Nutraceuticals for the Treatment of Diabetic Retinopathy
Abstract
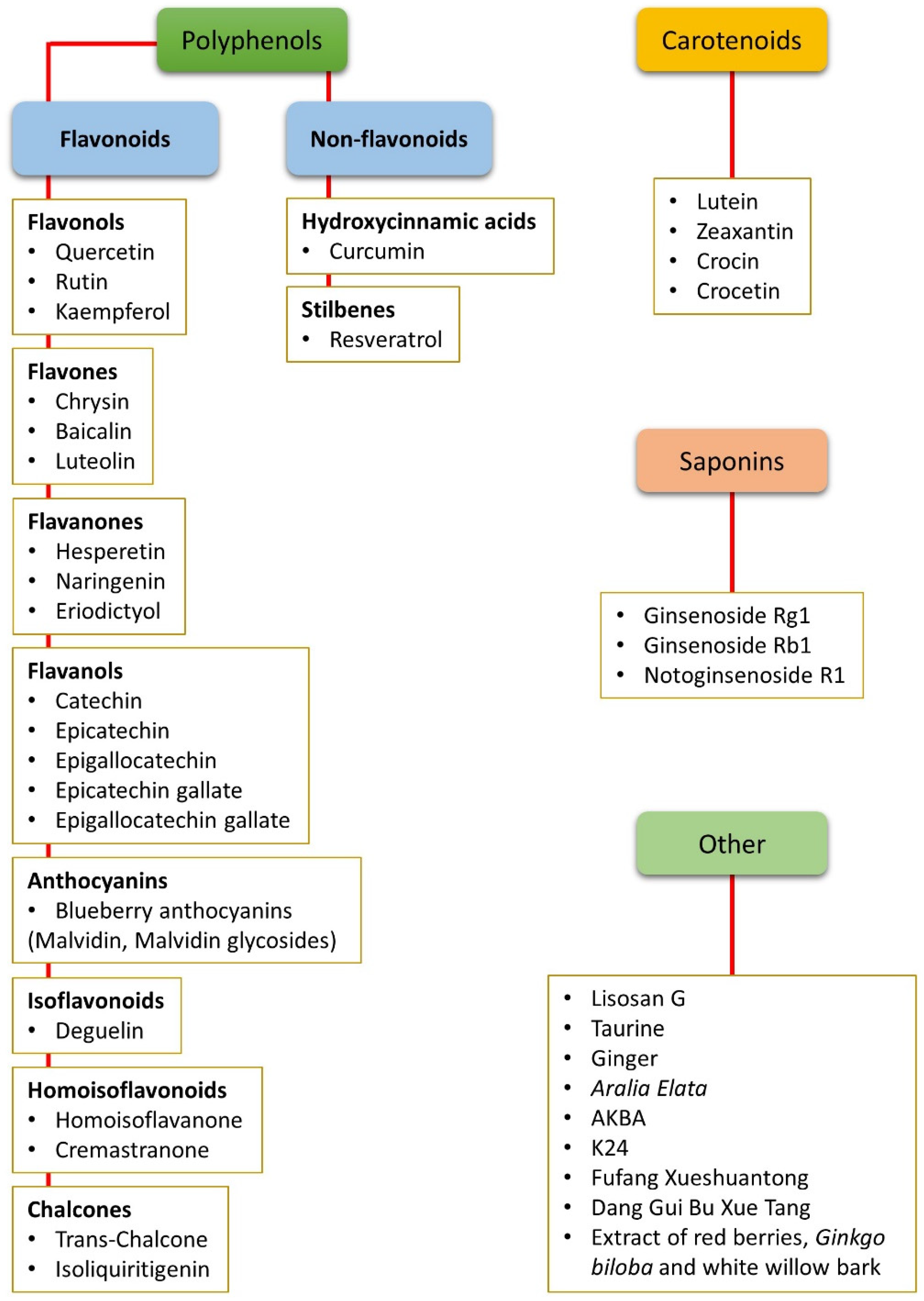
1. Introduction
2. Nutraceuticals and Oxidative Stress
2.1. Non-Flavonoid Polyphenols
2.2. Flavonoid Polyphenols
2.3. Carotenoids
2.4. Saponins
2.5. Other Compounds
3. Nutraceuticals and Inflammation
3.1. Non-Flavonoid Polyphenols
3.2. Flavonoid Polyphenols
3.3. Carotenoids
3.4. Other Compounds
3.5. Relationships between Inflammation and Oxidative Stress
4. Nutraceuticals and Neurodegeneration
4.1. Non-Flavonoid Polyphenols
4.2. Flavonoid Polyphenols
4.3. Carotenoids
4.4. Other Compounds
4.5. Relationships between Oxidative Stress, Inflammation, and Neurodegeneration
5. Nutraceuticals and Vascular Changes
5.1. Non-Flavonoid Polyphenols
5.2. Flavonoid Polyphenols
5.3. Carotenoids
5.4. Saponins
5.5. Other Compounds
5.6. Relationships between Oxidative Stress, Inflammation, Neurodegeneration, and Vascular Damage
6. Clinical Studies
7. Bioavailability of Nutraceuticals
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Curtis, T.; Gardiner, T.; Stitt, A. Microvascular lesions of diabetic retinopathy: Clues towards understanding pathogenesis? Eye 2009, 23, 1496. [Google Scholar] [CrossRef] [PubMed]
- Harrison, W.W.; Bearse, M.A.; Ng, J.S.; Jewell, N.P.; Barez, S.; Burger, D.; Schneck, M.E.; Adams, A.J. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Investig. Ophthalmol. Vis. Sci. 2011, 52, 772–777. [Google Scholar] [CrossRef]
- Hernández, C.; Dal Monte, M.; Simó, R.; Casini, G. Neuroprotection as a therapeutic target for diabetic retinopathy. J. Diabetes Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Simo, R.; Hernandez, C. Neurodegeneration in the diabetic eye: New insights and therapeutic perspectives. Trends Endocrinol. Metab. 2014, 25, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018. [Google Scholar] [CrossRef]
- Tarr, J.M.; Kaul, K.; Chopra, M.; Kohner, E.M.; Chibber, R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013. [Google Scholar] [CrossRef]
- Wang, W.; Lo, A. Diabetic retinopathy: Pathophysiology and treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Chan, P.-S. Oxidative stress and diabetic retinopathy. Exp. Diabetes Res. 2007, 2007. [Google Scholar] [CrossRef] [PubMed]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Sundstrom, J.M.; Antonetti, D.A. Ocular anti-VEGF therapy for diabetic retinopathy: The role of VEGF in the pathogenesis of diabetic retinopathy. Diabetes Care 2014, 37, 893–899. [Google Scholar] [CrossRef]
- Zhao, Y.; Singh, R.P. The role of anti-vascular endothelial growth factor (anti-VEGF) in the management of proliferative diabetic retinopathy. Drugs Context 2018, 7. [Google Scholar] [CrossRef]
- Amato, R.; Biagioni, M.; Cammalleri, M.; Dal Monte, M.; Casini, G. VEGF as a survival factor in ex vivo models of early diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3066–3076. [Google Scholar] [CrossRef]
- Behl, T.; Kotwani, A. Exploring the various aspects of the pathological role of vascular endothelial growth factor (VEGF) in diabetic retinopathy. Pharmacol. Res. 2015, 99, 137–148. [Google Scholar] [CrossRef]
- Witmer, A.; Vrensen, G.; Van Noorden, C.; Schlingemann, R. Vascular endothelial growth factors and angiogenesis in eye disease. Prog. Retin. Eye Res. 2003, 22, 1–29. [Google Scholar] [CrossRef]
- Brower, V. Nutraceuticals: Poised for a healthy slice of the healthcare market? Nat. Biotechnol. 1998, 16, 728. [Google Scholar] [CrossRef]
- Milatovic, D.; Zaja-Milatovic, S.; Gupta, R.C. Oxidative Stress and Excitotoxicity: Antioxidants from Nutraceuticals. In Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2016; pp. 401–413. [Google Scholar]
- Aggarwal, B.B.; Van Kuiken, M.E.; Iyer, L.H.; Harikumar, K.B.; Sung, B. Molecular targets of nutraceuticals derived from dietary spices: Potential role in suppression of inflammation and tumorigenesis. Exp. Biol. Med. 2009, 234, 825–849. [Google Scholar] [CrossRef]
- Chauhan, B.; Kumar, G.; Kalam, N.; Ansari, S.H. Current concepts and prospects of herbal nutraceutical: A review. J. Adv. Pharm. Technol. Res. 2013, 4, 4. [Google Scholar] [CrossRef]
- Kalra, E.K. Nutraceutical-definition and introduction. AAPS Pharmsci 2003, 5, 27–28. [Google Scholar] [CrossRef]
- Calderon, G.; Juarez, O.; Hernandez, G.; Punzo, S.; De la Cruz, Z. Oxidative stress and diabetic retinopathy: Development and treatment. Eye 2017, 31, 1122. [Google Scholar] [CrossRef]
- Tokarz, P.; Kaarniranta, K.; Blasiak, J. Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related macular degeneration (AMD). Biogerontology 2013, 14, 461–482. [Google Scholar] [CrossRef]
- Ahmadinejad, F.; Geir Møller, S.; Hashemzadeh-Chaleshtori, M.; Bidkhori, G.; Jami, M.-S. Molecular mechanisms behind free radical scavengers function against oxidative stress. Antioxidants 2017, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Cui, K.; Luo, X.; Xu, K.; Murthy, M.V. Role of oxidative stress in neurodegeneration: Recent developments in assay methods for oxidative stress and nutraceutical antioxidants. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 771–799. [Google Scholar] [CrossRef]
- Wu, M.Y.; Yiang, G.T.; Lai, T.T.; Li, C.J. The Oxidative Stress and Mitochondrial Dysfunction during the Pathogenesis of Diabetic Retinopathy. Oxid. Med. Cell. Longev. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Parsamanesh, N.; Moossavi, M.; Bahrami, A.; Butler, A.E.; Sahebkar, A. Therapeutic potential of curcumin in diabetic complications. Pharmacol. Res. 2018. [Google Scholar] [CrossRef]
- Peddada, K.V.; Verma, V.; Nebbioso, M. Therapeutic potential of curcumin in major retinal pathologies. Int. Ophthalmol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Sun, Y.; Huang, K.; Zheng, L. Curcumin, a potential therapeutic candidate for retinal diseases. Mol. Nutr. Food Res. 2013, 57, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Premanand, C.; Rema, M.; Sameer, M.Z.; Sujatha, M.; Balasubramanyam, M. Effect of curcumin on proliferation of human retinal endothelial cells under in vitro conditions. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2179–2184. [Google Scholar] [CrossRef]
- Woo, J.M.; Shin, D.-Y.; Lee, S.J.; Joe, Y.; Zheng, M.; Yim, J.H.; Callaway, Z.; Chung, H.T. Curcumin protects retinal pigment epithelial cells against oxidative stress via induction of heme oxygenase-1 expression and reduction of reactive oxygen. Mol. Vis. 2012, 18, 901. [Google Scholar]
- Platania, C.B.M.; Fidilio, A.; Lazzara, F.; Piazza, C.; Geraci, F.; Giurdanella, G.; Leggio, G.M.; Salomone, S.; Drago, F.; Bucolo, C. Retinal Protection and Distribution of Curcumin in Vitro and in Vivo. Front. Pharm. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zou, X.; Cao, K.; Xu, J.; Yue, T.; Dai, F.; Zhou, B.; Lu, W.; Feng, Z.; Liu, J. Curcumin analog 1, 5-bis (2-trifluoromethylphenyl)-1, 4-pentadien-3-one exhibits enhanced ability on Nrf2 activation and protection against acrolein-induced ARPE-19 cell toxicity. Toxicol. Appl. Pharmacol. 2013, 272, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, X.; Fan, H.; Liu, Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009, 1282, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Mazzone, M.G.; Giuliano, F.; Vinciguerra, M.; Basile, G.; Barchitta, M.; Agodi, A. Curcumin Modulates DNA Methyltransferase Functions in a Cellular Model of Diabetic Retinopathy. Oxid. Med. Cell. Longev. 2018, 2. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.-F.; Zhang, Q.; Liu, X.-Z. Protective effects of curcumin on retinal Müller cell in early diabetic rats. Int. J. Ophthalmol. 2013, 6, 422. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Kumar, B.; Nag, T.C.; Agrawal, S.S.; Agrawal, R.; Agrawal, P.; Saxena, R.; Srivastava, S. Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J. Ocul. Pharmacol. Ther. 2011, 27, 123–130. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kanwar, M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr. Metab. 2007, 4, 8. [Google Scholar] [CrossRef]
- Soufi, F.G.; Mohammad-nejad, D.; Ahmadieh, H. Resveratrol improves diabetic retinopathy possibly through oxidative stress–nuclear factor κB–apoptosis pathway. Pharmacol. Rep. 2012, 64, 1505–1514. [Google Scholar] [CrossRef]
- Al-Hussaini, H.; Kilarkaje, N. Effects of trans-resveratrol on type 1 diabetes-induced inhibition of retinoic acid metabolism pathway in retinal pigment epithelium of Dark Agouti rats. Eur. J. Pharm. 2018, 834, 142–151. [Google Scholar] [CrossRef]
- Panche, A.; Diwan, A.; Chandra, S. Flavonoids: An overview. J. Nutr. Sci. 2016, 5. [Google Scholar] [CrossRef]
- Kumar, B.; Gupta, S.K.; Nag, T.C.; Srivastava, S.; Saxena, R.; Jha, K.A.; Srinivasan, B.P. Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp. Eye Res. 2014, 125, 193–202. [Google Scholar] [CrossRef]
- Kumar, B.; Gupta, S.K.; Srinivasan, B.; Nag, T.C.; Srivastava, S.; Saxena, R.; Jha, K.A. Hesperetin rescues retinal oxidative stress, neuroinflammation and apoptosis in diabetic rats. Microvasc. Res. 2013, 87, 65–74. [Google Scholar] [CrossRef]
- Kumar, B.; Gupta, S.K.; Nag, T.C.; Srivastava, S.; Saxena, R. Green tea prevents hyperglycemia-induced retinal oxidative stress and inflammation in streptozotocin-induced diabetic rats. Ophthalmic Res. 2012, 47, 103–108. [Google Scholar] [CrossRef]
- Sampath, C.; Sang, S.; Ahmedna, M. In vitro and in vivo inhibition of aldose reductase and advanced glycation end products by phloretin, epigallocatechin 3-gallate and [6]-gingerol. Biomed. Pharmacother. 2016, 84, 502–513. [Google Scholar] [CrossRef]
- Lv, P.; Yu, J.; Xu, X.; Lu, T.; Xu, F. Eriodictyol inhibits high glucose-induced oxidative stress and inflammation in retinal ganglial cells. J. Cell. Biochem. 2018, 14, 27848. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yan, Z.; Li, D.; Ma, Y.; Zhou, J.; Sui, Z. Antioxidant and Anti-Inflammatory Effects of Blueberry Anthocyanins on High Glucose-Induced Human Retinal Capillary Endothelial Cells. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Huang, L.; Yu, J. Effects of blueberry anthocyanins on retinal oxidative stress and inflammation in diabetes through Nrf2/HO-1 signaling. J. Neuroimmunol. 2016, 301, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.A.; Landrum, J.T.; Hime, G.W.; Cains, A.; Zamor, J. Stereochemistry of the human macular carotenoids. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2033–2040. [Google Scholar]
- Khachik, F.; Bernstein, P.S.; Garland, D.L. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1802–1811. [Google Scholar]
- Scripsema, N.K.; Hu, D.-N.; Rosen, R.B. Lutein, zeaxanthin, and meso-zeaxanthin in the clinical management of eye disease. J. Ophthalmol. 2015. [Google Scholar] [CrossRef]
- Jia, Y.-P.; Sun, L.; Yu, H.-S.; Liang, L.-P.; Li, W.; Ding, H.; Song, X.-B.; Zhang, L.-J. The pharmacological effects of lutein and zeaxanthin on visual disorders and cognition diseases. Molecules 2017, 22, 610. [Google Scholar] [CrossRef]
- Li, B.; Ahmed, F.; Bernstein, P.S. Studies on the singlet oxygen scavenging mechanism of human macular pigment. Arch. Biochem. Biophys. 2010, 504, 56–60. [Google Scholar] [CrossRef]
- Neelam, K.; Goenadi, C.J.; Lun, K.; Yip, C.C.; Eong, K.-G.A. Putative protective role of lutein and zeaxanthin in diabetic retinopathy. Br. J. Ophthalmol. 2017, 101, 551–558. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Menon, B.; Gierhart, D.L. Beneficial effect of zeaxanthin on retinal metabolic abnormalities in diabetic rats. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1645–1651. [Google Scholar] [CrossRef]
- Sasaki, M.; Ozawa, Y.; Kurihara, T.; Kubota, S.; Yuki, K.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia 2010, 53, 971–979. [Google Scholar] [CrossRef]
- José Bagur, M.; Alonso Salinas, G.; Jiménez-Monreal, A.; Chaouqi, S.; Llorens, S.; Martínez-Tomé, M.; Alonso, G. Saffron: An Old Medicinal Plant and a Potential Novel Functional Food. Molecules 2018, 23, 30. [Google Scholar] [CrossRef]
- Yamauchi, M.; Tsuruma, K.; Imai, S.; Nakanishi, T.; Umigai, N.; Shimazawa, M.; Hara, H. Crocetin prevents retinal degeneration induced by oxidative and endoplasmic reticulum stresses via inhibition of caspase activity. Eur. J. Pharmacol. 2011, 650, 110–119. [Google Scholar] [CrossRef]
- Yang, X.; Huo, F.; Liu, B.; Liu, J.; Chen, T.; Li, J.; Zhu, Z.; Lv, B. Crocin inhibits oxidative stress and pro-inflammatory response of microglial cells associated with diabetic retinopathy through the activation of PI3K/Akt signaling pathway. J. Mol. Neurosci. 2017, 61, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Querques, M.A.; Chakrabarti, S. North American Ginseng (Panax quinquefolius) prevents hyperglycemia and associated pancreatic abnormalities in diabetes. J. Med. Food 2013, 16, 587–592. [Google Scholar] [CrossRef]
- Fan, Y.; Qiao, Y.; Huang, J.; Tang, M. Protective effects of Panax notoginseng saponins against high glucose-induced oxidative injury in rat retinal capillary endothelial cells. Evid.-Based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef]
- Fan, C.; Qiao, Y.; Tang, M. Notoginsenoside R1 attenuates high glucose-induced endothelial damage in rat retinal capillary endothelial cells by modulating the intracellular redox state. Drug Des. Dev. Ther. 2017, 11, 3343. [Google Scholar] [CrossRef] [PubMed]
- Amato, R.; Rossino, M.G.; Cammalleri, M.; Locri, F.; Pucci, L.; Monte, M.D.; Casini, G. Lisosan G Protects the Retina from Neurovascular Damage in Experimental Diabetic Retinopathy. Nutrients 2018, 10, 1932. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Poulaki, V.; Le, M.L.; Koizumi, K.; Esser, C.; Janicki, H.; Schraermeyer, U.; Kociok, N.; Fauser, S.; Kirchhof, B. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004, 18, 1450–1452. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.S. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. J. Diabetes Res. 2007. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Kern, T.S. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Lieth, E.; Gardner, T.W.; Barber, A.J.; Antonetti, D.A. Retinal neurodegeneration: Early pathology in diabetes. Clin. Exp. Ophthalmol. Viewp. 2000, 28, 3–8. [Google Scholar] [CrossRef]
- Lieth, E.; Barber, A.J.; Xu, B.; Dice, C.; Ratz, M.J.; Tanase, D.; Strother, J.M. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes 1998, 47, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, M.; Gerhardinger, C.; Lorenzi, M. Muller cell changes in human diabetic retinopathy. Diabetes 1998, 47, 445–449. [Google Scholar] [CrossRef]
- Bringmann, A.; Iandiev, I.; Pannicke, T.; Wurm, A.; Hollborn, M.; Wiedemann, P.; Osborne, N.N.; Reichenbach, A. Cellular signaling and factors involved in Muller cell gliosis: Neuroprotective and detrimental effects. Prog. Retin. Eye Res. 2009, 28, 423–451. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Suk, J.E.; Patrick, C.; Bae, E.J.; Cho, J.H.; Rho, S.; Hwang, D.; Masliah, E.; Lee, S.J. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J. Biol. Chem. 2010, 285, 9262–9272. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target 2017, 2, 14. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Kulkarni, Y.A. NF-kappabeta: A Potential Target in the Management of Vascular Complications of Diabetes. Front. Pharm. 2017, 8. [Google Scholar] [CrossRef]
- Li, J.; Wang, P.; Ying, J.; Chen, Z.; Yu, S. Curcumin Attenuates Retinal Vascular Leakage by Inhibiting Calcium/Calmodulin-Dependent Protein Kinase II Activity in Streptozotocin-Induced Diabetes. Cell Physiol Biochem 2016, 39, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.D.T.; Tripathy, G. Pharmacognostic evaluation of curcumin on diabetic retinopathy in alloxan-induced diabetes through NF-KB and Brn3a related mechanism. Pharmacogn. J. 2018, 10, 324–332. [Google Scholar] [CrossRef]
- Shome, S.; Talukdar, A.D.; Choudhury, M.D.; Bhattacharya, M.K.; Upadhyaya, H. Curcumin as potential therapeutic natural product: A nanobiotechnological perspective. J Pharm Pharm. 2016, 68, 1481–1500. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Cooper, N.G. Glutamate-induced NFkappaB activation in the retina. Investig. Ophthalmol. Vis. Sci. 2009, 50, 917–925. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Meng, J.; Li, H.; Wei, H.; Bi, F.; Liu, S.; Tang, K.; Guo, H.; Liu, W. Resveratrol exhibits an effect on attenuating retina inflammatory condition and damage of diabetic retinopathy via PON1. Exp Eye Res 2019, 181, 356–366. [Google Scholar] [CrossRef]
- Sohn, E.; Kim, J.; Kim, C.S.; Lee, Y.M.; Kim, J.S. Extract of Polygonum cuspidatum Attenuates Diabetic Retinopathy by Inhibiting the High-Mobility Group Box-1 (HMGB1) Signaling Pathway in Streptozotocin-Induced Diabetic Rats. Nutrients 2016, 8, 140. [Google Scholar] [CrossRef]
- Kubota, S.; Kurihara, T.; Mochimaru, H.; Satofuka, S.; Noda, K.; Ozawa, Y.; Oike, Y.; Ishida, S.; Tsubota, K. Prevention of ocular inflammation in endotoxin-induced uveitis with resveratrol by inhibiting oxidative damage and nuclear factor-kappaB activation. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3512–3519. [Google Scholar] [CrossRef] [PubMed]
- Kubota, S.; Ozawa, Y.; Kurihara, T.; Sasaki, M.; Yuki, K.; Miyake, S.; Noda, K.; Ishida, S.; Tsubota, K. Roles of AMP-activated protein kinase in diabetes-induced retinal inflammation. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9142–9148. [Google Scholar] [CrossRef] [PubMed]
- Bagul, P.K.; Deepthi, N.; Sultana, R.; Banerjee, S.K. Resveratrol ameliorates cardiac oxidative stress in diabetes through deacetylation of NFkB-p65 and histone 3. J. Nutr. Biochem. 2015, 26, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, Y.S.; Kang, S.S.; Cho, G.J.; Choi, W.S. Resveratrol inhibits neuronal apoptosis and elevated Ca2+/calmodulin-dependent protein kinase II activity in diabetic mouse retina. Diabetes 2010, 59, 1825–1835. [Google Scholar] [CrossRef]
- Lee, M.; Yun, S.; Lee, H.; Yang, J. Quercetin Mitigates Inflammatory Responses Induced by Vascular Endothelial Growth Factor in Mouse Retinal Photoreceptor Cells through Suppression of Nuclear Factor Kappa B. Int. J. Mol. Sci. 2017, 18, 2497. [Google Scholar] [CrossRef]
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Eriodictyol prevents early retinal and plasma abnormalities in streptozotocin-induced diabetic rats. Biochem Pharm. 2012, 84, 88–92. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Jin, W.; Xing, Y.; Yang, A. Catechin Weakens Diabetic Retinopathy by Inhibiting the Expression of NF-kappaB Signaling Pathway-Mediated Inflammatory Factors. Ann. Clin. Lab. Sci. 2018, 48, 594–600. [Google Scholar] [PubMed]
- Silva, K.C.; Rosales, M.A.; Hamassaki, D.E.; Saito, K.C.; Faria, A.M.; Ribeiro, P.A.; Faria, J.B.; Faria, J.M. Green tea is neuroprotective in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1325–1336. [Google Scholar] [CrossRef]
- Al-Gayyar, M.M.; Matragoon, S.; Pillai, B.A.; Ali, T.K.; Abdelsaid, M.A.; El-Remessy, A.B. Epicatechin blocks pro-nerve growth factor (proNGF)-mediated retinal neurodegeneration via inhibition of p75 neurotrophin receptor expression in a rat model of diabetes [corrected]. Diabetologia 2011, 54, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Dongare, S.; Gupta, S.K.; Mathur, R.; Saxena, R.; Mathur, S.; Agarwal, R.; Nag, T.C.; Srivastava, S.; Kumar, P. Zingiber officinale attenuates retinal microvascular changes in diabetic rats via anti-inflammatory and antiangiogenic mechanisms. Mol. Vis. 2016, 22, 599–609. [Google Scholar]
- Tzeng, T.F.; Liou, S.S.; Tzeng, Y.C.; Liu, I.M. Zerumbone, a Phytochemical of Subtropical Ginger, Protects against Hyperglycemia-Induced Retinal Damage in Experimental Diabetic Rats. Nutrients 2016, 8, 449. [Google Scholar] [CrossRef] [PubMed]
- Giusti, L.; Gabriele, M.; Penno, G.; Garofolo, M.; Longo, V.; Del Prato, S.; Lucchesi, D.; Pucci, L. A Fermented Whole Grain Prevents Lipopolysaccharides-Induced Dysfunction in Human Endothelial Progenitor Cells. Oxid. Med. Cell. Longev. 2017. [Google Scholar] [CrossRef] [PubMed]
- La Marca, M.; Beffy, P.; Pugliese, A.; Longo, V. Fermented wheat powder induces the antioxidant and detoxifying system in primary rat hepatocytes. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Yoo, W.S.; Choi, M.; Chung, I.; Yoo, J.M.; Choi, W.S. Increased O-GlcNAcylation of NF-kappaB Enhances Retinal Ganglion Cell Death in Streptozotocin-induced Diabetic Retinopathy. Curr. Eye Res. 2016, 41, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, M.J.; Choi, M.Y.; Kim, Y.S.; Yoo, J.M.; Hong, E.K.; Ju, S.; Choi, W.S. Aralia elata inhibits neurodegeneration by downregulating O-GlcNAcylation of NF-kappaB in diabetic mice. Int. J. Ophthalmol. 2017, 10, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Marrazzo, G.; Platania, C.B.; Drago, F.; Leggio, G.M.; Salomone, S. Fortified extract of red berry, Ginkgo biloba, and white willow bark in experimental early diabetic retinopathy. J. Diabetes Res. 2013. [Google Scholar] [CrossRef]
- Gill, R.; Tsung, A.; Billiar, T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic. Biol. Med. 2010, 48, 1121–1132. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Ran, Z.; Zhang, Y.; Wen, X.; Ma, J. Curcumin inhibits high glucoseinduced inflammatory injury in human retinal pigment epithelial cells through the ROSPI3K/AKT/mTOR signaling pathway. Mol. Med. Rep. 2018, 12. [Google Scholar] [CrossRef]
- Amato, R.; Catalani, E.; Dal Monte, M.; Cammalleri, M.; Di Renzo, I.; Perrotta, C.; Cervia, D.; Casini, G. Autophagy-mediated neuroprotection induced by octreotide in an ex vivo model of early diabetic retinopathy. Pharm. Res. 2018, 128, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J. Diabetic retinopathy: Recent advances towards understanding neurodegeneration and vision loss. Sci. China Life Sci. 2015, 58, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J.; Baccouche, B. Neurodegeneration in diabetic retinopathy: Potential for novel therapies. Vis. Res. 2017, 139, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Ola, M.S.; Nawaz, M.I.; Khan, H.A.; Alhomida, A.S. Neurodegeneration and neuroprotection in diabetic retinopathy. Int. J. Mol. Sci. 2013, 14, 2559–2572. [Google Scholar] [CrossRef] [PubMed]
- Ola, M.S.; Alhomida, A.S. Neurodegeneration in diabetic retina and its potential drug targets. Curr. Neuropharmacol. 2014, 12, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.; El-Remessy, A.B. Imbalance of the Nerve Growth Factor and Its Precursor: Implication in Diabetic Retinopathy. J. Clin. Exp. Ophthalmol. 2015, 6, 2155–9570. [Google Scholar] [CrossRef] [PubMed]
- Ola, M.S.; Nawaz, M.I.; El-Asrar, A.A.; Abouammoh, M.; Alhomida, A.S. Reduced levels of brain derived neurotrophic factor (BDNF) in the serum of diabetic retinopathy patients and in the retina of diabetic rats. Cell. Mol. Neurobiol. 2013, 33, 359–367. [Google Scholar] [CrossRef]
- Li, Q.; Puro, D.G. Diabetes-induced dysfunction of the glutamate transporter in retinal Muller cells. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3109–3116. [Google Scholar]
- Solanki, I.; Parihar, P.; Mansuri, M.L.; Parihar, M.S. Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv. Nutr. 2015, 6, 64–72. [Google Scholar] [CrossRef]
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008, 3, 115–126. [Google Scholar] [CrossRef]
- Yang, F.; Yu, J.; Ke, F.; Lan, M.; Li, D.; Tan, K.; Ling, J.; Wang, Y.; Wu, K. Curcumin Alleviates Diabetic Retinopathy in Experimental Diabetic Rats. Ophthalmic Res. 2018, 60, 43–54. [Google Scholar] [CrossRef]
- Shakeri, A.; Cicero, A.F.G.; Panahi, Y.; Mohajeri, M.; Sahebkar, A. Curcumin: A naturally occurring autophagy modulator. J. Cell. Physiol. 2018, 21, 27404. [Google Scholar] [CrossRef]
- Sheu, S.J.; Chen, J.L.; Bee, Y.S.; Chen, Y.A.; Lin, S.H.; Shu, C.W. Differential autophagic effects of vital dyes in retinal pigment epithelial ARPE-19 and photoreceptor 661W cells. PLoS ONE 2017, 12, e0174736. [Google Scholar] [CrossRef] [PubMed]
- Ola, M.S.; Ahmed, M.M.; Shams, S.; Al-Rejaie, S.S. Neuroprotective effects of quercetin in diabetic rat retina. Saudi J. Biol. Sci. 2017, 24, 1186–1194. [Google Scholar] [CrossRef]
- Qu, L.; Liang, X.; Gu, B.; Liu, W. Quercetin alleviates high glucose-induced Schwann cell damage by autophagy. Neural Regen. Res. 2014, 9, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Regitz, C.; Dussling, L.M.; Wenzel, U. Amyloid-beta (Abeta(1)(-)(4)(2))-induced paralysis in Caenorhabditis elegans is inhibited by the polyphenol quercetin through activation of protein degradation pathways. Mol. Nutr. Food Res. 2014, 58, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Somerset, S.M.; Johannot, L. Dietary flavonoid sources in Australian adults. Nutr. Cancer 2008, 60, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; Graziani, I.; De Zio, D.; Dini, L.; Centonze, D.; Rotilio, G.; Ciriolo, M.R. Neuroprotection of kaempferol by autophagy in models of rotenone-mediated acute toxicity: Possible implications for Parkinson’s disease. Neurobiol. Aging 2012, 33, 767–785. [Google Scholar] [CrossRef]
- Havsteen, B. Flavonoids, a class of natural products of high pharmacological potency. Biochem. Pharm. 1983, 32, 1141–1148. [Google Scholar] [CrossRef]
- Nakayama, M.; Aihara, M.; Chen, Y.N.; Araie, M.; Tomita-Yokotani, K.; Iwashina, T. Neuroprotective effects of flavonoids on hypoxia-, glutamate-, and oxidative stress-induced retinal ganglion cell death. Mol. Vis. 2011, 17, 1784–1793. [Google Scholar]
- Ola, M.S.; Ahmed, M.M.; Ahmad, R.; Abuohashish, H.M.; Al-Rejaie, S.S.; Alhomida, A.S. Neuroprotective Effects of Rutin in Streptozotocin-Induced Diabetic Rat Retina. J. Mol. Neurosci. 2015, 56, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.K.; Lee, E.J.; Kim, Y.H.; Kim, D.Y.; Oh, H.; Kim, S.I.; Kang, Y.H. Chrysin Ameliorates Malfunction of Retinoid Visual Cycle through Blocking Activation of AGE-RAGE-ER Stress in Glucose-Stimulated Retinal Pigment Epithelial Cells and Diabetic Eyes. Nutrients 2018, 10, 1046. [Google Scholar] [CrossRef]
- Shimouchi, A.; Yokota, H.; Ono, S.; Matsumoto, C.; Tamai, T.; Takumi, H.; Narayanan, S.P.; Kimura, S.; Kobayashi, H.; Caldwell, R.B.; et al. Neuroprotective effect of water-dispersible hesperetin in retinal ischemia reperfusion injury. Jpn. J. Ophthalmol. 2016, 60, 51–61. [Google Scholar] [CrossRef]
- Al-Dosari, D.I.; Ahmed, M.M.; Al-Rejaie, S.S.; Alhomida, A.S.; Ola, M.S. Flavonoid Naringenin Attenuates Oxidative Stress, Apoptosis and Improves Neurotrophic Effects in the Diabetic Rat Retina. Nutrients 2017, 9, 1161. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Ma, C.M.; Han, Y.Z.; Li, Y.; Liu, C.; Duan, Z.H.; Wang, H.L.; Liu, D.Q.; Liu, R.H. Protective Effect of Naringenin on Glutamate-Induced Neurotoxicity in Cultured Hippocampal Cells. Arch. Biol. Sci. 2015, 67, 639–646. [Google Scholar] [CrossRef]
- Sasaki, M.; Yuki, K.; Kurihara, T.; Miyake, S.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K.; Ozawa, Y. Biological role of lutein in the light-induced retinal degeneration. J. Nutr. Biochem. 2012, 23, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Sasaki, M.; Takahashi, N.; Kamoshita, M.; Miyake, S.; Tsubota, K. Neuroprotective Effects of Lutein in the Retina. Curr. Pharm. Des. 2012, 18, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.K.; Lee, Y.J.; Colitz, C.M.; Chang, C.J.; Lin, C.T. The protective effects of Lycium barbarum and Chrysanthemum morifolum on diabetic retinopathies in rats. Vet. Ophthalmol. 2012, 15, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Yang, D.; Yeung, C.M.; Yu, W.Y.; Chang, R.C.C.; So, K.F.; Wong, D.; Lo, A.C.Y. Lycium Barbarum Polysaccharides Reduce Neuronal Damage, Blood-Retinal Barrier Disruption and Oxidative Stress in Retinal Ischemia/Reperfusion Injury. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Song, M.K.; Roufogalis, B.D.; Huang, T.H.W. Reversal of the Caspase-Dependent Apoptotic Cytotoxicity Pathway by Taurine from Lycium barbarum (Goji Berry) in Human Retinal Pigment Epithelial Cells: Potential Benefit in Diabetic Retinopathy. Evid.-Based Complement. Altern. Med. 2012. [Google Scholar] [CrossRef]
- Kelsey, N.A.; Wilkins, H.M.; Linseman, D.A. Nutraceutical Antioxidants as Novel Neuroprotective Agents. Molecules 2010, 15, 7792–7814. [Google Scholar] [CrossRef]
- Stitt, A.W.; Lois, N.; Medina, R.J.; Adamson, P.; Curtis, T.M. Advances in our understanding of diabetic retinopathy. Clin. Sci. 2013, 125. [Google Scholar] [CrossRef] [PubMed]
- Frey, T.; Antonetti, D.A. Alterations to the blood-retinal barrier in diabetes: Cytokines and reactive oxygen species. Antioxid. Redox Signal. 2011, 15, 1271–1284. [Google Scholar] [CrossRef]
- Eshaq, R.S.; Aldalati, A.M.Z.; Alexander, J.S.; Harris, N.R. Diabetic retinopathy: Breaking the barrier. Pathophysiology 2017, 24, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Kusuhara, S.; Fukushima, Y.; Ogura, S.; Inoue, N.; Uemura, A. Pathophysiology of Diabetic Retinopathy: The Old and the New. Diabetes Metab. J. 2018, 42, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Barber, A.J.; Hollinger, L.A.; Wolpert, E.B.; Gardner, T.W. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J. Biol. Chem. 1999, 274, 23463–23467. [Google Scholar] [CrossRef]
- Murakami, T.; Felinski, E.A.; Antonetti, D.A. Occludin Phosphorylation and Ubiquitination Regulate Tight Junction Trafficking and Vascular Endothelial Growth Factor-induced Permeability. J. Biol. Chem. 2009, 284, 21036–21046. [Google Scholar] [CrossRef]
- Esser, S.; Lampugnani, M.G.; Corada, M.; Dejana, E.; Risau, W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J. Cell Sci. 1998, 111, 1853–1865. [Google Scholar]
- Aveleira, C.A.; Lin, C.M.; Abcouwer, S.F.; Ambrosio, A.F.; Antonetti, D.A. TNF-alpha signals through PKCzeta/NF-kappaB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes 2010, 59, 2872–2882. [Google Scholar] [CrossRef]
- Bamforth, S.D.; Lightman, S.L.; Greenwood, J. Interleukin-1 beta-induced disruption of the retinal vascular barrier of the central nervous system is mediated through leukocyte recruitment and histamine. Am. J. Pathol. 1997, 150, 329–340. [Google Scholar] [PubMed]
- Kowluru, R.A.; Zhong, Q.; Santos, J.M. Matrix metalloproteinases in diabetic retinopathy: Potential role of MMP-9. Expert Opin. Investig. Drugs 2012, 21, 797–805. [Google Scholar] [CrossRef]
- Hammes, H.P.; Lin, J.H.; Renner, O.; Shani, M.; Lundqvist, A.; Betsholtz, C.; Brownlee, M.; Deutsch, U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes 2002, 51, 3107–3112. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, L.J.; Yu, J.; Wang, H.J.; Zhang, F.; Liu, Q.; Wu, J. Involvement of Advanced Glycation End Products in the Pathogenesis of Diabetic Retinopathy. Cell. Physiol. Biochem. 2018, 48, 705–717. [Google Scholar] [CrossRef]
- Ejaz, S. Importance of pericytes and mechanisms of pericyte loss during diabetes retinopathy. Diabetes Obes. Metab. 2008, 10, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Beltramo, E.; Porta, M. Pericyte Loss in Diabetic Retinopathy: Mechanisms and Consequences. Curr. Med. Chem. 2013, 20, 3218–3225. [Google Scholar] [CrossRef]
- Loukovaara, S.; Gucciardo, E.; Repo, P.; Vihinen, H.; Lohi, J.; Jokitalo, E.; Salven, P.; Lehti, K. Indications of lymphatic endothelial differentiation and endothelial progenitor cell activation in the pathology of proliferative diabetic retinopathy. Acta Ophthalmol. 2015, 93, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Amin, S.; Roy, S. Retinal fibrosis in diabetic retinopathy. Exp. Eye Res. 2016, 142, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.P.; Simo, R.; et al. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef] [PubMed]
- Adamis, A.P.; Miller, J.W.; Bernal, M.T.; Damico, D.J.; Folkman, J.; Yeo, T.K.; Yeo, K.T. Increased Vascular Endothelial Growth-Factor Levels in the Vitreous of Eyes with Proliferative Diabetic-Retinopathy. Am. J. Ophthalmol. 1994, 118, 445–450. [Google Scholar] [CrossRef]
- Osaadon, P.; Fagan, X.J.; Lifshitz, T.; Levy, J. A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye 2014, 28, 510–520. [Google Scholar] [CrossRef]
- Wang, L.L.; Li, C.Z.; Guo, H.; Kern, T.S.; Huang, K.; Zheng, L. Curcumin Inhibits Neuronal and Vascular Degeneration in Retina after Ischemia and Reperfusion Injury. PLoS ONE 2011, 6, e23194. [Google Scholar] [CrossRef]
- Mrudula, T.; Suryanarayana, P.; Srinivas, P.N.B.S.; Reddy, G.B. Effect of curcumin on hyperglycemia-induced vascular endothelial growth factor expression in streptozotocin-induced diabetic rat retina. Biochem. Biophys. Res. Commun. 2007, 361, 528–532. [Google Scholar] [CrossRef]
- Xie, P.; Zhang, W.; Yuan, S.; Chen, Z.; Yang, Q.; Yuan, D.; Wang, F.; Liu, Q. Suppression of experimental choroidal neovascularization by curcumin in mice. PLoS ONE 2012, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Choi, E.Y.; Lee, S.C.; Koh, H.J.; Lee, J.H.; Chung, J.H. Resveratrol Inhibits Hypoxia-Induced Vascular Endothelial Growth Factor Expression and Pathological Neovascularization. Yonsei Med. J. 2015, 56, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, Y.S.; Roh, G.S.; Choi, W.S.; Cho, G.J. Resveratrol blocks diabetes-induced early vascular lesions and vascular endothelial growth factor induction in mouse retinas. Acta Ophthalmol. 2012, 90, E31–E37. [Google Scholar] [CrossRef]
- Li, W.L.; Jiang, D.Y. Effect of Resveratrol on Bcl-2 and VEGF Expression in Oxygen-Induced Retinopathy of Prematurity. J. Pediatric Ophthalmol. Strabismus 2012, 49, 230–235. [Google Scholar] [CrossRef]
- Nagai, N.; Kubota, S.; Tsubota, K.; Ozawa, Y. Resveratrol prevents the development of choroidal neovascularization by modulating AMP-activated protein kinase in macrophages and other cell types. J. Nutr. Biochem. 2014, 25, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Guerin, K.I.; Chen, J.; Michan, S.; Stahl, A.; Krah, N.M.; Seaward, M.R.; Dennison, R.J.; Juan, A.M.; Hatton, C.J.; et al. Resveratrol Inhibits Pathologic Retinal Neovascularization in Vldlr(-/-) Mice. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2809–2816. [Google Scholar] [CrossRef]
- Popescu, M.; Bogdan, C.; Pintea, A.; Rugina, D.; Ionescu, C. Antiangiogenic cytokines as potential new therapeutic targets for resveratrol in diabetic retinopathy. Drug Des. Dev. 2018, 12, 1985–1996. [Google Scholar] [CrossRef]
- Chen, B.; He, T.; Xing, Y.Q.; Cao, T. Effects of quercetin on the expression of MCP-1, MMP-9 and VEGF in rats with diabetic retinopathy. Exp. Ther. Med. 2017, 14, 6022–6026. [Google Scholar] [CrossRef]
- Li, F.T.; Bai, Y.J.; Zhao, M.; Huang, L.Z.; Li, S.S.; Li, X.X.; Chen, Y. Quercetin Inhibits Vascular Endothelial Growth Factor-Induced Choroidal and Retinal Angiogenesis in vitro. Ophthalmic Res. 2015, 53, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, X.X.; Xing, N.Z.; Cao, X.G. Quercetin inhibits choroidal and retinal angiogenesis in vitro. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 373–378. [Google Scholar] [CrossRef]
- Kang, M.K.; Park, S.H.; Kim, Y.H.; Lee, E.J.; Antika, L.D.; Kim, D.Y.; Choi, Y.J.; Kang, Y.H. Dietary Compound Chrysin Inhibits Retinal Neovascularization with Abnormal Capillaries in db/db Mice. Nutrients 2016, 8, 782. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, Y.H.; Lee, S.C.; Kim, M.H.; Lee, J.H. Inhibitory Effect of Chrysin (5,7-Dihydroxyflavone) on Experimental Choroidal Neovascularization in Rats. Ophthalmic Res. 2016, 56, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Jun, J.H.; Jung, E.H.; Koo, B.A.; Kim, Y.S. Epigalloccatechin-3-gallate Inhibits Ocular Neovascularization and Vascular Permeability in Human Retinal Pigment Epithelial and Human Retinal Microvascular Endothelial Cells via Suppression of MMP-9 and VEGF Activation. Molecules 2014, 19, 12150–12172. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, C.S.; Moon, M.K.; Kim, J.S. Epicatechin breaks preformed glycated serum albumin and reverses the retinal accumulation of advanced glycation end products. Eur. J. Pharmacol. 2015, 748, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Hasebe-Takenaka, Y.; Ueda, T.; Nakanishi-Ueda, T.; Kosuge, S.; Aburada, M.; Shimada, T.; Ikeya, Y.; Onda, H.; Ogura, H.; et al. Effects of green tea fractions on oxygen-induced retinal neovascularization in the neonatal rat. J. Clin. Biochem. Nutr. 2007, 41, 43–49. [Google Scholar] [CrossRef]
- Kumar, B.; Gupta, S.K.; Srinivasan, B.P.; Nag, T.C.; Srivastava, S.; Saxena, R. Hesperetin ameliorates hyperglycemia induced retinal vasculopathy via anti-angiogenic effects in experimental diabetic rats. Vasc. Pharmacol. 2012, 57, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, W.Y.; Chiou, G.C.Y. Effect of naringenin on NaIO3-induced retinal pigment epithelium degeneration and laser-induced choroidal neovascularization in rats. Int. J. Ophthalmol. 2010, 3, 5–8. [Google Scholar] [CrossRef]
- Xu, X.R.; Yu, H.T.; Hang, L.; Shao, Y.; Ding, S.H.; Yang, X.W. Preparation of Naringenin/beta-Cyclodextrin Complex and Its More Potent Alleviative Effect on Choroidal Neovascularization in Rats. Biomed Res. Int. 2014. [Google Scholar] [CrossRef]
- Yang, S.J.; Jo, H.; Kim, J.G.; Jung, S.H. Baicalin Attenuates Laser-Induced Choroidal Neovascularization. Curr. Eye Res. 2014, 39, 745–751. [Google Scholar] [CrossRef]
- Park, S.W.; Cho, C.S.; Jun, H.O.; Ryu, N.H.; Kim, J.H.; Yu, Y.S.; Kim, J.S.; Kim, J.H. Anti-Angiogenic Effect of Luteolin on Retinal Neovascularization via Blockade of Reactive Oxygen Species Production. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7718–7726. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Yu, Y.S.; Park, K.H.; Kang, H.J.; Lee, H.Y.; Kim, K.W. Antiangiogenic effect of deguelin on choroidal neovascularization. J. Pharmacol. Exp. Ther. 2008, 324, 643–647. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Yu, Y.S.; Shin, J.Y.; Lee, H.Y.; Kim, K.W. Deguelin inhibits retinal neovascularization by down-regulation of HIF-1 alpha in oxygen-induced retinopathy. J. Cell. Mol. Med. 2008, 12, 2407–2415. [Google Scholar] [CrossRef]
- An, H.; Lee, S.; Lee, J.M.; Jo, D.H.; Kim, J.; Jeong, Y.S.; Heo, M.J.; Cho, C.S.; Choi, H.; Seo, J.H.; et al. Novel Hypoxia-Inducible Factor 1alpha (HIF-1alpha) Inhibitors for Angiogenesis-Related Ocular Diseases: Discovery of a Novel Scaffold via Ring-Truncation Strategy. J. Med. Chem. 2018, 61, 9266–9286. [Google Scholar] [CrossRef]
- Basavarajappa, H.D.; Lee, B.; Lee, H.; Sulaiman, R.S.; An, H.; Magana, C.; Shadmand, M.; Vayl, A.; Rajashekhar, G.; Kim, E.Y.; et al. Synthesis and Biological Evaluation of Novel Homoisoflavonoids for Retinal Neovascularization. J. Med. Chem. 2015, 58, 5015–5027. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Yu, Y.S.; Jun, H.O.; Kwon, H.J.; Park, K.H.; Kim, K.W. Inhibition of choroidal neovascularization by homoisoflavanone, a new angiogenesis inhibitor. Mol. Vis. 2008, 14, 556–561. [Google Scholar]
- Lamoke, F.; Labazi, M.; Montemari, A.; Parisi, G.; Varano, M.; Bartoli, M. Trans-Chalcone prevents VEGF expression and retinal neovascularization in the ischemic retina. Exp. Eye Res. 2011, 93, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Jhanji, V.; Liu, H.M.; Law, K.; Lee, V.Y.W.; Huang, S.F.; Pang, C.P.; Yam, G.H.F. Isoliquiritigenin from licorice root suppressed neovascularisation in experimental ocular angiogenesis models. Br. J. Ophthalmol. 2011, 95, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, C.S.; Lee, Y.M.; Sohn, E.; Jo, K.; Kim, J.S. Vaccinium myrtillus extract prevents or delays the onset of diabetes-induced blood-retinal barrier breakdown. Int. J. Food Sci. Nutr. 2015, 66, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Yanai, R.; Chen, S.; Uchi, S.H.; Nanri, T.; Connor, K.M.; Kimura, K. Attenuation of choroidal neovascularization by dietary intake of omega-3 long-chain polyunsaturated fatty acids and lutein in mice. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Maeng, Y.S.; Maharjan, S.; Kim, J.H.; Park, J.H.; Yu, Y.S.; Kim, Y.M.; Kwon, Y.G. Rk1, a Ginsenoside, Is a New Blocker of Vascular Leakage Acting through Actin Structure Remodeling. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.W.; Wan, X.S.; Shao, N.; Ye, R.Y.; Zhang, N.; Zhang, Y.J. Protective and anti-angiopathy effects of ginsenoside Re against diabetes mellitus via the activation of p38 MAPK, ERK1/2 and JNK signaling. Mol. Med. Rep. 2016, 14, 4849–4856. [Google Scholar] [CrossRef] [PubMed]
- Lulli, M.; Cammalleri, M.; Fornaciari, I.; Casini, G.; Dal Monte, M. Acetyl-11-keto-beta-boswellic acid reduces retinal angiogenesis in a mouse model of oxygen-induced retinopathy. Exp. Eye Res. 2015, 135, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, J.; Kim, C.S.; Jo, K.; Yoo, N.H.; Sohn, E.; Kim, J.S. Anti-glycation and anti-angiogenic activities of 5′-methoxybiphenyl-3,4,3′-triol, a novel phytochemical component of Osteomeles schwerinae. Eur. J. Pharmacol. 2015, 760, 172–178. [Google Scholar] [CrossRef]
- Hong, T.Y.; Tzeng, T.F.; Liou, S.S.; Liu, I.M. The ethanol extract of Zingiber zerumbet rhizomes mitigates vascular lesions in the diabetic retina. Vasc. Pharmacol. 2016, 76, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.H.; Huang, J.M.; Li, W.; Tang, M.K. Protective Effects of Fufang Xueshuantong on Diabetic Retinopathy in Rats. Evid.-Based Complement. Altern. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.J.; Yu, S.Y.; Tang, M.K.; Duan, H.H.; Huang, J.M. A combination of the main constituents of Fufang Xueshuantong Capsules shows protective effects against streptozotocin-induced retinal lesions in rats. J. Ethnopharmacol. 2016, 182, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.H.; Guo, Y.J.; Li, X.J.; Li, X.M.; Li, Z.P.; Xue, M.; Ou, Z.M.; Liu, M.; Yang, M.X.; Liu, S.H.; et al. An Aqueous Extract of Radix Astragali, Angelica sinensis, and Panax notoginseng Is Effective in Preventing Diabetic Retinopathy. Evid.-Based Complement. Altern. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.J.; Hu, Y.N.; Lin, S.; Ma, W.J.; Li, X.R. Application of Lutein and Zeaxanthin in nonproliferative diabetic retinopathy. Int. J. Ophthalmol. 2011, 4, 303–306. [Google Scholar] [CrossRef]
- Zhang, P.C.; Wu, C.R.; Wang, Z.L.; Wang, L.Y.; Han, Y.; Sun, S.L.; Li, Q.S.; Ma, L. Effect of lutein supplementation on visual function in nonproliferative diabetic retinopathy. Asia Pac. J. Clin. Nutr. 2017, 26, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Sepahi, S.; Mohajeri, S.A.; Hosseini, S.M.; Khodaverdi, E.; Shoeibi, N.; Namdari, M.; Tabassi, S.A.S. Effects of Crocin on Diabetic Maculopathy: A Placebo-Controlled Randomized Clinical Trial. Am. J. Ophthalmol. 2018, 190, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Brazionis, L.; Rowley, K.; Itsiopoulos, C.; O’Dea, K. Plasma carotenoids and diabetic retinopathy. Br. J. Nutr. 2009, 101, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.C.; Rosen, R.B.; Maia, M.; Prata, T.S.; Dorairaj, S.; Farah, M.E.; Sallum, J. Macular Pigment Optical Density Measured by Dual-Wavelength Autofluorescence Imaging in Diabetic and Nondiabetic Patients: A Comparative Study. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5840–5845. [Google Scholar] [CrossRef] [PubMed]
- Moschos, M.M.; Dettoraki, M.; Tsatsos, M.; Kitsos, G.; Kalogeropoulos, C. Effect of carotenoids dietary supplementation on macular function in diabetic patients. Eye Vis. 2017, 4. [Google Scholar] [CrossRef]
- Cuomo, J.; Appendino, G.; Dern, A.S.; Schneider, E.; McKinnon, T.P.; Brown, M.J.; Togni, S.; Dixon, B.M. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J. Nat. Prod. 2011, 74, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Steigerwalt, R.; Nebbioso, M.; Appendino, G.; Belcaro, G.; Ciammaichella, G.; Cornelli, U.; Luzzi, R.; Togni, S.; Dugall, M.; Cesarone, M.R.; et al. Meriva (R), a lecithinized curcumin delivery system, in diabetic microangiopathy and retinopathy. Panminerva Med. 2012, 54, 11–16. [Google Scholar] [PubMed]
- Ma, Q.H.; Chen, D.D.; Sun, H.P.; Yan, N.; Xu, Y.; Pan, C.W. Regular Chinese Green Tea Consumption Is Protective for Diabetic Retinopathy: A Clinic-Based Case-Control Study. J. Diabetes Res. 2015. [Google Scholar] [CrossRef]
- Acosta, E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr. Opin. Colloid Interface Sci. 2009, 14, 3–15. [Google Scholar] [CrossRef]
- Huang, Q.R.; Yu, H.L.; Ru, Q.M. Bioavailability and Delivery of Nutraceuticals Using Nanotechnology. J. Food Sci. 2010, 75, R50–R57. [Google Scholar] [CrossRef]
- Ting, Y.W.; Jiang, Y.; Ho, C.T.; Huang, Q.R. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J. Funct. Foods 2014, 7, 112–128. [Google Scholar] [CrossRef]
- Yao, M.F.; McClements, D.J.; Xiao, H. Improving oral bioavailability of nutraceuticals by engineered nanoparticle-based delivery systems. Curr. Opin. Food Sci. 2015, 2, 14–19. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Granata, G.; Paterniti, I.; Geraci, C.; Cunsolo, F.; Esposito, E.; Cordaro, M.; Blanco, A.R.; Cuzzocrea, S.; Consoli, G.M.L. Potential Eye Drop Based on a Calix[4]arene Nanoassembly for Curcumin Delivery: Enhanced Drug Solubility, Stability, and Anti Inflammatory Effect. Mol. Pharm. 2017, 14, 1610–1622. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.M.; Pahlitzsch, M.; Guo, L.; Balendra, S.; Shah, P.; Ravindran, N.; Malaguarnera, G.; Sisa, C.; Shamsher, E.; Hamze, H.; et al. Topical Curcumin Nanocarriers are Neuroprotective in Eye Disease. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Rotches-Ribalta, M.; Andres-Lacueva, C.; Estruch, R.; Escribano, E.; Urpi-Sarda, M. Pharmacokinetics of resveratrol metabolic profile in healthy humans after moderate consumption of red wine and grape extract tablets. Pharmacol. Res. 2012, 66, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Bonechi, C.; Martini, S.; Ciani, L.; Lamponi, S.; Rebmann, H.; Rossi, C.; Ristori, S. Using Liposomes as Carriers for Polyphenolic Compounds: The Case of Trans-Resveratrol. PLoS ONE 2012, 7, e41438. [Google Scholar] [CrossRef]
- Jung, K.H.; Lee, J.H.; Park, J.W.; Quach, C.H.T.; Moon, S.H.; Cho, Y.S.; Lee, K.H. Resveratrol-loaded polymeric nanoparticles suppress glucose metabolism and tumor growth in vitro and in vivo. Int. J. Pharm. 2015, 478, 251–257. [Google Scholar] [CrossRef]
- Summerlin, N.; Soo, E.; Thakur, S.; Qu, Z.; Jambhrunkar, S.; Popat, A. Resveratrol nanoformulations: Challenges and opportunities. Int. J. Pharm. 2015, 479, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.H.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef]
- Granja, A.; Frias, I.; Neves, A.R.; Pinheiro, M.; Reis, S. Therapeutic Potential of Epigallocatechin Gallate Nanodelivery Systems. Biomed Res. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Minnelli, C.; Moretti, P.; Fulgenzi, G.; Mariani, P.; Laudadio, E.; Armeni, T.; Galeazzi, R.; Mobbili, G. A Poloxamer-407 modified liposome encapsulating epigallocatechin-3-gallate in the presence of magnesium: Characterization and protective effect against oxidative damage. Int. J. Pharm. 2018, 552, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Li, Q.; Rubakhin, S.S.; Sweedler, J.V.; Smith, J.W.; Neuringer, M.; Kuchan, M.; Erdman, J.W., Jr. (13)C-lutein is differentially distributed in tissues of an adult female rhesus macaque following a single oral administration: A pilot study. Nutr. Res. 2019, 61, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Chittasupho, C.; Posritong, P.; Ariyawong, P. Stability, Cytotoxicity, and Retinal Pigment Epithelial Cell Binding of Hyaluronic Acid-Coated PLGA Nanoparticles Encapsulating Lutein. AAPS Pharmscitech 2018, 20. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossino, M.G.; Casini, G. Nutraceuticals for the Treatment of Diabetic Retinopathy. Nutrients 2019, 11, 771. https://doi.org/10.3390/nu11040771
Rossino MG, Casini G. Nutraceuticals for the Treatment of Diabetic Retinopathy. Nutrients. 2019; 11(4):771. https://doi.org/10.3390/nu11040771
Chicago/Turabian StyleRossino, Maria Grazia, and Giovanni Casini. 2019. "Nutraceuticals for the Treatment of Diabetic Retinopathy" Nutrients 11, no. 4: 771. https://doi.org/10.3390/nu11040771
APA StyleRossino, M. G., & Casini, G. (2019). Nutraceuticals for the Treatment of Diabetic Retinopathy. Nutrients, 11(4), 771. https://doi.org/10.3390/nu11040771





