection. [Google Scholar]
- Moramarco, S.; Amerio, G.; Kasengele Chipoma, J.; Nielsen-Saines, K.; Palombi, L.; Buonomo, E. Filling the Gaps for Enhancing the Effectiveness of Community-Based Programs Combining Treatment and Prevention of Child Malnutrition: Results from the Rainbow Project 2015–17 in Zambia. Int. J. Environ. Res Public Health 2018, 15, 1807. [Google Scholar] [CrossRef]
- Prado, E.L.; Dewey, K.G. Nutrition and brain development in early life. Nutr. Rev. 2014, 72, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Levitsky, D.A.; Strupp, B.J. Malnutrition and the brain: changing concepts, changing concerns. J. Nutr. 1995, 125, 2212S–2220S. [Google Scholar] [CrossRef]
- Sika, M.K.; Adu-Afarwuah, S.; Young, R.R.; Oaks, B.M.; Tamakloe, S.M.; Ocansey, M.E.; Okronipa, H.; Prado, E.L.; Dewey, K.G. Maternal–Infant Supplementation with Small-Quantity Lipid-Based Nutrient Supplements Does Not Affect Child Blood Pressure at 4–6 Y in Ghana: Follow-up of a Randomized Trial. J. Nutr. 2019, 149, 522–531. [Google Scholar]
- Null, C.; Stewart, C.P.; Pickering, A.J.; Dentz, H.N.; Arnold, B.F.; Arnold, C.D.; Benjamin-Chung, J.; Clasen, T.; Dewey, K.G.; Fernald, L.C.H.; et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: a cluster-randomised controlled trial. The Lancet Glob. Health 2018, 6, e316–e329. [Google Scholar] [CrossRef]

| SP | CON | |
|---|---|---|
| (n = 187) | (n = 180) | |
| Child characteristics | ||
| Age at follow up (months) | 43.0 ± 4.5 | 43.5 ± 5.0 |
| Child female (%) | 52.1 | 46.5 |
| Stunting at baseline (%) | 42.1 | 43.0 |
| Underweight at baseline (%) | 19.5 | 24.3 |
| Wasting at baseline (%) | 9.7 | 10.7 |
| Dietary diversity score (0–7) | 5.2 ± 1.0 | 5.2 ± 1.0 |
| HIV-positive at baseline (%) | 3.1 | 2.5 |
| Child exclusively breastfed for 6 months (%) | 89.5 | 90.1 |
| Length of exclusive breastfeeding (months) | 5.9 ± 0.5 | 5.8 ± 0.5 |
| Maternal characteristics | ||
| Maternal age at baseline (years) | 28.1 ± 6.5 | 27.6 ± 7.5 |
| Maternal height at baseline (cm) | 152.5 ± 12.8 | 154.1 ± 10.6 |
| Maternal weight at baseline (kg) | 49.7 ± 7.3 | 49.4 ± 8.5 |
| Maternal education at baseline (years) | 6.1 ± 4.7 | 5.9 ± 4.3 |
| Household characteristics | ||
| Farmer (%) | 61.7 | 69.1 |
| Number of household members at baseline (persons) | 5.8 ± 2.1 | 5.7 ± 2.5 |
| Number of household members under the age of 5 at baseline (persons) | 2.2 ± 0.9 | 2.2 ± 1.1 |
| Households which had access to electricity at baseline (%) | 1.1 | 1.1 |
| Outcome: Standardized z Score Measuring | Motor Development | Mental Development | Gross Motor Development | Fine Motor Development | Language Skill | Personal-Social Skills |
|---|---|---|---|---|---|---|
| All children | ||||||
| Effect size | 0.42 *** | 0.33 *** | 0.37 *** | 0.38 *** | 0.24 ** | 0.28 ** |
| 95% CI | (0.22, 0.63) | (0.12, 0.54) | (0.16, 0.58) | (0.18, 0.59) | (0.03, 0.45) | (0.06, 0.49) |
| Children with HAZ < −2.0 at baseline (n = 141) | ||||||
| Effect size | 0.57 *** | 0.37 ** | 0.55 *** | 0.46 *** | 0.22 | 0.41 ** |
| 95% CI | (0.25, 0.89) | (0.04, 0.70) | (0.23, 0.88) | (0.12, 0.80) | (−0.11, 0.55) | (0.05, 0.77) |
| Children with HAZ > −2.0 at baseline (n = 190) | ||||||
| Effect size | 0.36 ** | 0.23 | 0.25 * | 0.35 ** | 0.15 | 0.20 |
| 95% CI | (0.07, 0.64) | (−0.07, 0.52) | (−0.04, 0.54) | (0.07, 0.63) | (−0.12, 0.43) | (−0.11, 0.51) |
| Children with dietary diversity score < median at baseline (n = 128) | ||||||
| Effect size | 0.75 *** | 0.41 ** | 0.66 *** | 0.67 *** | 0.34 ** | 0.27 |
| 95% CI | (0.40, 1.09) | (0.07, 0.76) | (0.27, 1.04) | (0.36, 0.98) | (0.03, 0.66) | (−0.09, 0.62) |
| Children with dietary diversity score > median at baseline (n = 209) | ||||||
| Effect size | 0.20 | 0.27 * | 0.22 | 0.13 | 0.16 | 0.32 ** |
| 95% CI | (−0.07, 0.47) | (−0.01, 0.55) | (−0.06, 0.49) | (−0.14, 0.39) | (−0.12, 0.43) | (0.03, 0.61) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masuda, K.; Chitundu, M. Multiple Micronutrient Supplementation Using Spirulina platensis during the First 1000 Days is Positively Associated with Development in Children under Five Years: A Follow up of A Randomized Trial in Zambia. Nutrients 2019, 11, 730. https://doi.org/10.3390/nu11040730
Masuda K, Chitundu M. Multiple Micronutrient Supplementation Using Spirulina platensis during the First 1000 Days is Positively Associated with Development in Children under Five Years: A Follow up of A Randomized Trial in Zambia. Nutrients. 2019; 11(4):730. https://doi.org/10.3390/nu11040730
Chicago/Turabian StyleMasuda, Kazuya, and Maureen Chitundu. 2019. "Multiple Micronutrient Supplementation Using Spirulina platensis during the First 1000 Days is Positively Associated with Development in Children under Five Years: A Follow up of A Randomized Trial in Zambia" Nutrients 11, no. 4: 730. https://doi.org/10.3390/nu11040730
APA StyleMasuda, K., & Chitundu, M. (2019). Multiple Micronutrient Supplementation Using Spirulina platensis during the First 1000 Days is Positively Associated with Development in Children under Five Years: A Follow up of A Randomized Trial in Zambia. Nutrients, 11(4), 730. https://doi.org/10.3390/nu11040730





