Nationwide Online Survey Enables the Reevaluation of the Safety of Coleus forskohlii Extract Intake Based on the Adverse Event Frequencies
Abstract
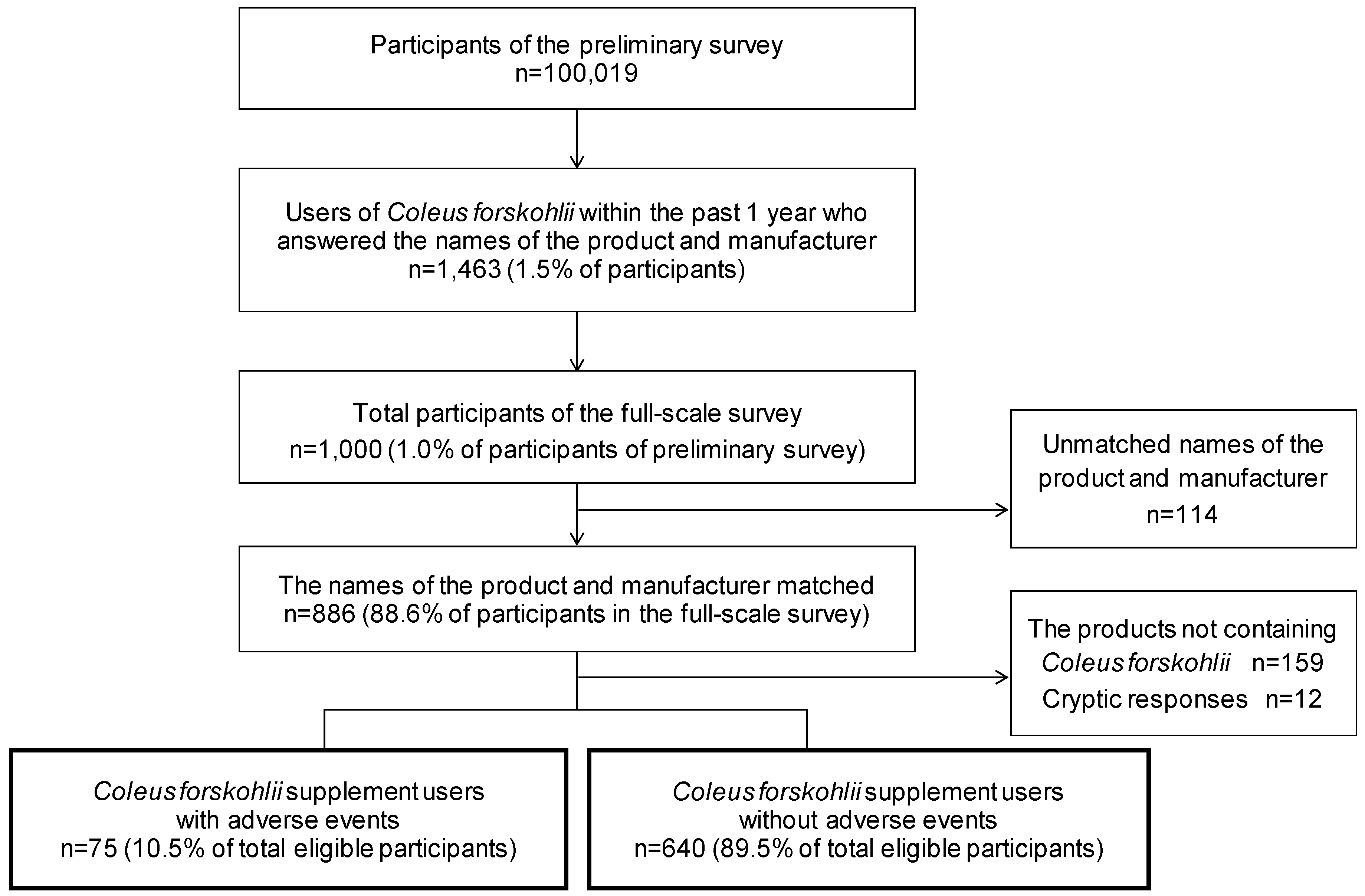
:1. Introduction
2. Materials and Methods
2.1. Online Survey Procedure
2.2. Study Population and Questionnaire
2.3. Analysis of the Survey Data
3. Results
3.1. Characteristics
3.2. Adverse Symptoms
3.3. Relationship between the Suggested Amount of Coleus forskohlii Extract and Frequency of Diarrhea
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, J.; Seo, J.E.; Wang, S.; Ashby, K.; Ballard, R.; Yu, D.; Ning, B.; Agarwal, R.; Borlak, J.; Tong, W.; et al. The Development of a Database for Herbal and Dietary Supplement Induced Liver Toxicity. Int. J. Mol. Sci. 2018, 19, 2955. [Google Scholar] [CrossRef]
- De Boer, Y.S.; Sherker, A.H. Herbal and Dietary Supplement-Induced Liver Injury. Clin. Liver Dis. 2017, 21, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.J.; Khan, I.; Bjornsson, E.; Seeff, L.B.; Serrano, J.; Hoofnagle, J.H. Liver injury from herbal and dietary supplements. Hepatology 2017, 65, 363–373. [Google Scholar] [CrossRef]
- Chiba, T.; Sato, Y.; Nakanishi, T.; Yokotani, K.; Suzuki, S.; Umegaki, K. Inappropriate usage of dietary supplements in patients by miscommunication with physicians in Japan. Nutrients 2014, 6, 5392–5404. [Google Scholar] [CrossRef]
- Nishijima, C.; Chiba, T.; Sato, Y.; Yamada, H.; Umegaki, K. Nationwide Online Survey Method to Estimate Ongoing Adverse Events Caused by Supplement Use: Application to Diarrhea. Shokuhin Eiseigaku Zasshi 2018, 59, 106–113. [Google Scholar] [CrossRef]
- Badyal, D.K.; Desai, C. Animal use in pharmacology education and research: The changing scenario. Ind. J. Pharmacol. 2014, 46, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Ukelis, U.; Kramer, P.J.; Olejniczak, K.; Mueller, S.O. Replacement of in vivo acute oral toxicity studies by in vitro cytotoxicity methods: Opportunities, limits and regulatory status. RTP 2008, 51, 108–118. [Google Scholar] [CrossRef]
- Restani, P.; Di Lorenzo, C.; Garcia-Alvarez, A.; Badea, M.; Ceschi, A.; Egan, B.; Dima, L.; Lude, S.; Maggi, F.M.; Marculescu, A.; et al. Adverse Effects of Plant Food Supplements Self-Reported by Consumers in the PlantLIBRA Survey Involving Six European Countries. PLoS ONE 2016, 11, e0150089. [Google Scholar] [CrossRef] [PubMed]
- Timbo, B.B.; Ross, M.P.; McCarthy, P.V.; Lin, C.T. Dietary supplements in a national survey: Prevalence of use and reports of adverse events. J. Am. Diet. Assoc. 2006, 106, 1966–1974. [Google Scholar] [CrossRef]
- Umegaki, K.; Yamada, H.; Chiba, T.; Nakanishi, T.; Sato, Y.; Fukuyama, S. Information sources for causality assessment of health problems related to health foods and their usefulness. Shokuhin Eiseigaku Zasshi 2013, 54, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, P.; Howlett, J.; Boeing, H.; Cockburn, A.; Constable, A.; Davi, A.; de Jong, N.; Moseley, B.; Oberdorfer, R.; Robertson, C.; et al. The application of post-market monitoring to novel foods. Food Chem. Toxicol. 2008, 46, 9–33. [Google Scholar] [CrossRef]
- Nishijima, C.; Sato, Y.; Chiba, T.; Umegaki, K. Nationwide Online Survey to Complement the Current Voluntary Reporting System for Adverse Events Associated with Dietary Supplements: Application to the Case of Skin Manifestations. J. Nutr. Sci. Vitaminol. 2018, 64, 277–283. [Google Scholar] [CrossRef]
- Ammon, H.P.; Muller, A.B. Forskolin: From an ayurvedic remedy to a modern agent. Planta Med. 1985, 51, 473–477. [Google Scholar] [CrossRef]
- Kamohara, S.; Terasaki, Y.; Horikoshi, I.; Sunayama, S. Safety of a Coleus forskohlii formulation in healthy volunteers. Pers. Med. Univ. 2015, 4, 63–65. [Google Scholar] [CrossRef]
- Umegaki, K.; Yamazaki, Y.; Yokotani, K.; Chiba, T.; Sato, Y.; Shimura, F. Induction of fatty liver by Coleus forskohlii extract through enhancement of de novo triglyceride synthesis in mice. Toxicol. Rep. 2014, 1, 787–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virgona, N.; Yokotani, K.; Yamazaki, Y.; Shimura, F.; Chiba, T.; Taki, Y.; Yamada, S.; Shinozuka, K.; Murata, M.; Umegaki, K. Coleus forskohlii extract induces hepatic cytochrome P450 enzymes in mice. Food Chem. Toxicol. 2012, 50, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Virgona, N.; Taki, Y.; Yamada, S.; Umegaki, K. Dietary Coleus forskohlii extract generates dose-related hepatotoxicity in mice. J. Appl. Toxicol. 2013, 33, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Cassinese, C.; de Combarieu, E.; Falzoni, M.; Fuzzati, N.; Pace, R.; Sardone, N. New liquid chromatography method with ultraviolet detection for analysis of anthocyanins and anthocyanidins in Vaccinium myrtillus fruit dry extracts and commercial preparations. J. AOAC Int. 2007, 90, 911–919. [Google Scholar] [PubMed]
- Virgona, N.; Taki, Y.; Umegaki, K. A rapid HPLC with evaporative light scattering method for quantification of forskolin in multi-herbal weight-loss solid oral dosage forms. Pharmazie 2010, 65, 322–326. [Google Scholar]
- Heitkemper, M.; Jarrett, M. Irritable bowel syndrome: Does gender matter? J. Psychosom. Res. 2008, 64, 583–587. [Google Scholar] [CrossRef]
- Meleine, M.; Matricon, J. Gender-related differences in irritable bowel syndrome: Potential mechanisms of sex hormones. World J. Gastroenterol. 2014, 20, 6725–6743. [Google Scholar] [CrossRef] [PubMed]
- Seamon, K.B.; Daly, J.W. Forskolin: A unique diterpene activator of cyclic AMP-generating systems. J. Cyclic Nucleotide Res. 1981, 7, 201–224. [Google Scholar] [PubMed]
- Allen, D.O.; Ahmed, B.; Naseer, K. Relationships between cyclic AMP levels and lipolysis in fat cells after isoproterenol and forskolin stimulation. J. Pharmacol. Exp. Ther. 1986, 238, 659–664. [Google Scholar]
- Okuda, H.; Morimoto, C.; Tsujita, T. Relationship between cyclic AMP production and lipolysis induced by forskolin in rat fat cells. J. Lipid Res. 1992, 33, 225–231. [Google Scholar] [PubMed]
- Godard, M.P.; Johnson, B.A.; Richmond, S.R. Body composition and hormonal adaptations associated with forskolin consumption in overweight and obese men. Obes. Res. 2005, 13, 1335–1343. [Google Scholar] [CrossRef]
- Henderson, S.; Magu, B.; Rasmussen, C.; Lancaster, S.; Kerksick, C.; Smith, P.; Melton, C.; Cowan, P.; Greenwood, M.; Earnest, C.; et al. Effects of coleus forskohlii supplementation on body composition and hematological profiles in mildly overweight women. J. Int. Soc. Sports Nutr. 2005, 2, 54–62. [Google Scholar] [CrossRef]
- Vanden Broeck, D.; Horvath, C.; De Wolf, M.J. Vibrio cholerae: Cholera toxin. Int. J. Biochem. Cell Biol. 2007, 39, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Danan, G.; Teschke, R. Drug-Induced Liver Injury: Why is the Roussel Uclaf Causality Assessment Method (RUCAM) Still Used 25 Years After Its Launch? Drug Saf. 2018, 41, 735–743. [Google Scholar] [CrossRef]
- Genuis, S.J.; Schwalfenberg, G.; Siy, A.K.; Rodushkin, I. Toxic element contamination of natural health products and pharmaceutical preparations. PLoS ONE 2012, 7, e49676. [Google Scholar] [CrossRef]
- Newmaster, S.G.; Grguric, M.; Shanmughanandhan, D.; Ramalingam, S.; Ragupathy, S. DNA barcoding detects contamination and substitution in North American herbal products. BMC Med. 2013, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, R.B.; Fong, H.H.; Farnsworth, N.R. Ensuring the safety of botanical dietary supplements. Am. J. Clin. Nutr. 2008, 87, 509S–513S. [Google Scholar] [CrossRef] [PubMed]
- Oketch-Rabah, H.A.; Marles, R.J.; Brinckmann, J.A. Cinnamon and Cassia Nomenclature Confusion: A Challenge to the Applicability of Clinical Data. Clin. Pharmacol. Ther. 2018, 104, 435–445. [Google Scholar] [CrossRef] [PubMed]

| All | Users with Experience of Adverse Events (n = 75) | Users without Experience of Adverse Events (n = 640) | p | |||||
|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | |||
| Sex | Male | 326 | (45.6) | 31 | (41.3) | 295 | (46.1) | 0.434 |
| Female | 389 | (54.4) | 44 | (58.7) | 345 | (53.9) | ||
| Age (Male) | 20–29 | 10 | (3.1) | 2 | (6.5) | 8 | (2.7) | 0.574 |
| 30–39 | 84 | (25.8) | 9 | (29.0) | 75 | (25.4) | ||
| 40–49 | 120 | (36.8) | 9 | (29.0) | 111 | (37.6) | ||
| 50–59 | 86 | (26.4) | 8 | (25.8) | 78 | (26.4) | ||
| over 60 | 26 | (8.0) | 3 | (9.7) | 23 | (7.8) | ||
| (Female) | 20–29 | 39 | (10.0) | 5 | (11.4) | 34 | (9.9) | 0.129 |
| 30–39 | 120 | (30.8) | 21 | (47.7) | 99 | (28.7) | ||
| 40–49 | 144 | (37.0) | 12 | (27.3) | 132 | (38.3) | ||
| 50–59 | 64 | (16.5) | 5 | (11.4) | 59 | (17.1) | ||
| over 60 | 22 | (5.7) | 1 | (2.3) | 21 | (6.1) | ||
| Residential area | ||||||||
| Hokkaido | 40 | (5.6) | 5 | (6.7) | 35 | (5.5) | 0.292 | |
| Tohoku | 41 | (5.7) | 8 | (10.7) | 33 | (5.2) | ||
| Kanto | 247 | (34.5) | 28 | (37.3) | 219 | (34.2) | ||
| Chubu | 116 | (16.2) | 10 | (13.3) | 106 | (16.6) | ||
| Kinki | 155 | (21.7) | 13 | (17.3) | 142 | (22.2) | ||
| Chugoku, Shikoku | 57 | (8.0) | 3 | (4.0) | 54 | (8.4) | ||
| Kyushu, Okinawa | 59 | (8.3) | 8 | (10.7) | 51 | (8.0) | ||
| Duration | <1 week | 15 | (2.1) | 5 | (6.7) | 10 | (1.6) | <0.001 |
| 1 week–1 month | 70 | (9.8) | 22 | (29.3) | 48 | (7.5) | ||
| 1–3 months | 174 | (24.3) | 20 | (26.7) | 154 | (24.1) | ||
| 3–6 months | 135 | (18.9) | 10 | (13.3) | 125 | (19.5) | ||
| 6 months–1 year | 85 | (11.9) | 5 | (6.7) | 80 | (12.5) | ||
| over 1 year | 204 | (28.5) | 13 | (17.3) | 191 | (29.8) | ||
| Did not remember | 32 | (4.5) | 0 | 32 | (5.0) | |||
| Frequency | Almost every day | 556 | (77.8) | 59 | (78.7) | 497 | (77.7) | 0.371 |
| Every other day | 82 | (11.5) | 11 | (14.7) | 71 | (11.1) | ||
| 1–2 days/week | 55 | (7.7) | 2 | (2.7) | 53 | (8.3) | ||
| <1 day/week | 17 | (2.4) | 2 | (2.7) | 15 | (2.3) | ||
| Others | 5 | (0.7) | 1 | (1.3) | 4 | (0.6) | ||
| Intake level compared with the amount suggested | ||||||||
| Less | 27 | (3.8) | 6 | (8.0) | 21 | (3.3) | 0.048 | |
| Equal | 670 | (93.7) | 69 | (92.0) | 601 | (93.9) | ||
| More | 18 | (2.5) | 0 | (0.0) | 18 | (2.8) | ||
| Health status (multiple answer) | ||||||||
| In good health but had concerns about own health | 106 | (14.8) | 16 | (21.3) | 90 | (14.1) | 0.094 | |
| Having a treatment for chronic disease | 74 | (10.3) | 9 | (12.0) | 65 | (10.2) | 0.620 | |
| Taking a nonprescription drug | 28 | (3.9) | 2 | (2.7) | 26 | (4.1) | 0.758 | |
| Concomitantly with other health foods | 297 | (41.5) | 27 | (36.0) | 270 | (42.2) | 0.304 | |
| None of the above | 296 | (41.4) | 29 | (38.7) | 267 | (41.7) | 0.612 | |
| Purpose of use (multiple answer) | ||||||||
| Maintenance of health | 205 | (28.7) | 14 | (18.7) | 191 | (29.8) | 0.043 | |
| Weight loss | 619 | (86.6) | 68 | (90.7) | 551 | (86.1) | 0.272 | |
| Improvement of health | 98 | (13.7) | 10 | (13.3) | 88 | (13.8) | 0.921 | |
| Disease prevention | 16 | (2.2) | 3 | (4.0) | 13 | (2.0) | 0.231 | |
| Beauty care | 49 | (6.9) | 2 | (2.7) | 47 | (7.3) | 0.152 | |
| Prevalence | Use of the Product after the Onset of Adverse Symptoms | |||||||
|---|---|---|---|---|---|---|---|---|
| Continued without Change | Continued with Reduced Amount/Frequency | Stopped | Went to a Hospital | |||||
| n1 | % 1 | n | n | n | n | |||
| Experience of adverse events | ||||||||
| Have experienced | 75 | 10.5 | 22 | 33 | 20 | 1 | ||
| Symptoms 2 | ||||||||
| Diarrhea | 61 | 81.3 | 18 | 28 | 15 | 0 | ||
| Nausea and vomiting | 11 | 14.7 | 3 | 3 | 5 | 0 | ||
| Headache | 9 | 12.0 | 3 | 3 | 3 | 0 | ||
| Constipation | 5 | 6.7 | 1 | 3 | 1 | 1 | ||
| Anthema and itching | 3 | 4.0 | 0 | 2 | 1 | 1 | ||
| Fatigue | 5 | 6.7 | 0 | 2 | 3 | 0 | ||
| Loss of appetite | 3 | 4.0 | 0 | 2 | 1 | 0 | ||
| Palpitations | 2 | 2.7 | 1 | 0 | 1 | 0 | ||
| Worsening of liver function test | 1 | 1.3 | 0 | 1 | 0 | 0 | ||
| Worsening of other clinical test values | 0 | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishijima, C.; Chiba, T.; Sato, Y.; Umegaki, K. Nationwide Online Survey Enables the Reevaluation of the Safety of Coleus forskohlii Extract Intake Based on the Adverse Event Frequencies. Nutrients 2019, 11, 866. https://doi.org/10.3390/nu11040866
Nishijima C, Chiba T, Sato Y, Umegaki K. Nationwide Online Survey Enables the Reevaluation of the Safety of Coleus forskohlii Extract Intake Based on the Adverse Event Frequencies. Nutrients. 2019; 11(4):866. https://doi.org/10.3390/nu11040866
Chicago/Turabian StyleNishijima, Chiharu, Tsuyoshi Chiba, Yoko Sato, and Keizo Umegaki. 2019. "Nationwide Online Survey Enables the Reevaluation of the Safety of Coleus forskohlii Extract Intake Based on the Adverse Event Frequencies" Nutrients 11, no. 4: 866. https://doi.org/10.3390/nu11040866
APA StyleNishijima, C., Chiba, T., Sato, Y., & Umegaki, K. (2019). Nationwide Online Survey Enables the Reevaluation of the Safety of Coleus forskohlii Extract Intake Based on the Adverse Event Frequencies. Nutrients, 11(4), 866. https://doi.org/10.3390/nu11040866





