Melatonin Improves Fatty Liver Syndrome by Inhibiting the Lipogenesis Pathway in Hamsters with High-Fat Diet-Induced Hyperlipidemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and the Experimental Design
2.2. Biochemical Parameter Measurements
2.3. Real-Time Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
2.4. Hepatic Fatty Acid Metabolic Enzyme Measurements
2.5. Histologic Analysis
2.6. Statistical Analysis
3. Results
3.1. Effects of Melatonin on BM and Tissue Weights
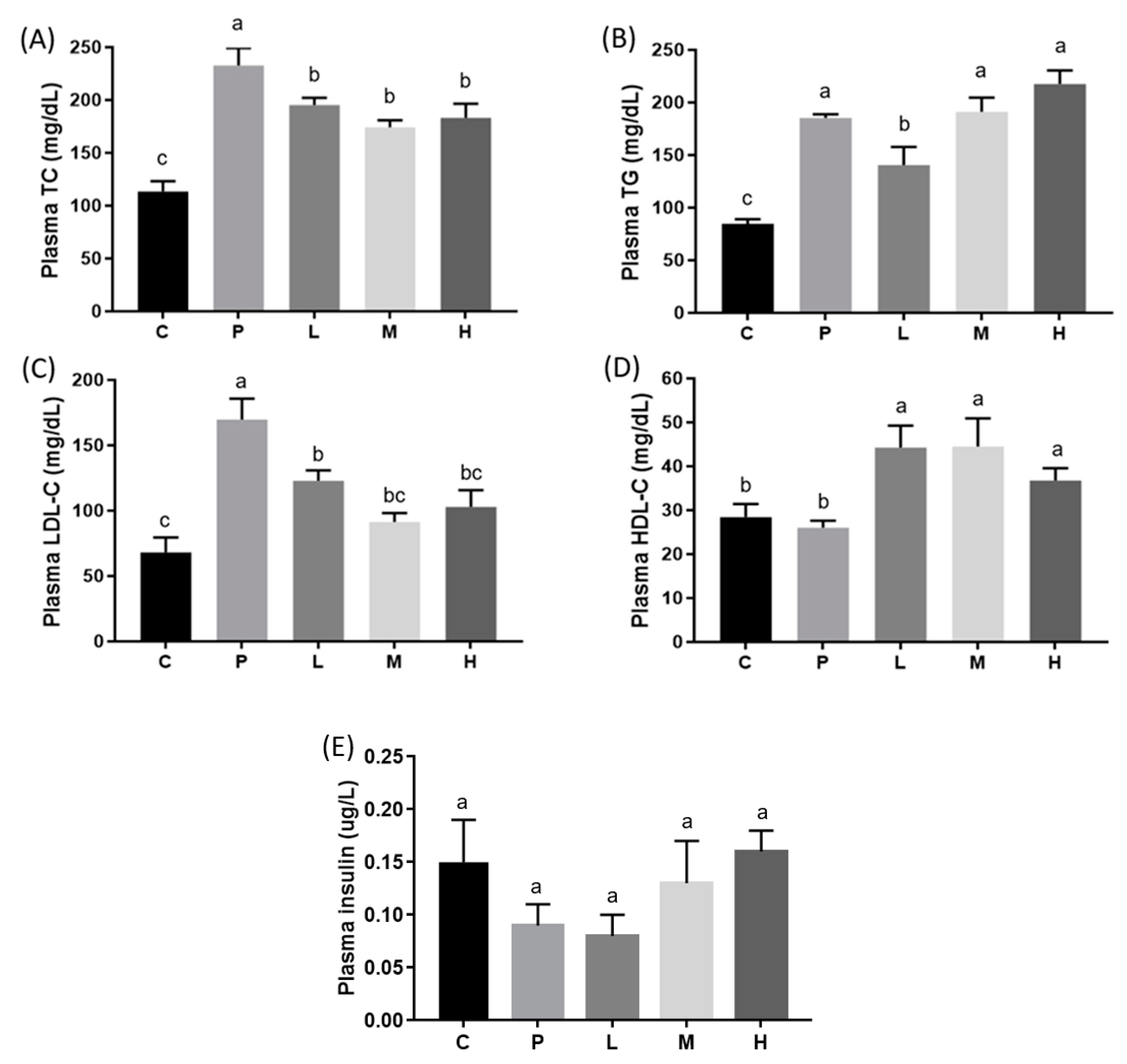
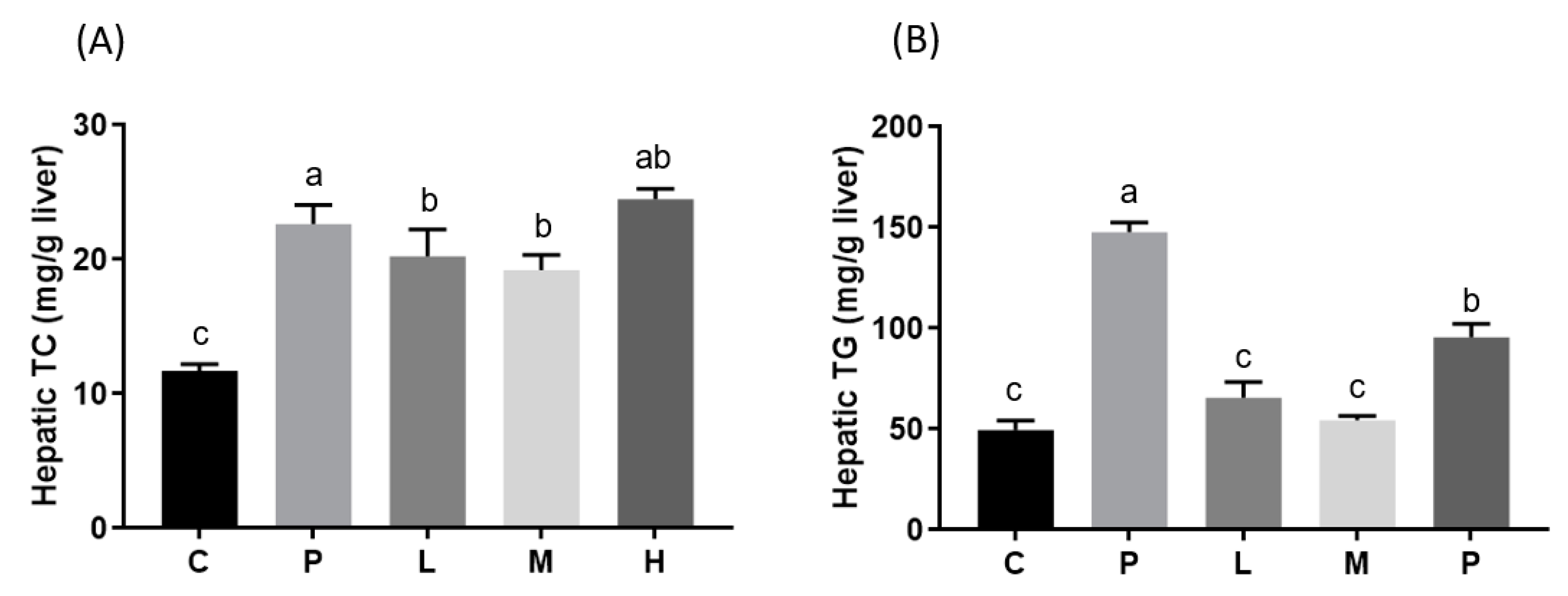
3.2. Effects of Melatonin on Blood and Hepatic Biochemical Parameters
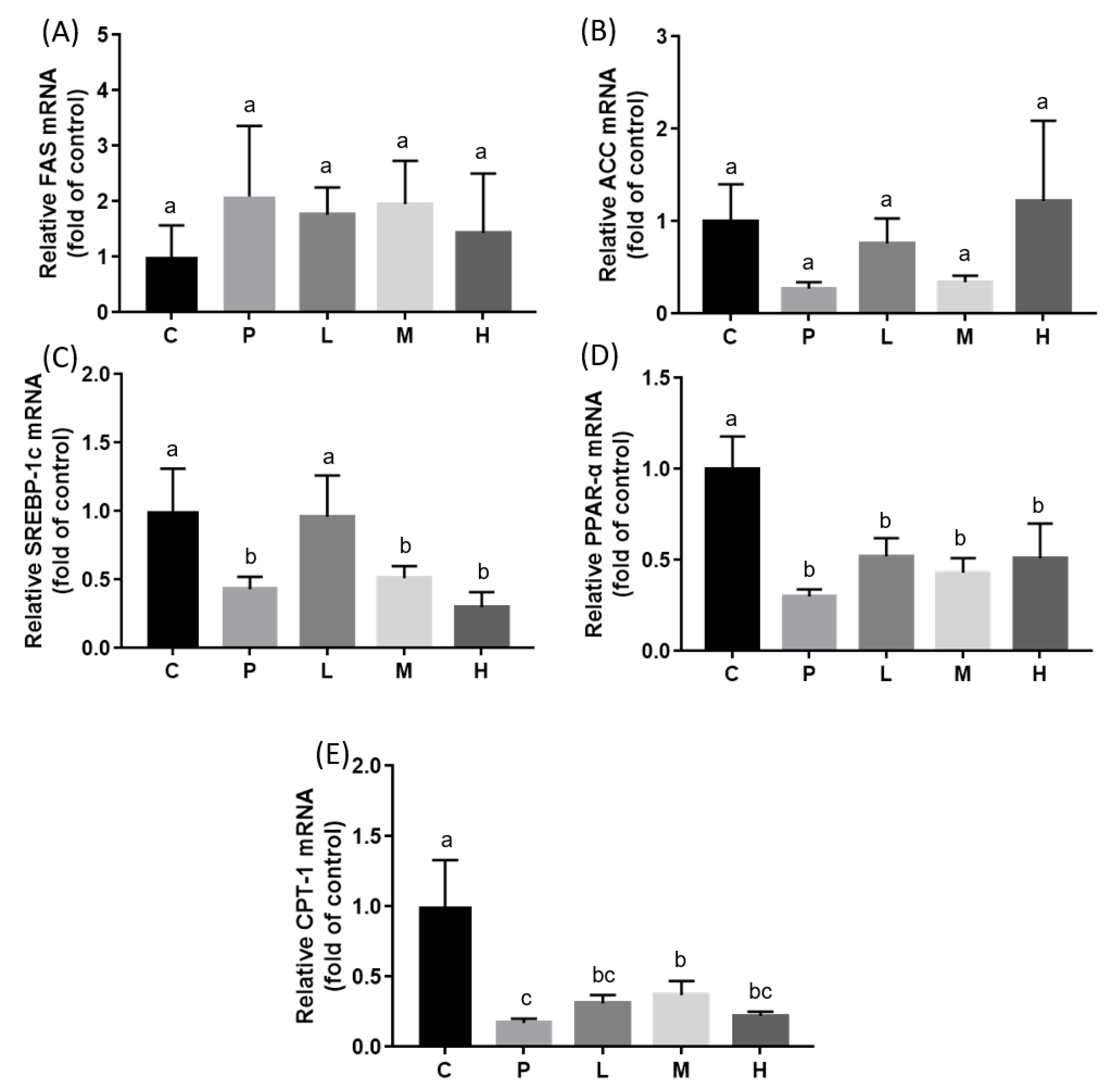
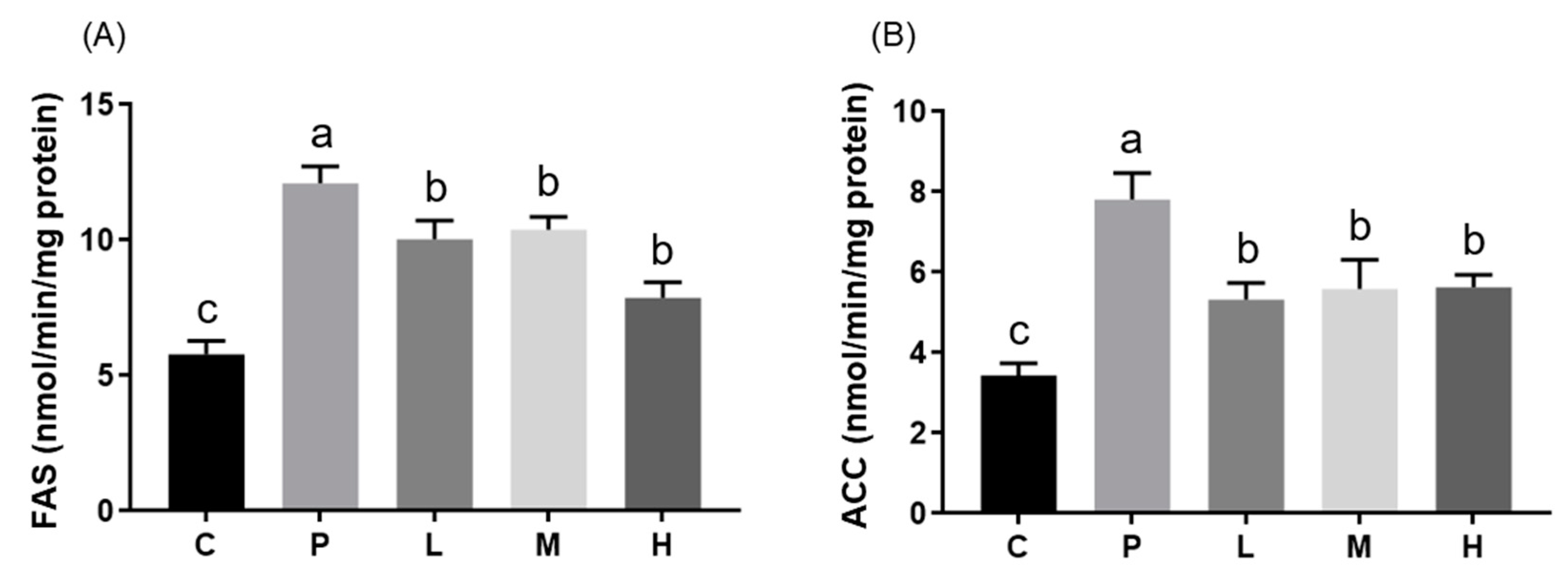
3.3. Effects of Melatonin on Hepatic mRNA Expressions and Enzyme Activities
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pi-Sunyer, F.X. The obesity epidemic: Pathophysiology and consequences of obesity. Obes. Res. 2002, 10, 97S–104S. [Google Scholar] [CrossRef]
- Kelly, T.; Yang, W.; Chen, C.S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Hardeland, R.; Lopez-Burillo, S.; Mayo, J.C.; Sainz, R.M.; Reiter, R.J. Melatonin: A hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J. Pineal. Res. 2003, 34, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocr. J. 1993, 1, 57–60. [Google Scholar]
- Ramis, M.R.; Esteban, S.; Miralles, A.; Tan, D.X.; Reiter, R.J. Protective effects of melatonin and mitochondria-targeted antioxidants against oxidative stress: A review. Curr. Med. Chem. 2015, 22, 2690–2711. [Google Scholar] [CrossRef] [PubMed]
- Rozov, S.V.; Filatova, E.V.; Orlov, A.A.; Volkova, A.V.; Zhloba, A.R.; Blashko, E.L.; Pozdeyev, N.V. N1-acetyl-N2-formyl-5-methoxykynuramine is a product of melatonin oxidation in rats. J. Pineal. Res. 2003, 35, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M.; Hardeland, R. The melatonin metabolite N1-acetyl-5-methoxykynuramine is a potent singlet oxygen scavenger. J. Pineal Res. 2009, 46, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.; Afeche, S.C.; Tan, D.X.; Reiter, R.J. Melatonin, energy metabolism, and obesity: A review. J. Pineal Res. 2014, 56, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.B.; Machado, U.F.; Bartol, I.; Seraphim, P.M.; Sumida, D.H.; Moraes, S.M.; Hell, N.S.; Okamoto, M.M.; Saad, M.J.; Carvalho, C.R.; et al. Pinealectomy causes glucose intolerance and decreases adipose cell responsiveness to insulin in rats. Am. J. Physiol. 1998, 275, E934–E941. [Google Scholar] [CrossRef]
- Nogueira, T.C.; Lellis-Santos, C.; Jesus, D.S.; Taneda, M.; Rodrigues, S.C.; Amaral, F.G.; Lopes, A.M.S.; Cipolla-Neto, J.; Bordin, S.; Anhê, G.F. Absence of melatonin induces night-time hepatic insulin resistance and increased gluconeogenesis due to stimulation of nocturnal unfolded protein response. Endocrinology 2011, 152, 1253–1263. [Google Scholar] [CrossRef]
- Prunet-Marcassus, B.; Desbazeille, M.; Bros, A.; Louche, K.; Delagrange, P.; Renard, P.; Casteilla, L.; Pénicaud, L. Melatonin reduces body weight gain in Sprague Dawley rats with diet-induced obesity. Endocrinology 2003, 144, 5347–5352. [Google Scholar] [CrossRef]
- Bravo, E.; Cantafora, A.; Calcabrini, A.; Ortu, G. Why prefer the golden Syrian hamster (Mesocricetus auratus) to the Wistar rat in experimental studies on plasma lipoprotein metabolism? Comp. Biochem. Physiol. B Comp. Biochem. 1994, 107, 347–355. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Numa, S.; Ringelmann, E.; Lynen, F. Zur Hemmung der Acetyl-CoA-Carboxylase durch Fettsaure-Coenzym A-Verbindungen. Biochem. Z. 1965, 343, 243–257. [Google Scholar]
- Wongchitrat, P.; Klosen, P.; Pannengpetch, S.; Kitidee, K.; Govitrapong, P.; Isarankura-Na-Ayudhya, C. High-fat diet-induced plasma protein and liver changes in obese rats can be attenuated by melatonin supplementation. Nutr. Res. 2017, 42, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Nduhirabandi, F.; Du Toit, E.F.; Blackhurst, D.; Marais, D.; Lochner, A. Chronic melatonin consumption prevents obesity-related metabolic abnormalities and protects the heart against myocardial ischemia and reperfusion injury in a prediabetic model of diet-induced obesity. J. Pineal Res. 2011, 50, 171–182. [Google Scholar] [CrossRef]
- Harpsøe, N.G.; Andersen, L.P.H.; Gögenur, I.; Rosenberg, J. Clinical pharmacokinetics of melatonin: A systematic review. Eur. J. Clin. Pharmacol. 2015, 71, 901–909. [Google Scholar] [CrossRef]
- Kitagawa, A.; Ohta, Y.; Ohashi, K. Melatonin improves metabolic syndrome induced by high fructose intake in rats. J. Pineal Res. 2012, 52, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.R. Effect of melatonin on cholesterol absorption in rats. J. Pineal Res. 2007, 42, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.; Tang, P. Effect of melatonin on the maintenance of cholesterol homeostasis in the rat. Endocr. Res. 1995, 21, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Mullerwieland, D.; Behnke, B.; Koopmann, K.; Krone, W. Melatonin inhibits LDL receptor activity and cholesterol-synthesis in freshly isolated human mononuclear leukocytes. Biochem. Biophys. Res. Commun. 1994, 203, 416–421. [Google Scholar] [CrossRef]
- Pan, M.; Song, Y.L.; Xu, J.M.; Gan, H.Z. Melatonin ameliorates nonalcoholic fatty liver induced by high-fat diet in rats. J. Pineal Res. 2006, 41, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Postic, C.; Girard, J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: Lessons from genetically engineered mice. J. Clin. Investig. 2008, 118, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, C.; Zhao, M.; Shi, C.E.; Zhu, R.M.; Wang, H.; Zhao, H.; Wei, W.; Li, J.B.; Xu, D.X. Melatonin alleviates lipopolysaccharide-induced hepatic SREBP-1c activation and lipid accumulation in mice. J. Pineal Res. 2011, 51, 416–425. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Pagano, G.; Pacini, G.; Musso, G.; Gambino, R.; Mecca, F.; Depetris, N.; Cassader, M.; David, E.; Cavallo-Perin, P.; Rizzetto, M. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: Further evidence for an etiologic association. Hepatology 2002, 35, 367–372. [Google Scholar] [CrossRef]
- Kainuma, M.; Fujimoto, M.; Sekiya, N.; Tsuneyama, K.; Cheng, C.; Takano, Y.; Terasawa, K.; Shimada, Y. Cholesterol-fed rabbit as a unique model of nonalcoholic, nonobese, non-insulin-resistant fatty liver disease with characteristic fibrosis. J. Gastroenterol. 2006, 41, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.I.; Yoon, D.W.; Yu, J.H.; Kim, N.H.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Choi, K.M.; Baik, S.H.; Choi, D.S. Melatonin improves insulin resistance and hepatic steatosis through attenuation of alpha-2-HS-glycoprotein. J. Pineal Res. 2018, e12493. [Google Scholar] [CrossRef]


| (g/kg Diet) | Control Diet | High-Fat Diet |
|---|---|---|
| Casein | 140 | 140 |
| L-Cysteine | 1.8 | 1.8 |
| Corn starch | 620.7 | 558.7 |
| Sucrose | 100 | 100 |
| Cellulose | 50 | 50 |
| Soybean oil | 40 | 40 |
| Lard | 0 | 110 |
| AIN-93 mineral mix | 35 | 35 |
| AIN-93 vitamin mix | 10 | 10 |
| Choline bitartrate | 2.5 | 2.5 |
| Cholesterol | 0 | 2 |
| Calories (kcal/kg diet) | 3850 | 4592 |
| C | P | L | M | H | |
|---|---|---|---|---|---|
| Initial BM (g) | 137.0 ± 3.1 | 142.2 ± 4.3 | 135.8 ± 3.7 | 142.8 ± 5.6 | 144.1 ± 6.0 |
| Final BM (g) | 141.2 ± 1.7 b | 159.4 ± 4.6 ab | 154.2 ± 6.3 ab | 153.9 ± 7.8 ab | 162.4 ± 9.1 a |
| Total weight gain (g) | 4.1 ± 2.3 b | 17.2 ± 3.5 a | 18.4 ± 3.5 a | 11.1 ± 6.8 ab | 18.3 ± 3.9 a |
| Food intake (g/day) | 7.7 ± 0.11 | 7.8 ± 0.22 | 7.3 ± 0.32 | 7.7 ± 0.15 | 7.3 ± 0.18 |
| Energy intake (kcal/day) | 29.8 ± 0.41 b | 36.0 ± 0.99 a | 33.7 ± 1.49 a | 35.5 ± 0.70 a | 33.7 ± 0.81 a |
| Feed efficiency (%) | 7.6 ± 4.2 b | 31.2 ± 6.3 ab | 34.8 ± 6.6 a | 20.5 ± 12.5 ab | 35.7 ± 7.6 a |
| Liver weight (g) | 3.97 ± 0.22 c | 8.25 ± 0.25 a | 6.80 ± 0.47 b | 7.15 ± 0.24 b | 7.92 ± 0.45 ab |
| Kidney weight (g) | 0.86 ± 0.02 | 0.92 ± 0.06 | 0.92 ± 0.04 | 0.95 ± 0.04 | 0.92 ± 0.03 |
| Inguinal fat weight (g) | 3.50 ± 0.40 | 4.46 ± 0.61 | 4.08 ± 0.76 | 4.51 ± 0.51 | 5.06 ± 0.64 |
| Epididymal fat weight (g) | 2.71 ± 0.14 | 4.13 ± 0.74 | 3.05 ± 0.36 | 3.13 ± 0.39 | 3.39 ± 0.35 |
| Retroperitoneal fat weight (g) | 2.10 ± 0.11 | 2.75 ± 0.24 | 2.30 ± 0.31 | 2.86 ± 0.22 | 2.57 ± 0.33 |
| Relative liver weight (g/100 g BM) | 2.82 ± 0.14 c | 5.20 ± 0.31 a | 4.35 ± 0.12 b | 4.49 ± 0.06 b | 4.90 ± 0.27 ab |
| Relative epididymal fat weight (g/100 g BM) | 1.93 ± 0.08 | 2.55 ± 0.41 | 1.94 ± 0.17 | 1.93 ± 0.19 | 2.09 ± 0.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, T.-H.; Tung, Y.-T.; Yang, T.-H.; Chien, Y.-W. Melatonin Improves Fatty Liver Syndrome by Inhibiting the Lipogenesis Pathway in Hamsters with High-Fat Diet-Induced Hyperlipidemia. Nutrients 2019, 11, 748. https://doi.org/10.3390/nu11040748
Ou T-H, Tung Y-T, Yang T-H, Chien Y-W. Melatonin Improves Fatty Liver Syndrome by Inhibiting the Lipogenesis Pathway in Hamsters with High-Fat Diet-Induced Hyperlipidemia. Nutrients. 2019; 11(4):748. https://doi.org/10.3390/nu11040748
Chicago/Turabian StyleOu, Tzu-Hsuan, Yu-Tang Tung, Ting-Hsuan Yang, and Yi-Wen Chien. 2019. "Melatonin Improves Fatty Liver Syndrome by Inhibiting the Lipogenesis Pathway in Hamsters with High-Fat Diet-Induced Hyperlipidemia" Nutrients 11, no. 4: 748. https://doi.org/10.3390/nu11040748
APA StyleOu, T.-H., Tung, Y.-T., Yang, T.-H., & Chien, Y.-W. (2019). Melatonin Improves Fatty Liver Syndrome by Inhibiting the Lipogenesis Pathway in Hamsters with High-Fat Diet-Induced Hyperlipidemia. Nutrients, 11(4), 748. https://doi.org/10.3390/nu11040748






