Mechanisms of Carotenoid Intestinal Absorption: Where Do We Stand?
Abstract
:1. Introduction
2. Digestion Process of Carotenoids
3. Carotenoid Absorption through the Enterocyte
3.1. Apical Transport Across the Brush Border Membrane of the Enterocyte
3.2. Cytosolic Transport and Intracellular Metabolism
3.3. Secretion Through the Basolateral Membrane of the Enterocyte
4. Regulation of Carotenoid Transporter Expressions in the Enterocyte
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ABCA1 | ATP binding cassette A1 |
| ABCB1 | ATB binding cassette B1 |
| ABCG5 | ATP binding cassette G5 |
| BCO1 | β-carotene-oxygenase 1 |
| BCO2 | β-carotene-oxygenase 2 |
| CD36 | CD36 molecule |
| CEH | cholesterol ester hydrolase |
| DGAT1 | diacylglycerol acyltransferase 1 |
| HR-LBP | human retinal lutein-binding protein |
| ISX | intestine-specific homebox |
| FABP | fatty-acid-binding protein |
| HDL | high-density lipoproteins |
| LRAT | lecithin retinol acyltransferase |
| NPC1L1 | NPC1-like transporter 1 |
| SR-BI | scavenger receptor class B type 1 |
References
- Khachik, F.; Spangler, C.J.; Smith, J.C., Jr.; Canfield, L.M.; Steck, A.; Pfander, H. Identification, quantification, and relative concentrations of carotenoids and their metabolites in human milk and serum. Anal. Chem. 1997, 69, 1873–1881. [Google Scholar] [PubMed]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The role of carotenoids in the prevention of human pathologies. Biomed. Pharmacother. 2004, 58, 100–110. [Google Scholar] [CrossRef]
- Engelmann, N.J.; Clinton, S.K.; Erdman, J.W., Jr. Nutritional aspects of phytoene and phytofluene, carotenoid precursors to lycopene. Adv. Nutr. 2011, 2, 51–61. [Google Scholar] [PubMed]
- Moise, A.R.; Al-Babili, S.; Wurtzel, E.T. Mechanistic aspects of carotenoid biosynthesis. Chem. Rev. 2014, 114, 164–193. [Google Scholar] [CrossRef] [PubMed]
- Breithaupt, D.E.; Bamedi, A.; Wirt, U. Carotenol fatty acid esters: Easy substrates for digestive enzymes? Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 132, 721–728. [Google Scholar] [CrossRef]
- Bowen, P.E.; Herbst-Espinosa, S.M.; Hussain, E.A.; Stacewicz-Sapuntzakis, M. Esterification does not impair lutein bioavailability in humans. J. Nutr. 2002, 132, 3668–3673. [Google Scholar] [CrossRef]
- Shi, J.; Le Maguer, M. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit. Rev. Biotechnol. 2000, 20, 293–334. [Google Scholar] [CrossRef]
- Rodriguez, E.B.; Rodriguez-Amaya, D.B. Formation of apocarotenals and epoxycarotenoids from beta-carotene by chemical reactions and by autoxidation in model systems and processed foods. Food Chem. 2007, 101, 563–572. [Google Scholar] [CrossRef]
- Kopec, R.E.; Riedl, K.M.; Harrison, E.H.; Curley, R.W., Jr.; Hruszkewycz, D.P.; Clinton, S.K.; Schwartz, S.J. Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. J. Agric. Food Chem. 2010, 58, 3290–3296. [Google Scholar] [CrossRef]
- Reboul, E.; Richelle, M.; Perrot, E.; Desmoulins-Malezet, C.; Pirisi, V.; Borel, P. Bioaccessibility of carotenoids and vitamin e from their main dietary sources. J. Agric. Food Chem. 2006, 54, 8749–8755. [Google Scholar] [CrossRef] [PubMed]
- Mapelli-Brahm, P.; Corte-Real, J.; Melendez-Martinez, A.J.; Bohn, T. Bioaccessibility of phytoene and phytofluene is superior to other carotenoids from selected fruit and vegetable juices. Food Chem. 2017, 229, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I.; Yeum, K.J. Carotenoid-radical interactions. Biochem. Biophys. Res. Commun. 2003, 305, 754–760. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—Implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef] [PubMed]
- Scripsema, N.K.; Hu, D.N.; Rosen, R.B. Lutein, zeaxanthin, and meso-zeaxanthin in the clinical management of eye disease. J. Ophthalmol. 2015, 2015, 865179. [Google Scholar] [CrossRef]
- Borel, P.; Grolier, P.; Armand, M.; Partier, A.; Lafont, H.; Lairon, D.; Azais-Braesco, V. Carotenoids in biological emulsions: Solubility, surface-to-core distribution, and release from lipid droplets. J. Lipid Res. 1996, 37, 250–261. [Google Scholar] [PubMed]
- Tyssandier, V.; Reboul, E.; Dumas, J.F.; Bouteloup-Demange, C.; Armand, M.; Marcand, J.; Sallas, M.; Borel, P. Processing of vegetable-borne carotenoids in the human stomach and duodenum. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G913–G923. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Fraser, P.D.; Long, M.; Bramley, P.M.; Rice-Evans, C. Isomerization of lycopene in the gastric milieu. Biochem. Biophys. Res. Commun. 2001, 281, 576–581. [Google Scholar] [CrossRef]
- Kopec, R.E.; Gleize, B.; Borel, P.; Desmarchelier, C.; Caris-Veyrat, C. Are lutein, lycopene, and beta-carotene lost through the digestive process? Food Funct. 2017, 8, 1494–1503. [Google Scholar] [CrossRef]
- Dhuique-Mayer, C.; Borel, P.; Reboul, E.; Caporiccio, B.; Besancon, P.; Amiot, M.J. Beta-cryptoxanthin from citrus juices: Assessment of bioaccessibility using an in vitro digestion/caco-2 cell culture model. Br. J. Nutr. 2007, 97, 883–890. [Google Scholar] [CrossRef]
- Reboul, E. Absorption of vitamin a and carotenoids by the enterocyte: Focus on transport proteins. Nutrients 2013, 5, 3563–3581. [Google Scholar] [CrossRef]
- Mensi, A.; Borel, P.; Goncalves, A.; Nowicki, M.; Gleize, B.; Roi, S.; Chobert, J.M.; Haertle, T.; Reboul, E. Beta-lactoglobulin as a vector for beta-carotene food fortification. J. Agric. Food Chem. 2014, 62, 5916–5924. [Google Scholar] [CrossRef]
- Phan, C.T.; Tso, P. Intestinal lipid absorption and transport. Front. Biosci. 2001, 6, D299–D319. [Google Scholar] [CrossRef] [PubMed]
- van Lieshout, M.; West, C.E.; van Breemen, R.B. Isotopic tracer techniques for studying the bioavailability and bioefficacy of dietary carotenoids, particularly beta-carotene, in humans: A review. Am. J. Clin. Nutr. 2003, 77, 12–28. [Google Scholar] [CrossRef]
- Van Loo-Bouwman, C.A.; Naber, T.H.; van Breemen, R.B.; Zhu, D.; Dicke, H.; Siebelink, E.; Hulshof, P.J.; Russel, F.G.; Schaafsma, G.; West, C.E. Vitamin a equivalency and apparent absorption of beta-carotene in ileostomy subjects using a dual-isotope dilution technique. Br. J. Nutr. 2010, 103, 1836–1843. [Google Scholar] [CrossRef]
- Borel, P.; Grolier, P.; Mekki, N.; Boirie, Y.; Rochette, Y.; Le Roy, B.; Alexandre-Gouabau, M.C.; Lairon, D.; Azais-Braesco, V. Low and high responders to pharmacological doses of beta-carotene: Proportion in the population, mechanisms involved and consequences on beta-carotene metabolism. J. Lipid Res. 1998, 39, 2250–2260. [Google Scholar]
- During, A.; Hussain, M.M.; Morel, D.W.; Harrison, E.H. Carotenoid uptake and secretion by caco-2 cells: Beta-carotene isomer selectivity and carotenoid interactions. J. Lipid Res. 2002, 43, 1086–1095. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Desmarchelier, C.; Margier, M.; Reboul, E.; Melendez Martinez, A.J.; Borel, P. Phytoene and phytofluene isolated from a tomato extract are readily incorporated in mixed micelles and absorbed by caco-2 cells, as compared to lycopene, and sr-bi is involved in their cellular uptake. Mol. Nutr. Food Res. 2018, 62, e1800703. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, C.; Sumser, E.; Wernet, M.F.; Von Lintig, J. A class b scavenger receptor mediates the cellular uptake of carotenoids in drosophila. Proc. Natl. Acad. Sci. USA 2002, 99, 10581–10586. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E.; Soayfane, Z.; Goncalves, A.; Cantiello, M.; Bott, R.; Nauze, M.; Terce, F.; Collet, X.; Comera, C. Respective contributions of intestinal niemann-pick c1-like 1 and scavenger receptor class b type i to cholesterol and tocopherol uptake: In vivo v. In vitro studies. Br. J. Nutr. 2012, 107, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Hauser, H.; Dyer, J.H.; Nandy, A.; Vega, M.A.; Werder, M.; Bieliauskaite, E.; Weber, F.E.; Compassi, S.; Gemperli, A.; Boffelli, D.; et al. Identification of a receptor mediating absorption of dietary cholesterol in the intestine. Biochemistry 1998, 37, 17843–17850. [Google Scholar] [CrossRef] [PubMed]
- Bietrix, F.; Yan, D.; Nauze, M.; Rolland, C.; Bertrand-Michel, J.; Comera, C.; Schaak, S.; Barbaras, R.; Groen, A.K.; Perret, B.; et al. Accelerated lipid absorption in mice overexpressing intestinal sr-bi. J. Biol. Chem. 2006, 281, 7214–7219. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Drover, V.A.; Knopfel, M.; Dhanasekaran, P.; Hauser, H.; Phillips, M.C. Influence of class b scavenger receptors on cholesterol flux across the brush border membrane and intestinal absorption. J. Lipid Res. 2009, 50, 2235–2244. [Google Scholar] [CrossRef]
- Saddar, S.; Carriere, V.; Lee, W.R.; Tanigaki, K.; Yuhanna, I.S.; Parathath, S.; Morel, E.; Warrier, M.; Sawyer, J.K.; Gerard, R.D.; et al. Scavenger receptor class b type i is a plasma membrane cholesterol sensor. Circ. Res. 2013, 112, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Lino, M.; Farr, S.; Baker, C.; Fuller, M.; Trigatti, B.; Adeli, K. Intestinal scavenger receptor class b type i as a novel regulator of chylomicron production in healthy and diet-induced obese states. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G350–G359. [Google Scholar] [CrossRef]
- Moussa, M.; Landrier, J.F.; Reboul, E.; Ghiringhelli, O.; Comera, C.; Collet, X.; Frohlich, K.; Bohm, V.; Borel, P. Lycopene absorption in human intestinal cells and in mice involves scavenger receptor class b type i but not niemann-pick c1-like 1. J. Nutr. 2008, 138, 1432–1436. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Lietz, G.; Goncalves, A.; Szabo de Edelenyi, F.; Lecompte, S.; Curtis, P.; Goumidi, L.; Caslake, M.J.; Miles, E.A.; Packard, C.; et al. Cd36 and sr-bi are involved in cellular uptake of provitamin a carotenoids by caco-2 and hek cells, and some of their genetic variants are associated with plasma concentrations of these micronutrients in humans. J. Nutr. 2013, 143, 448–456. [Google Scholar] [CrossRef]
- Reboul, E.; Goncalves, A.; Comera, C.; Bott, R.; Nowicki, M.; Landrier, J.F.; Jourdheuil-Rahmani, D.; Dufour, C.; Collet, X.; Borel, P. Vitamin d intestinal absorption is not a simple passive diffusion: Evidences for involvement of cholesterol transporters. Mol. Nutr. Food Res. 2011, 55, 691–702. [Google Scholar] [CrossRef]
- Reboul, E.; Klein, A.; Bietrix, F.; Gleize, B.; Malezet-Desmoulins, C.; Schneider, M.; Margotat, A.; Lagrost, L.; Collet, X.; Borel, P. Scavenger receptor class b type i (sr-bi) is involved in vitamin e transport across the enterocyte. J. Biol. Chem. 2006, 281, 4739–4745. [Google Scholar] [CrossRef]
- Goncalves, A.; Margier, M.; Roi, S.; Collet, X.; Niot, I.; Goupy, P.; Caris-Veyrat, C.; Reboul, E. Intestinal scavenger receptors are involved in vitamin k1 absorption. J. Biol. Chem. 2014, 289, 30743–30752. [Google Scholar] [CrossRef]
- Terpstra, V.; van Amersfoort, E.S.; van Velzen, A.G.; Kuiper, J.; van Berkel, T.J. Hepatic and extrahepatic scavenger receptors: Function in relation to disease. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1860–1872. [Google Scholar] [CrossRef]
- Drover, V.A.; Nguyen, D.V.; Bastie, C.C.; Darlington, Y.F.; Abumrad, N.A.; Pessin, J.E.; London, E.; Sahoo, D.; Phillips, M.C. Cd36 mediates both cellular uptake of very long chain fatty acids and their intestinal absorption in mice. J. Biol. Chem. 2008, 283, 13108–13115. [Google Scholar] [CrossRef] [PubMed]
- Rigotti, A.; Acton, S.L.; Krieger, M. The class b scavenger receptors sr-bi and cd36 are receptors for anionic phospholipids. J. Biol. Chem. 1995, 270, 16221–16224. [Google Scholar] [CrossRef] [PubMed]
- Endemann, G.; Stanton, L.W.; Madden, K.S.; Bryant, C.M.; White, R.T.; Protter, A.A. Cd36 is a receptor for oxidized low density lipoprotein. J. Biol. Chem. 1993, 268, 11811–11816. [Google Scholar] [PubMed]
- Buttet, M.; Traynard, V.; Tran, T.T.; Besnard, P.; Poirier, H.; Niot, I. From fatty-acid sensing to chylomicron synthesis: Role of intestinal lipid-binding proteins. Biochimie 2014, 96, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.; Roi, S.; Nowicki, M.; Niot, I.; Reboul, E. Cluster-determinant 36 (cd36) impacts on vitamin e postprandial response. Mol. Nutr. Food Res. 2014, 58, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Moussa, M.; Gouranton, E.; Gleize, B.; El Yazidi, C.; Niot, I.; Besnard, P.; Borel, P.; Landrier, J.F. Cd36 is involved in lycopene and lutein uptake by adipocytes and adipose tissue cultures. Mol. Nutr. Food Res. 2011, 55, 578–584. [Google Scholar] [CrossRef]
- van Bennekum, A.; Werder, M.; Thuahnai, S.T.; Han, C.H.; Duong, P.; Williams, D.L.; Wettstein, P.; Schulthess, G.; Phillips, M.C.; Hauser, H. Class b scavenger receptor-mediated intestinal absorption of dietary beta-carotene and cholesterol. Biochemistry 2005, 44, 4517–4525. [Google Scholar] [CrossRef]
- Davis, H.R., Jr.; Altmann, S.W. Niemann-pick c1 like 1 (npc1l1) an intestinal sterol transporter. Biochim. Biophys. Acta 2009, 1791, 679–683. [Google Scholar] [CrossRef]
- During, A.; Dawson, H.D.; Harrison, E.H. Carotenoid transport is decreased and expression of the lipid transporters sr-bi, npc1l1, and abca1 is downregulated in caco-2 cells treated with ezetimibe. J. Nutr. 2005, 135, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Suzuki, R.; Kobayashi, M.; Itagaki, S.; Hirano, T.; Noda, T.; Mizuno, S.; Sugawara, M.; Iseki, K. Involvement of cholesterol membrane transporter niemann-pick c1-like 1 in the intestinal absorption of lutein. J. Pharm. Pharm. Sci. 2012, 15, 256–264. [Google Scholar] [CrossRef]
- Margier, M.; Collet, X.; le May, C.; Desmarchelier, C.; Andre, F.; Lebrun, C.; Defoort, C.; Bluteau, A.; Borel, P.; Lespine, A.; et al. Abcb1 (p-glycoprotein) regulates vitamin d absorption and contributes to its transintestinal efflux. FASEB J. 2019, 33, 2084–2094. [Google Scholar] [CrossRef] [PubMed]
- Herron, K.L.; McGrane, M.M.; Waters, D.; Lofgren, I.E.; Clark, R.M.; Ordovas, J.M.; Fernandez, M.L. The abcg5 polymorphism contributes to individual responses to dietary cholesterol and carotenoids in eggs. J. Nutr. 2006, 136, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Tabunoki, H.; Sugiyama, H.; Tanaka, Y.; Fujii, H.; Banno, Y.; Jouni, Z.E.; Kobayashi, M.; Sato, R.; Maekawa, H.; Tsuchida, K. Isolation, characterization, and cdna sequence of a carotenoid binding protein from the silk gland of bombyx mori larvae. J. Biol. Chem. 2002, 277, 32133–32140. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.; Li, B.; Sharifzadeh, M.; Gellermann, W.; Frederick, J.M.; Tsuchida, K.; Bernstein, P.S. Purification and partial characterization of a lutein-binding protein from human retina. Biochemistry 2009, 48, 4798–4807. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Moussa, M.; Reboul, E.; Lyan, B.; Defoort, C.; Vincent-Baudry, S.; Maillot, M.; Gastaldi, M.; Darmon, M.; Portugal, H.; et al. Human fasting plasma concentrations of vitamin e and carotenoids, and their association with genetic variants in apo c-iii, cholesteryl ester transfer protein, hepatic lipase, intestinal fatty acid binding protein and microsomal triacylglycerol transfer protein. Br. J. Nutr. 2009, 101, 680–687. [Google Scholar] [PubMed]
- Castenmiller, J.J.M.; West, C.E. Bioavailability and bioconversion of carotenoids. Annu. Rev. Nutr. 1998, 18, 19–38. [Google Scholar] [CrossRef] [PubMed]
- dela Sena, C.; Riedl, K.M.; Narayanasamy, S.; Curley, R.W., Jr.; Schwartz, S.J.; Harrison, E.H. The human enzyme that converts dietary provitamin a carotenoids to vitamin a is a dioxygenase. J. Biol. Chem. 2014, 289, 13661–13666. [Google Scholar] [CrossRef] [PubMed]
- Lobo, G.P.; Amengual, J.; Palczewski, G.; Babino, D.; von Lintig, J. Mammalian carotenoid-oxygenases: Key players for carotenoid function and homeostasis. Biochim. Biophys. Acta 2012, 1821, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J.; Widjaja-Adhi, M.A.; Rodriguez-Santiago, S.; Hessel, S.; Golczak, M.; Palczewski, K.; von Lintig, J. Two carotenoid oxygenases contribute to mammalian provitamin a metabolism. J. Biol. Chem. 2013, 288, 34081–34096. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, S.M.; Wongsiriroj, N.; Libien, J.; Vogel, S.; Goldberg, I.J.; Baehr, W.; Palczewski, K.; Blaner, W.S. Retinoid absorption and storage is impaired in mice lacking lecithin:Retinol acyltransferase (lrat). J. Biol. Chem. 2005, 280, 35647–35657. [Google Scholar] [CrossRef]
- Wongsiriroj, N.; Piantedosi, R.; Palczewski, K.; Goldberg, I.J.; Johnston, T.P.; Li, E.; Blaner, W.S. The molecular basis of retinoid absorption: A genetic dissection. J. Biol. Chem. 2008, 283, 13510–13519. [Google Scholar] [CrossRef]
- Palczewski, G.; Amengual, J.; Hoppel, C.L.; von Lintig, J. Evidence for compartmentalization of mammalian carotenoid metabolism. FASEB J. 2014, 28, 4457–4469. [Google Scholar] [CrossRef] [Green Version]
- Kopec, R.E.; Caris-Veyrat, C.; Nowicki, M.; Gleize, B.; Carail, M.; Borel, P. Production of asymmetric oxidative metabolites of [13c]-beta-carotene during digestion in the gastrointestinal lumen of healthy men. Am. J. Clin. Nutr. 2018, 108, 803–813. [Google Scholar] [CrossRef]
- You, C.S.; Parker, R.S.; Goodman, K.J.; Swanson, J.E.; Corso, T.N. Evidence of cis-trans isomerization of 9-cis-beta-carotene during absorption in humans. Am. J. Clin. Nutr. 1996, 64, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Richelle, M.; Sanchez, B.; Tavazzi, I.; Lambelet, P.; Bortlik, K.; Williamson, G. Lycopene isomerisation takes place within enterocytes during absorption in human subjects. Br. J. Nutr. 2010, 103, 1800–1807. [Google Scholar] [CrossRef] [Green Version]
- Reboul, E.; Trompier, D.; Moussa, M.; Klein, A.; Landrier, J.F.; Chimini, G.; Borel, P. Atp-binding cassette transporter a1 is significantly involved in the intestinal absorption of alpha- and gamma-tocopherol but not in that of retinyl palmitate in mice. Am. J. Clin. Nutr. 2009, 89, 177–184. [Google Scholar] [CrossRef]
- Niesor, E.J.; Chaput, E.; Mary, J.L.; Staempfli, A.; Topp, A.; Stauffer, A.; Wang, H.; Durrwell, A. Effect of compounds affecting abca1 expression and cetp activity on the hdl pathway involved in intestinal absorption of lutein and zeaxanthin. Lipids 2014, 49, 1233–1243. [Google Scholar] [CrossRef]
- Borel, P.; Desmarchelier, C.; Nowicki, M.; Bott, R. Lycopene bioavailability is associated with a combination of genetic variants. Free Radic. Biol. Med. 2015, 83, 238–244. [Google Scholar] [CrossRef]
- Borel, P.; Desmarchelier, C.; Nowicki, M.; Bott, R. A combination of single-nucleotide polymorphisms is associated with interindividual variability in dietary beta-carotene bioavailability in healthy men. J. Nutr. 2015, 145, 1740–1747. [Google Scholar] [CrossRef]
- Borel, P.; Desmarchelier, C.; Nowicki, M.; Bott, R.; Morange, S.; Lesavre, N. Interindividual variability of lutein bioavailability in healthy men: Characterization, genetic variants involved, and relation with fasting plasma lutein concentration. Am. J. Clin. Nutr. 2014, 100, 168–175. [Google Scholar] [CrossRef]
- Seino, Y.; Miki, T.; Kiyonari, H.; Abe, T.; Fujimoto, W.; Kimura, K.; Takeuchi, A.; Takahashi, Y.; Oiso, Y.; Iwanaga, T.; et al. Isx participates in the maintenance of vitamin a metabolism by regulation of beta-carotene 15,15′-monooxygenase (bcmo1) expression. J. Biol. Chem. 2008, 283, 4905–4911. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.Y.; Romer, A.I.; Hu, M.; Lepourcelet, M.; Mechoor, A.; Yesilaltay, A.; Krieger, M.; Gray, P.A.; Shivdasani, R.A. A dynamic expression survey identifies transcription factors relevant in mouse digestive tract development. Development 2006, 133, 4119–4129. [Google Scholar] [CrossRef] [Green Version]
- Lobo, G.P.; Hessel, S.; Eichinger, A.; Noy, N.; Moise, A.R.; Wyss, A.; Palczewski, K.; von Lintig, J. Isx is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin a production. FASEB J. 2010, 24, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.; Gleize, B.; Roi, S.; Nowicki, M.; Dhaussy, A.; Huertas, A.; Amiot, M.J.; Reboul, E. Fatty acids affect micellar properties and modulate vitamin d uptake and basolateral efflux in caco-2 cells. J. Nutr. Biochem. 2013, 24, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- de Vogel-van den Bosch, H.M.; de Wit, N.J.; Hooiveld, G.J.; Vermeulen, H.; van der Veen, J.N.; Houten, S.M.; Kuipers, F.; Muller, M.; van der Meer, R. A cholesterol-free, high-fat diet suppresses gene expression of cholesterol transporters in murine small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1171–G1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Yang, Y.; Braunstein, E.; Georgeson, K.E.; Harmon, C.M. Gut expression and regulation of fat/cd36: Possible role in fatty acid transport in rat enterocytes. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E916–E923. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.R., Jr.; Zhu, L.J.; Hoos, L.M.; Tetzloff, G.; Maguire, M.; Liu, J.; Yao, X.; Iyer, S.P.; Lam, M.H.; Lund, E.G.; et al. Niemann-pick c1 like 1 (npc1l1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 2004, 279, 33586–33592. [Google Scholar] [CrossRef]
- Jesch, E.D.; Seo, J.M.; Carr, T.P.; Lee, J.Y. Sitosterol reduces messenger rna and protein expression levels of niemann-pick c1-like 1 in fhs 74 int cells. Nutr. Res. 2009, 29, 859–866. [Google Scholar] [CrossRef]
- Brauner, R.; Johannes, C.; Ploessl, F.; Bracher, F.; Lorenz, R.L. Phytosterols reduce cholesterol absorption by inhibition of 27-hydroxycholesterol generation, liver x receptor alpha activation, and expression of the basolateral sterol exporter atp-binding cassette a1 in caco-2 enterocytes. J. Nutr. 2012, 142, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Alvaro, A.; Rosales, R.; Masana, L.; Vallve, J.C. Polyunsaturated fatty acids down-regulate in vitro expression of the key intestinal cholesterol absorption protein npc1l1: No effect of monounsaturated nor saturated fatty acids. J. Nutr. Biochem. 2010, 21, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, P.; Boddy, C.S.; Soni, V.; Saksena, S.; Dudeja, P.K.; Gill, R.K.; Alrefai, W.A. D-glucose modulates intestinal niemann-pick c1-like 1 (npc1l1) gene expression via transcriptional regulation. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G203–G210. [Google Scholar] [CrossRef] [PubMed]
- Boztepe, T.; Gulec, S. Investigation of the influence of high glucose on molecular and genetic responses: An in vitro study using a human intestine model. Genes Nutr. 2018, 13, 11. [Google Scholar] [CrossRef]
- Kim, B.; Park, Y.; Wegner, C.J.; Bolling, B.W.; Lee, J. Polyphenol-rich black chokeberry (aronia melanocarpa) extract regulates the expression of genes critical for intestinal cholesterol flux in caco-2 cells. J. Nutr. Biochem. 2013, 24, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zou, J.; Zhang, S.; Li, X.; Lu, M. Hypocholesterolemic activity of curcumin is mediated by down-regulating the expression of niemann-pick c1-like 1 in hamsters. J. Agric. Food Chem. 2017, 65, 276–280. [Google Scholar] [CrossRef]
- Hayashi, A.A.; Webb, J.; Choi, J.; Baker, C.; Lino, M.; Trigatti, B.; Trajcevski, K.E.; Hawke, T.J.; Adeli, K. Intestinal sr-bi is upregulated in insulin-resistant states and is associated with overproduction of intestinal apob48-containing lipoproteins. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G326–G337. [Google Scholar] [CrossRef] [PubMed]
- Voshol, P.J.; Schwarz, M.; Rigotti, A.; Krieger, M.; Groen, A.K.; Kuipers, F. Down-regulation of intestinal scavenger receptor class b, type i (sr-bi) expression in rodents under conditions of deficient bile delivery to the intestine. Biochem. J. 2001, 356, 317–325. [Google Scholar] [CrossRef]
- Duan, L.P.; Wang, H.H.; Ohashi, A.; Wang, D.Q. Role of intestinal sterol transporters abcg5, abcg8, and npc1l1 in cholesterol absorption in mice: Gender and age effects. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G269–G276. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, H.; Okoro, E.U.; Guo, Z. Up-regulation of cholesterol absorption is a mechanism for cholecystokinin-induced hypercholesterolemia. J. Biol. Chem. 2014, 289, 12989–12999. [Google Scholar] [CrossRef]
- Grenier, E.; Garofalo, C.; Delvin, E.; Levy, E. Modulatory role of pyy in transport and metabolism of cholesterol in intestinal epithelial cells. PLoS ONE 2012, 7, e40992. [Google Scholar] [CrossRef]
- Reboul, E. Vitamin e intestinal absorption: Regulation of membrane transport across the enterocyte. IUBMB Life 2019, 71, 416–423. [Google Scholar] [CrossRef]
- Desmarchelier, C.; Landrier, J.F.; Borel, P. Genetic factors involved in the bioavailability of tomato carotenoids. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 489–497. [Google Scholar] [CrossRef] [PubMed]

| Carotenoids | Molecular Structure | Examples of Food Sources (mg/100 g) [10,11] |
|---|---|---|
| Phytoene | 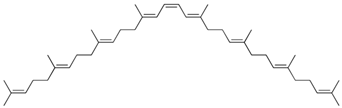 | Tomato juice: 2.24 Carrot juice: 0.94 |
| Phytofluene | 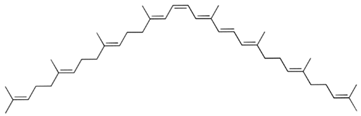 | Tomato juice: 0.86 Carrot juice: 0.59 |
| Lycopene | 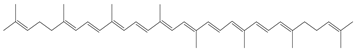 | Tomato sauce: 15.92 Tomatoes: 3.03 Watermelon: 4.87 |
| β-carotene |  | Raw carrot: 8.84 Canned carrot: 5.78 Cooked spinach: 5.24 |
| α-carotene |  | Carrot juice: 1.70 |
| β-cryptoxanthin |  | Sanguinello juice: 0.02 |
| Lutein | 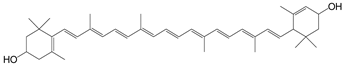 | Cooked spinach: 7.04 Lettuce: 2.64 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reboul, E. Mechanisms of Carotenoid Intestinal Absorption: Where Do We Stand? Nutrients 2019, 11, 838. https://doi.org/10.3390/nu11040838
Reboul E. Mechanisms of Carotenoid Intestinal Absorption: Where Do We Stand? Nutrients. 2019; 11(4):838. https://doi.org/10.3390/nu11040838
Chicago/Turabian StyleReboul, Emmanuelle. 2019. "Mechanisms of Carotenoid Intestinal Absorption: Where Do We Stand?" Nutrients 11, no. 4: 838. https://doi.org/10.3390/nu11040838
APA StyleReboul, E. (2019). Mechanisms of Carotenoid Intestinal Absorption: Where Do We Stand? Nutrients, 11(4), 838. https://doi.org/10.3390/nu11040838





