Exploring Common Culinary Herbs and Spices as Potential Anti-Quorum Sensing Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Extraction
2.2. Test Bacteria and Culture Media
2.3. Determination of the Antimicrobial Activities of Extracts
2.4. Determination of the Minimum Inhibitory Concentration
2.5. Preliminary Screening of Anti-Quorum Sensing (AQS) Activity
2.6. Quantitative AQS Activity
2.7. Identification of Major Active Compounds and Their AQS Activity
2.7.1. High Performance Thin Layer Chromatography (HPTLC) Analysis
2.7.2. HPTLC-Bio-Autography Assay
2.7.3. Identification of the Target Compounds Using UHPLC-MS Analysis
2.7.4. Isolation and Identification of the Active Compound
3. Results and Discussion
3.1. Crude Extract Yields
3.2. Qualitative Evaluation of AQS Activity
3.3. Determination of MICs
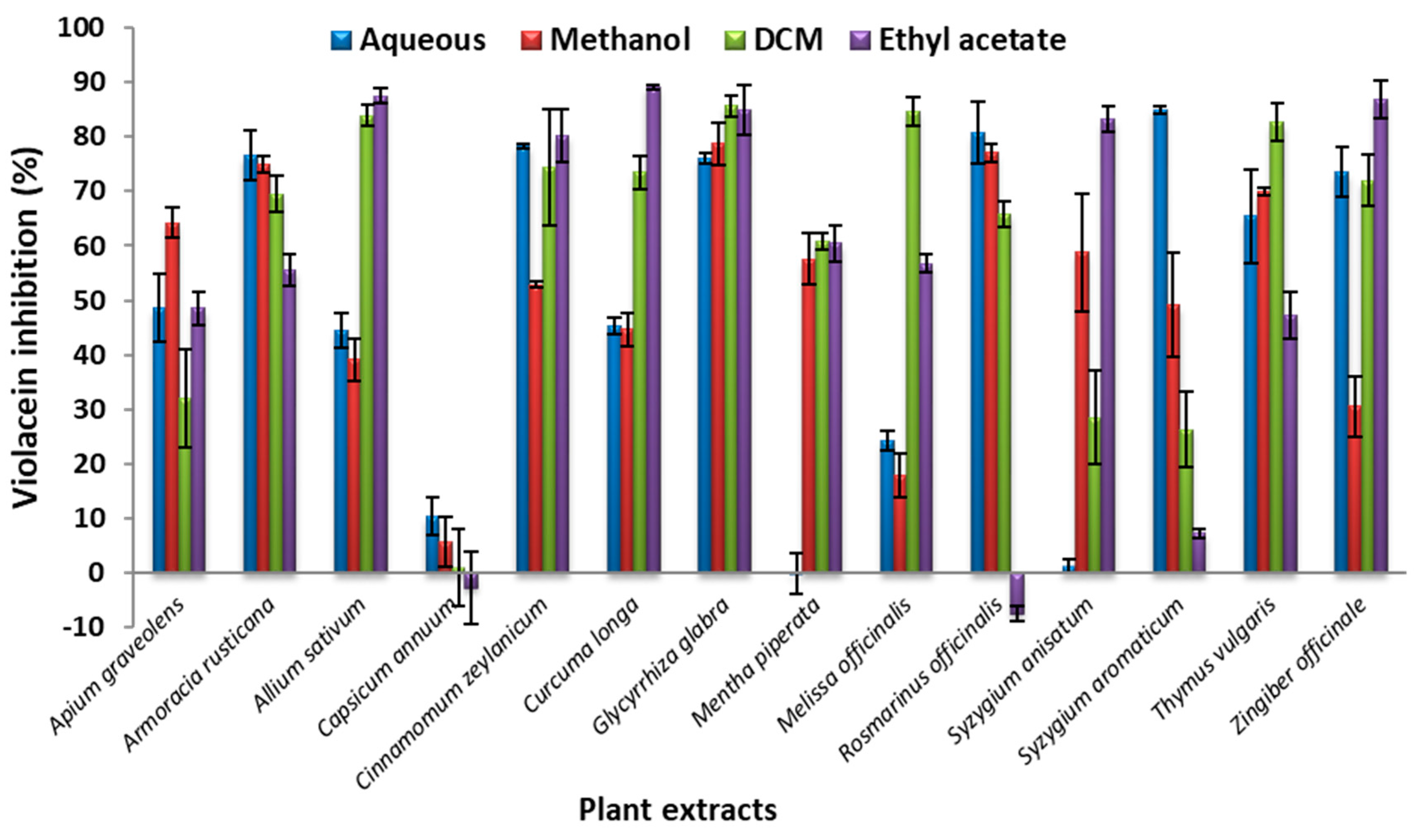
3.4. Quantitative Evaluation of AQS Activity
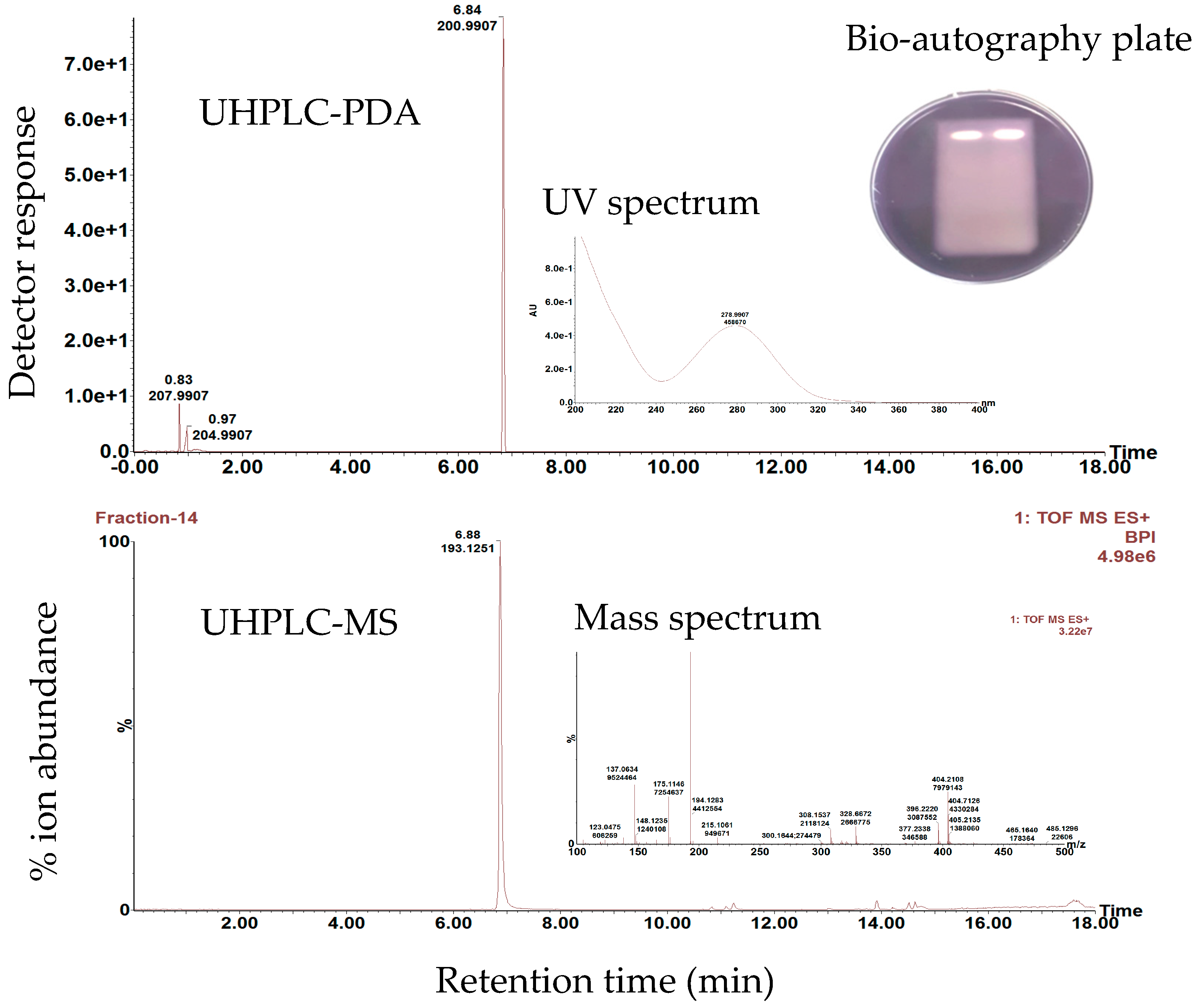
3.5. Identification and Isolation of Secondary Metabolites in Celery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AHL | acylhomoserine lactone |
| AQS | anti-quorum sensing |
| ATCC | American Type Culture Collection |
| DCM | Dichloromethane |
| DMSO | dimethyl sulfoxide |
| GRAS | Generally Regarded As Safe |
| HPTLC | high performance thin layer chromatography |
| I | Intermediate |
| INT | p-iodonitrotetrazolium violet |
| LB | Luria Bertani |
| LC-MS | liquid chromatography-mass spectrometry |
| MIC | minimum inhibitory concentration |
| NA | not active |
| PDA | photodiode array |
| prep-HPLC-MS | preparative high performance liquid chromatography-mass spectrometry |
| QS | quorum sensing |
| R | Resistant |
| Rf | retardation factor |
| S | Susceptible |
| UHPLC-QToF-MS | ultra high performance liquid chromatography-quadrupole Time-of-Flight-mass spectrometry |
| USFDA | United States food and Drug Administration |
References
- Asowata-Ayodele, A.M.; Afolayan, A.J.; Otunola, G.A. Ethnobotanical survey of culinary herbs and spices used in the traditional medicinal system of Nkonkobe Municipality, Eastern Cape, South Africa. S. Afr. J. Bot. 2016, 104, 69–75. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Rinaldi Alvarenga, J.F.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef]
- Weerakkody, N.S.; Caffin, N.; Turner, M.S.; Dykes, G.A. In vitro antimicrobial activity of less-utilized spice and herb extracts against selected food-borne bacteria. Food Control 2010, 21, 1408–1414. [Google Scholar] [CrossRef]
- Ariaee, N.; Vahedi, Z.; Vahedi, F. Inhibitory effects of cinnamon-water extract on human tumor cell lines. Asian Pac. J. Trop. Dis. 2014, 4, S975–S978. [Google Scholar] [CrossRef]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants—A mini review. J. Funct. Food 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.; Brooks, J.D.; Corke, H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol. 2007, 117, 112–119. [Google Scholar] [CrossRef]
- Oonmetta-aree, J.; Suzuki, T.; Gasaluck, P.; Eumkeb, G. Antimicrobial properties and action of galangal (Alpinia galangal Linn.) on Staphylococcus aureus. LTW Food Sci. Technol. 2006, 39, 1214–1220. [Google Scholar] [CrossRef]
- Kong, W.; Wei, R.; Logrieco, A.I.; Wei, J.; Wen, J.; Xiao, X.; Yang, M. Occurrence of toxigenic fungi and determination of mycotoxins by HPLC-FLD in functional foods and spices in China markets. Food Chem. 2014, 146, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Kaefer, M.C.; Milner, J.A. The role of herbs and spices in cancer prevention. J. Nutr. Biochem. 2008, 19, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Low Dog, T. A reason to season: The therapeutic benefits of spices and culinary herbs. Explore 2006, 2, 446–449. [Google Scholar] [CrossRef]
- Karadas, C.; Kara, D. Chemometric approach to evaluate trace metal concentrations in some spices and herbs. Food Chem. 2012, 130, 196–202. [Google Scholar] [CrossRef]
- Gonzalez, J.E.; Keshavan, N.D. Messing with bacterial quorum sensing. Microbiol. Mol. Biol. Rev. 2006, 70, 859–875. [Google Scholar] [CrossRef]
- Keshavan, N.D.; Chowdary, P.K.; Haines, D.C.; Gonzalez, J.E. L-canavanine made by Medicago sativa interferes with quorum sensing in Sinorhizobium meliloti. J. Bacteriol. 2005, 187, 8427–8436. [Google Scholar] [CrossRef]
- Truchado, P.; López-Gálvez, F.; Gil, M.I.; Tomás-Barberán, F.A.; Allende, A. Quorum sensing inhibitory and antimicrobial activities of honeys and the relationship with individual phenolics. Food Chem. 2009, 115, 1337–1344. [Google Scholar] [CrossRef]
- Kasote, D.; Ahmad, A.; Chen, W.; Combrinck, S.; Viljoen, A. HPTLC-MS as an efficient hyphenated technique for the rapid identification of antimicrobial compounds from propolis. Phytochem. Lett. 2015, 11, 326–331. [Google Scholar] [CrossRef]
- Nalina, T.; Rahim, A. The crude extract of Piper betel L. and its antibacterial effect towards Streptococcus mutans. Am. J. Biochem. Biotechnol. 2007, 3, 10–15. [Google Scholar]
- Ng, W.L.; Perez, L.; Cong, J.; Semmelhack, M.F.; Bassler, B.L. Broad spectrum pro-quorum-sensing molecules as inhibitors of virulence in Vibrios. PLoS Pathog. 2012, 8, e1002767. [Google Scholar] [CrossRef]
- Issac Abraham, S.V.P.; Palani, A.; Ramaswamy, B.R.; Shunmugiah, K.P.; Arumgam, V.R. Anti-quorum sensing and anti-biofilm potential of Capparis spinosa. Arch. Med. Res. 2011, 42, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.L.; Sam, C.K.; Yin, W.F.; Tan, L.Y.; Krishnan, T.; Chong, Y.M.; Chan, K. Plant-derived natural products as sources of anti-quorum sensing compounds. Sensors 2013, 13, 6217–6228. [Google Scholar] [CrossRef]
- Vasavi, H.S.; Arun, A.B.; Rekha, P.D. Anti-quorum sensing activity of flavonoid-rich fraction from Centella asiatica L. against Pseudomonas aeruginosa PAO1. J. Microbiol. Immunol. Inf. 2016, 49, 8–15. [Google Scholar] [CrossRef]
- Fenwick, G.R.; Hanley, A.B. The genus Allium. Part 2. Crit. Rev. Food Sci. Nutr. 1985, 22, 273–377. [Google Scholar]
- Abu-Lafi, S.; Dembitsky, J.W.; Goldshlag, P.; Hanus, L.; Dembitsky, V.M. The use of the ‘Cryogenic’ GC-MS and on-column injection for study of organosulfur compounds of the Allium sativum. J. Food Compos. Anal. 2004, 17, 235–245. [Google Scholar] [CrossRef]
- Jorge, V.G.; Ángel, J.R.; Adrián, T.S.; Francisco, A.C.; Anuar, S.G.; Samuel, E.S.; Ángel, S.O.; Emmanuel, H.N. Vasorelaxant activity of extracts obtained from Apium graveolens: Possible source for vasorelaxant molecules isolation with potential antihypertensive effect. Asian Pac. J. Trop. Biomed. 2013, 3, 776–779. [Google Scholar] [CrossRef]
- Hussain, I.M.T.; Ahmed, G.; Jahan, N.; Adiba, M. Unani description of Tukhme Karafs (seeds of Apium graveolens Linn) and its scientific reports. Int. Res. J. Biol. Sci. 2013, 2, 88–93. [Google Scholar]
- Bladh, W.K.; Olsson, K.M. Introduction and use of horseradish (Armoracia rusticana) as food and medicine from antiquity to the present: Emphasis on the Nordic countries. J. Herbs Spices Med. Plants 2011, 17, 197–213. [Google Scholar] [CrossRef]
- Tolan, I.; Ragoobirsingh, D.; Morrison, E.Y. Isolation and purification of the hypoglycaemic principle present in Capsicum frutescens. Phytother. Res. 2004, 18, 95–96. [Google Scholar] [CrossRef]
- Tundis, R.; Federica, M.; Marco, B.; Filomena, C.; Giancarlo, S.; Francesco, M.; Loizzo, M. Antioxidant and hypoglycaemic activities and their relationship to phytochemicals in Capsicum annuum cultivars during fruit development. LWT Food Sci. Technol. 2013, 53, 370–377. [Google Scholar] [CrossRef]
- Labban, L. Medicinal and pharmacological properties of turmeric (Curcuma longa): A review. Int. J. Pharm. Biomed. Sci. 2014, 5, 17–23. [Google Scholar]
- Chandrasekaran, C.V.; Deepak, H.B.; Thiyagarajan, P.; Kathiresan, S.; Sangli, G.K.; Deepak, M.; Amit, A. Dual inhibitory effect of Glycyrrhiza glabra (GutGard™) on COX and LOX products. Phytomedicine 2011, 18, 278–284. [Google Scholar] [CrossRef]
- Weitzel, C.; Petersen, M. Cloning and characterisation of rosmarinic acid synthase from Melissa officinalis L. Phytochemistry 2011, 72, 572–578. [Google Scholar] [CrossRef]
- Paul, R.; Data, A.K. An updated overview on peppermint (Mentha piperita L.). Int. Res. J. Pharm. 2011, 2, 1–10. [Google Scholar]
- Ventura-Martinez, R.; Rivero-Osorno, O.; Gomez, C.; González-Trujano, E.M. Spasmolytic activity of Rosmarinus officinalis L. involves calcium channels in the guinea pig ileum. J. Ethnopharmacol. 2011, 137, 528–532. [Google Scholar] [CrossRef]
- Hossain, M.A.; Harbi, S.R.A.; Weli, A.M.; Al-Riyami, Q.; Al-Sabahi, J. Comparison of chemical constituents and antimicrobial activities of three essential oils from three different brands’ clove samples collected from Gulf region. Asian Pac. J. Trop. Dis. 2014, 4, 262–268. [Google Scholar] [CrossRef]
- Dragland, S.; Senoo, H.; Wake, K. Several culinary and medicinal herbs are important sources of dietary antioxidants. J. Nutr. 2003, 133, 1286–1290. [Google Scholar] [CrossRef]
- Alqareer, A.; Alyahya, A.; Andersson, L. The effect of clove and benzocaine versus placebo as topical anesthetics. J. Dent. 2006, 34, 747–750. [Google Scholar] [CrossRef]
- Kozics, K.; Klusová, V.; Srančíková, A.; Mučaji, P.; Slameňová, D.; Hunáková, L.; Horváthová, E. Effects of Salvia officinalis and Thymus vulgaris on oxidant induced DNA damage and antioxidant status in HepG2 cells. Food Chem. 2013, 141, 2198–2206. [Google Scholar] [CrossRef]
- Karuppiah, P.; Rajaram, S. Antibacterial effect of Allium sativum cloves and Zingiber officinale rhizomes against multiple-drug resistant clinical pathogens. Asian Pac. J. Trop. Biomed. 2012, 2, 597–601. [Google Scholar] [CrossRef]
- Khan, M.S.; Zahin, M.; Hasan, S.; Husain, F.M.; Ahmad, I. Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to clove oil. Lett. Appl. Microbiol. 2009, 49, 354–360. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; CLSI: Wayne, PA, USA, 2007; Volume 27. [Google Scholar]
- EUCAST (European Committee for Antimicrobial Susceptibility Testing). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 9, 1–7. [Google Scholar]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef]
- McLean, R.J.; Pierson, L.S.; Fuqua, C. A simple screening protocol for the identification of quorum signal antagonists. J. Microbiol. Methods 2004, 58, 351–360. [Google Scholar] [CrossRef]
- McClean, K.H.; Winson, M.K.; Fish, L.; Taylor, A.; Chhabra, S.R.; Camara, M.; Daykin, M.; Lamb, J.H.; Swift, S.; Bycroft, B.W.; et al. Quorum sensing and Chromobacterium violaceum: Exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 1997, 143, 3703–3711. [Google Scholar] [CrossRef] [PubMed]
- Alkuraishy, H.M. Evaluation the antibacterial activity of aniseed: In vitro study. J. Clin. Res. Healthc. Man 2012, 3, 1–7. [Google Scholar]
- Alzoreky, N.S.; Nakahara, K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int. J. Food Microbiol. 2003, 80, 223–230. [Google Scholar] [CrossRef]
- Van Vuuren, S.F. Antimicrobial activity of South African medicinal plants. J. Ethnopharmacol. 2008, 119, 462–472. [Google Scholar] [CrossRef]
- Packiavathy, I.A.S.V.; Agilandeswari, P.; Musthafa, K.S.; Pandian, S.K.; Ravi, A.V. Antibiofilm and quorum sensing inhibitory potential of Cuminum cyminum and its secondary metabolite methyl eugenol against Gram negative bacterial pathogens. Food Res. Int. 2012, 45, 85–92. [Google Scholar] [CrossRef]
- Chenia, H.Y. Anti-quorum sensing potential of crude Kigelia africana fruit extracts. Sensors 2013, 13, 2802–2817. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhao, M.; Zhao, T.; Feng, M.; Chen, H.; Zhuang, M.; Lin, L. Bioactive profiles, antioxidant activities, nitrite scavenging capacities and protective effects on h2o2-injured pc12 cells of Glycyrrhiza glabra L. leaf and root extracts. Molecules 2014, 19, 9101–9113. [Google Scholar] [CrossRef]
- Dong, Y.H.; Zhang, L.H. Quorum sensing and quorum-quenching enzymes. J. Microbiol. 2005, 43, 101–109. [Google Scholar] [PubMed]
- Jakobsen, T.M.; van Gennip, M.; Phipps, R.K.; Shanmugham, M.S.; Christensen, L.D.; Alhede, M.; Skinersoe, M.E.; Rasmussen, T.B.; Friedrich, K.; Uthe, F.; et al. Ajoene, a sulphur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob. Agents Chemother. 2012, 56, 2314–2325. [Google Scholar] [CrossRef]
- Choo, J.H.; Rukayadi, Y.; Hwang, J.K. Inhibition of bacterial quorum sensing by vanilla extract. Lett. Appl. Microbiol. 2006, 42, 637–641. [Google Scholar] [CrossRef]
- Vattem, D.A.; Mihalik, K.; Crixell, S.H.; McLean, R.J. Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia 2007, 78, 302–310. [Google Scholar] [CrossRef]
- Krishnan, T.; Yin, W.F.; Chan, K.G. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa PAO1 by ayurveda spice clove (Syzygium aromaticum) bud extract. Sensors 2012, 12, 4016–4030. [Google Scholar] [CrossRef]
- Adonizio, A.L.; Downum, K.; Bennett, B.C.; Mathee, K. Anti-quorum sensing activity of medicinal plants in southern Florida. J. Ethnopharmacol. 2006, 105, 427–435. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, Y.; Hartman, T.G.; Rosen, R.T.; Ho, C.T. Free and glycosidically bound volatile compounds in fresh celery (Apium graveolens L.). J. Agric. Food Chem. 1990, 38, 1937–1940. [Google Scholar] [CrossRef]
- Ahluwalia, V.K.; Boyda, D.R.; Jain, A.K.; Khanduri, C.H.; Sharma, N.D. Furanocoumarin glucosides from the seeds of Apium graveolens. Phytochemistry 1988, 27, 1181–1183. [Google Scholar] [CrossRef]
- Kurobayashi, Y.; Kouno, E.; Fujita, A.; Morimitsu, Y.; Kubota, K. Potent odorants characterize the aroma quality of leaves and stalks in raw and boiled celery. Biosci. Biotechnol. Biochem. 2006, 70, 958–965. [Google Scholar] [CrossRef]
- Mihalik, K.; Chung, D.W.; Crixell, S.H.; McLean, R.J.C.; Vattem, D.A. Quorum sensing modulators of Pseudomonas aeruginosa characterized in Camellia sinensis. Asian J. Tradit. Med. 2008, 3, 12–23. [Google Scholar]
- Givskov, M.; de Nys, R.; Manefield, M.; Gram, L.; Maximilien, R.; Eberl, L.; Molin, S.; Steinberg, P.D.; Kjelleberg, S. Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J. Bacteriol. 1996, 178, 6618–6622. [Google Scholar] [CrossRef]
- Manefield, M.; de Nys, R.; Kumar, N.; Read, R.; Givskov, M.; Steinberg, P.; Kjelleberg, S. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology 1999, 145, 283–291. [Google Scholar] [CrossRef]

| Species Name | Family Name | Common Name | Medicinal Use | Reference |
|---|---|---|---|---|
| Allium sativum | Amaryllidaceae | Garlic | Treatment of various disorders such as respiratory ailments, asthma, pneumonia, diabetes, cardiovascular disorders and rheumatism | [22,23] |
| Apium graveolens | Apiaceae | Celery | Treat toothache, diarrhea, hypertension and-pulmonary disease. Used as stimulant, cardiac tonic, carminative, diuretic and antiseptic | [24,25] |
| Armoracia rusticana | Brassicaceae | Horse-radish | Cough, lung and heart disease, diuretic, digestion, wounds, shortness of breath, stomach problems, bronchitis, headache, high blood pressure and rheumatism | [26] |
| Capsicum annuum | Solanaceae | Cayenne pepper | Use in traditional medicine to alleviate gastric ulcers, rheumatism, alopecia, toothache and diabetes | [27,28] |
| Cinnamomum zeylanicum | Lauraceae | Cinnamon | Benefit for common cold, cardiovascular, neurodegenerative diseases and gastrointestinal disorders | [4] |
| Curcuma longa | Zingiberaceae | Turmeric | Anti-inflammatory and for the treatment of jaundice, menstrual difficulties, hematuria, hemorrhage, and colic. Applied topically for urticaria and skin allergy, viral hepatitis, inflammatory conditions of joints, sore throat and wounds | [29] |
| Glycyrrhiza glabra | Fabaceae | Liquorice | Used in medicines for its unique and diverse pharmacological properties viz., antiviral, anticancer, anti-ulcer, anti-diabetic, anti-inflammatory, anti-oxidant, immuno-stimulant, anti-allergenic | [30] |
| Melissa officinalis | Lamiaceae | Lemon balm | Treat infections of Herpes simplex | [31] |
| Mentha piperita | Lamiaceae | Peppermint (wild) | Used to treat coughs, bronchitis, inflammation of oral mucosa and throat, pulmonary tuberculosis, digestive complaints such as colic in infants, flatulence, diarrhea, indigestion, nausea, morning sickness and anorexia | [32] |
| Rosmarinus officinalis | Lamiaceae | Rosemary | Relief pain in renal colic and dysmenorrhoea, and as antispasmodic diuretic, antipyretic and as a mood stabilizer | [33] |
| Syzygium anisatum | Myrtaceae | Aniseed | Sedative and stimulant in cough medicines | [34] |
| Syzygium aromaticum | Myrtaceae | Cloves | Used to treat indigestion, flatulence, nausea, vomiting, diarrhea, cough, infertility, warts, hernias, ringworm, wounds, toothaches, athletes foot and other fungal infections | [34,35,36] |
| Thymus vulgaris | Lamiaceae | Thyme | Bronchial asthma, inflammatory affection, hepatotoxicity, atherosclerosis, ischaemic heart disease, cataracts, cancer, insufficient sperm mobility | [37] |
| Zingiber officinale | Zingiberaceae | Ginger | Used for cold-induced diseases, nausea, asthma, cough, colic, heart palpitation, swelling, dyspepsia, less appetite and rheumatism | [38] |
| Plants | Solvents | |||
|---|---|---|---|---|
| Aqueous | Methanol | DCM | Ethyl Acetate | |
| Allium sativum | 19.1% | 10.5% | 1.7% | 1.7% |
| Apium graveolens | 3.0% | 4.1% | 10.0% | 7.5% |
| Armoracia rusticana | 8.3% | 8.6% | 6.0% | 1.3% |
| Capsicum annuum | 13.9% | 6.4% | 6.7% | 3.3% |
| Cinnamomum zeylanicum | 4.0% | 3.3% | 3.1% | 1.7% |
| Curcuma longa | 3.3% | 7.5% | 2.8% | 2.2% |
| Glycyrrhiza glabra | 5.6% | 18.2% | 4.8% | 1.3% |
| Melissa officinalis | 8.3% | 11.1% | 6.6% | 10.8% |
| Mentha piperita | 10.8% | 19.5% | 1.7% | 4.3% |
| Rosmarinus officinalis | 8.2% | 10.6% | 8.4% | 6.0% |
| Syzygium anisatum | 10.1% | 4.8% | 5.4% | 5.3% |
| Syzygium aromaticum | 13.2% | 6.4% | 5.6% | 3.8% |
| Thymus vulgaris | 11.4% | 11.5% | 6.4% | 6.1% |
| Zingiber officinale | 7.0% | 6.4% | 3.3% | 5.8% |
| Plant Pecies | Zone Diameters (mm) and Associated Susceptibility Phenotypes | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aqueous Extract | Methanol Extract | Dichloromethane Extract | Ethyl Acetate Extract | |||||||||||||
| Concentration (mg/mL) | ||||||||||||||||
| 0.350 | 1.75 | 3.85 | 7.00 | 0.350 | 1.75 | 3.85 | 7.00 | 0.350 | 1.75 | 3.85 | 7.00 | 0.350 | 1.75 | 3.85 | 7.00 | |
| Allium sativum | 11(I) | 11(I) | 12(I) | 13(I) | 10(R) | 10(R) | 10(R) | 10(R) | 11(I) | 12(I) | 12(I) | 12(I) | 11(I) | 11(I) | 12(I) | 12(I) |
| Apium graveolens | 10(R) | 10(R) | 11(I) | 11(I) | 0(R) | 0(R) | 0(R) | 0(R) | 11(I) | 12(I) | 12(I) | 12(I) | 0(R) | 0(R) | 0(R) | 0(R) |
| Armoracia rusticana | 0(R) | 10(R) | 11(I) | 11(I) | 12(I) | 11(I) | 11(I) | 11(I) | 12(I) | 13(I) | 13(I) | 12 (I) | 10(R) | 11(I) | 12(I) | 14(S) |
| Capsicum annuum | 10(R) | 10(R) | 10(R) | 10(R) | 12(I) | 12(I) | 13(I) | 13(I) | 11(I) | 12(I) | 12(I) | 13(I) | 11(I) | 12(I) | 12(I) | 13(I) |
| Cinnamomum zeylanicum | 11(I) | 12(I) | 11(I) | 11(I) | 11(I) | 11(I) | 12(I) | 12(I) | 11(I) | 12(I) | 12(I) | 14(S) | 11(I) | 12(I) | 13(I) | 12(I) |
| Curcuma longa | 10(R) | 11(I) | 12(I) | 12(I) | 10(R) | 11(I) | 12(I) | 12(I) | 10(R) | 11(I) | 12(I) | 12(I) | 10(R) | 11(I) | 12(I) | 12(I) |
| Glycyrrhiza glabra | 10(R) | 11(I) | 12(I) | 13(I) | 15(S) | 15(S) | 15(S) | 15(S) | 10(R) | 10(R) | 10(R) | 11(I) | 13(I) | 13(I) | 13(I) | 13(I) |
| Melissa officinalis | 11(I) | 12(I) | 15(S) | 15(S) | 11(I) | 11(I) | 11(I) | 11(I) | 10(R) | 10(R) | 10(R) | 10(R) | 11(I) | 11(I) | 11(I) | 11(I) |
| Mentha piperita | 11(I) | 12(I) | 12(I) | 12(I) | 10(R) | 11(I) | 12(I) | 12(I) | 11(I) | 12(I) | 12(I) | 12(I) | 11(I) | 12(I) | 12(I) | 12(I) |
| Rosmarinus officinalis | 10(R) | 11(I) | 11(I) | 12(I) | 11(I) | 11(I) | 13(I) | 13(I) | 11(I) | 11(I) | 13(I) | 13(I) | 11(I) | 11(I) | 10(R) | 10(R) |
| Syzygium anisatum | 12(I) | 13(I) | 13(I) | 12(I) | 11(I) | 12(I) | 13(I) | 16(S) | 11(I) | 11(I) | 13(I) | 14(S) | 11(I) | 11(I) | 11(I) | 14(S) |
| Syzygium anisatum | 11(I) | 12(I) | 12(I) | 12(I) | 11(I) | 12(I) | 11(I) | 11(I) | 0(R) | 11(I) | 11(I) | 11(I) | 12(I) | 12(I) | 12(I) | 12(I) |
| Thymus vulgaris | 10(R) | 10(R) | 12(I) | 13(I) | 10(R) | 10(R) | 10(R) | 12(I) | 11(I) | 11(I) | 11(I) | 11(I) | 10(R) | 10(R) | 10(R) | 10(R) |
| Zingiber officinale | 11(I) | 12(I) | 13(I) | 13(I) | 12(I) | 12(I) | 12(I) | 14(S) | 12(I) | 13(I) | 13(I) | 13(I) | 12(I) | 13(I) | 13(I) | 13(I) |
| CIP5 # | 31(S) | 30(S) | 30(S) | 30(S) | ||||||||||||
| Eugenol | 12(I) | 22(S) | 24(S) | 28(S) | ||||||||||||
| Plants | MIC (mg/mL) against CV12472 | Anti-QS Activities | ||||
|---|---|---|---|---|---|---|
| Extracts | Active Plant Extract | AQS Zone of Inhibition (mm) | ||||
| Aqueous | Methanol | DCM | Ethyl Acetate | |||
| Allium sativum | 4 | 4 | 4 | 4 | NA | NA |
| Apium graveolens | 4 | 4 | 4 | 4 | ethyl acetate; methanol | 14; 12–15 |
| Armoracia rusticana | 2 | 4 | 4 | 4 | aqueous | 12–14 |
| Capsicum annuum | 4 | 4 | 4 | 4 | DCM | 13–14 |
| Cinnamomum zeylanicum | 4 | 2 | 2 | 2 | NA | NA |
| Curcuma longa | 4 | 2 | 2 | 2 | NA | NA |
| Glycyrrhiza glabra | 2 | 2 | 2 | 2 | aqueous; methanol | 19; 12 |
| Melissa officinalis | 2 | 2 | 2 | 2 | methanol | 9 |
| Mentha piperita | 4 | 4 | 4 | 4 | NA | NA |
| Rosmarinus officinalis | 2 | 2 | 4 | 4 | ethyl acetate | 12–13 |
| Syzygium anisatum | 2 | 2 | 2 | 2 | ethyl acetate; DCM | 14; 13 |
| Syzygium aromaticum | 2 | 2 | 2 | 2 | methanol | 13 |
| Thymus vulgaris | 2 | 2 | 2 | 2 | ethyl acetate; methanol | 13; 12 |
| Zingiber officinale | 4 | 2 | 2 | 2 | NA | NA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosa, S.; Chaudhary, S.K.; Chen, W.; Combrinck, S.; Viljoen, A. Exploring Common Culinary Herbs and Spices as Potential Anti-Quorum Sensing Agents. Nutrients 2019, 11, 739. https://doi.org/10.3390/nu11040739
Cosa S, Chaudhary SK, Chen W, Combrinck S, Viljoen A. Exploring Common Culinary Herbs and Spices as Potential Anti-Quorum Sensing Agents. Nutrients. 2019; 11(4):739. https://doi.org/10.3390/nu11040739
Chicago/Turabian StyleCosa, Sekelwa, Sushil Kumar Chaudhary, Weiyang Chen, Sandra Combrinck, and Alvaro Viljoen. 2019. "Exploring Common Culinary Herbs and Spices as Potential Anti-Quorum Sensing Agents" Nutrients 11, no. 4: 739. https://doi.org/10.3390/nu11040739
APA StyleCosa, S., Chaudhary, S. K., Chen, W., Combrinck, S., & Viljoen, A. (2019). Exploring Common Culinary Herbs and Spices as Potential Anti-Quorum Sensing Agents. Nutrients, 11(4), 739. https://doi.org/10.3390/nu11040739





