Plasma Transthyretin as A Biomarker of Sarcopenia in Elderly Subjects
Abstract
1. Introduction
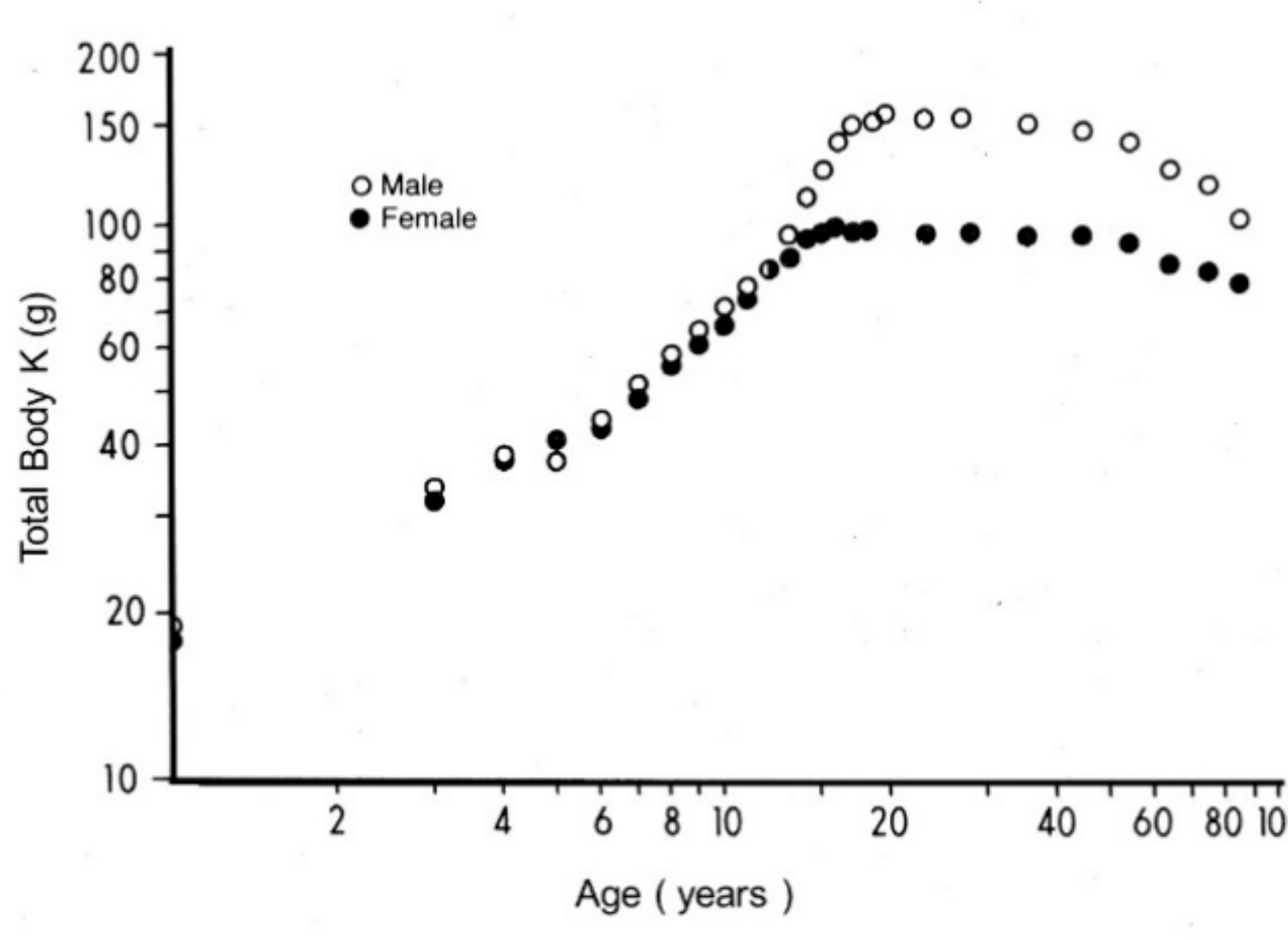
2. Body Composition Studies
3. Place of Sarcopenia in Body Composition Studies
4. Biochemical Assessment of Sarcopenia
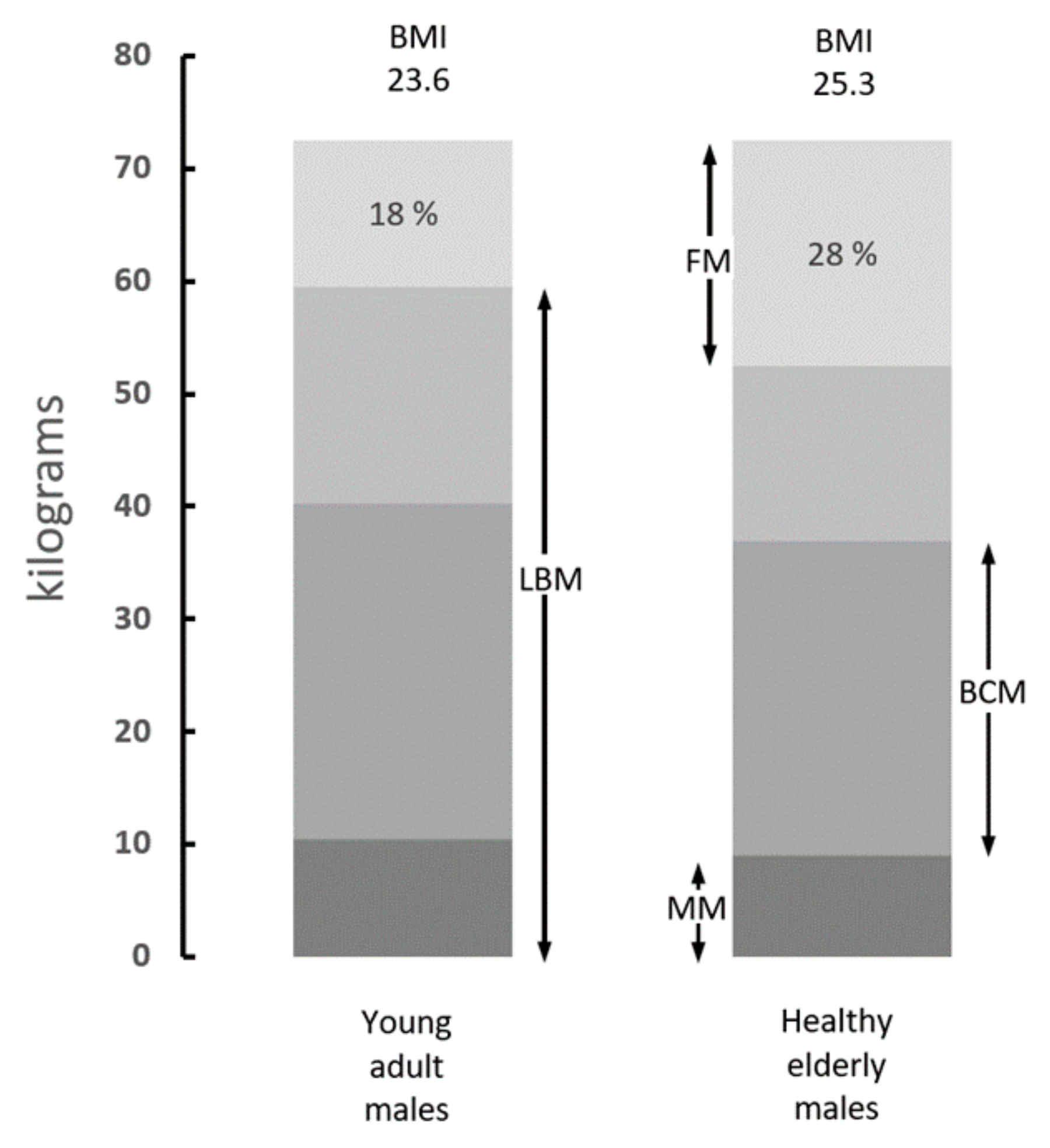
5. Is the Body Mass Index Correlated to Sarcopenic States?
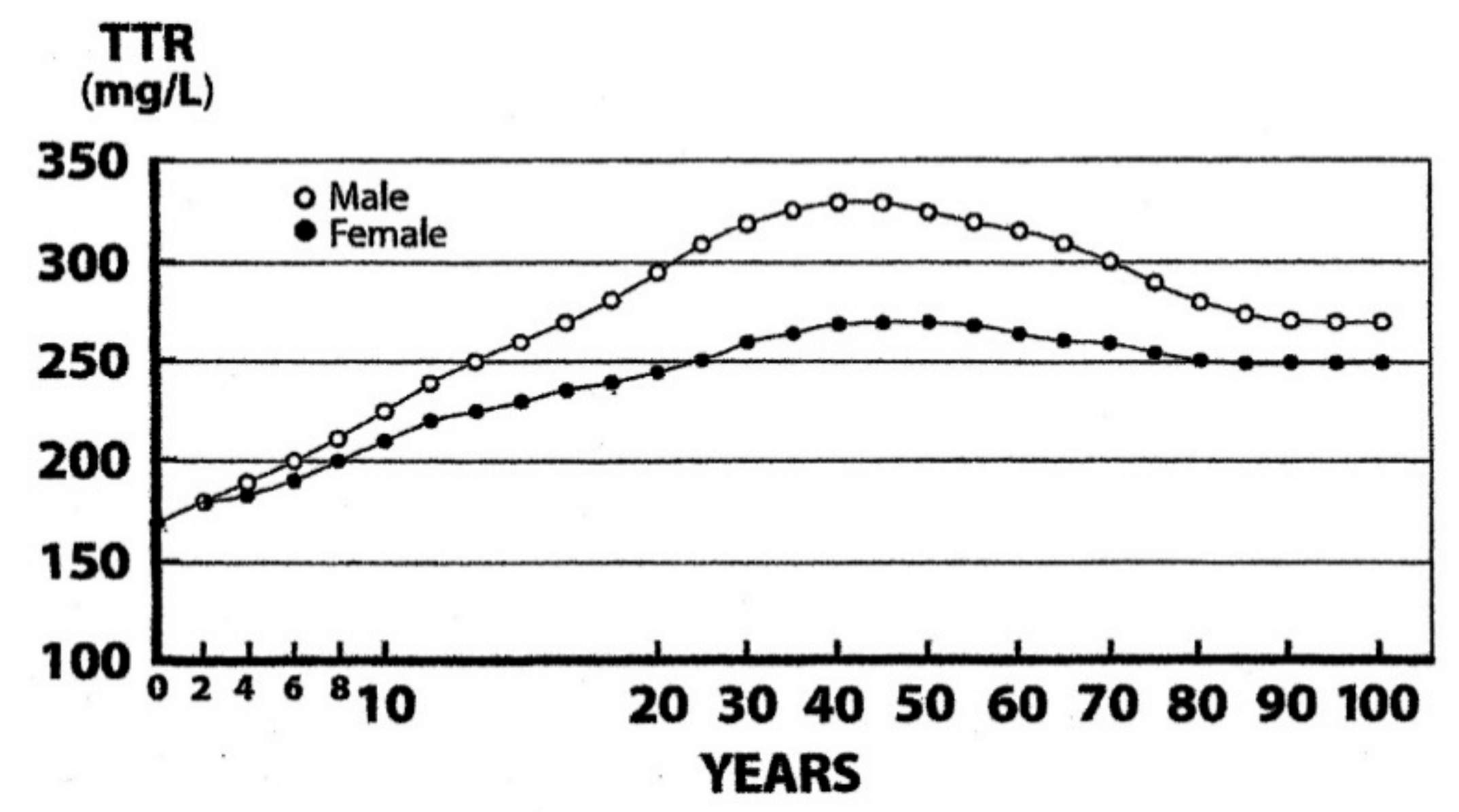
6. Measurement of TTR as A Surrogate Biomarker of LBM Components
7. TTR as A Biomarker of Sarcopenia in Elderly Persons
8. Concluding Remarks
Funding
Conflicts of Interest
References
- Rosenberg, H.I. Sarcopenia: Origins and clinical relevance. J. Nutr. 1977, 127, S1990–S1991. [Google Scholar]
- Jansen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef]
- Pichard, C.; Kyle, U.G.; Morabia, A.; Perrier, A.; Vermeulen, B.; Unger, P. Nutritional assessment: Lean body mass depletion at hospital admission is associated with an increased length of stay. Am. J. Clin. Nutr. 2004, 79, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Filippin, L.I.; Teixeira, V.N.; da Silva, M.P.; Miraglia, F.; da Silva, F.S. Sarcopenia: A predictor of mortality and the need for early diagnosis and intervention. Aging Clin. Exp. Res. 2015, 27, 249–254. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 19–31. [Google Scholar] [CrossRef]
- Dhillon, R.J.; Hasni, S. Pathogenesis and management of sarcopenia. Clin. Geriatr. Med. 2017, 33, 17–26. [Google Scholar] [CrossRef]
- Tosato, M.; Marzetti, E.; Cesari, M.; Savera, G.; Miller, R.R.; Bernabei, R.; Landi, F.; Calvani, R. Measurement of muscle mass in sarcopenia: From imaging to biochemical markers. Aging Clin. Exp. Res. 2017, 29, 19–27. [Google Scholar] [CrossRef]
- Forbes, G.B. Human Body Composition, Growth, Aging, Nutrition, and Activity; Springer: Berlin, Germany, 1987. [Google Scholar]
- Cohn, S.H.; Vartsky, D.; Yasumura, S.; Vaswani, A.N.; Ellis, K.J. Indexes of body cell mass: Nitrogen versus potassium. Am. J. Physiol. 1983, 244, E305–E310. [Google Scholar] [CrossRef] [PubMed]
- Ingenbleek, Y.; Young, V.R. Significance of transthyretin in protein metabolism. Clin. Chem. Lab. Med. 2002, 40, 1281–1291. [Google Scholar] [CrossRef]
- Brožek, J.; Grande, F. Body composition and basal metabolism in man: Correlation analysis versus physiological approach. Hum. Biol. 1955, 27, 22–31. [Google Scholar]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.M.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef]
- Illner, K.; Brinkmann, G.; Heller, M.; Bosy-Westphal, A.; Müller, M.J. Metabolically active components of fat free mass and resting energy expenditure in nonobese adults. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E308–E315. [Google Scholar] [CrossRef] [PubMed]
- Nakshabendi, I.M.; McKee, R.; Downie, S.; Russell, R.I.; Rennie, M.J. Rates of small intestinal mucosal protein synthesis in human jejunum and ileum. Am. J. Physiol. Endocrinol. Metab. 1999, 277, E1028–E1031. [Google Scholar] [CrossRef]
- McNurlan, M.A.; Sandgren, A.; Hunter, K.; Essén, P.; Garlick, P.J.; Wernerman, J. Protein synthesis rates of skeletal muscle, lymphocytes, and albumin with stress hormone infusion in healthy men. Metabolism 1996, 45, 1388–1394. [Google Scholar] [CrossRef]
- Sparti, A.; DeLany, J.P.; de la Bretonne, J.A.; Sander, G.E.; Bray, G.A. Relationship between resting metabolic rate and the composition of the fat-free mass. Metabolism 1997, 46, 1225–1230. [Google Scholar] [CrossRef]
- Guglielmi, G.; Ponti, F.; Agostini, M.; Amadori, M.; Battista, G.; Bazzocchi, A. The role of DXA in sarcopenia. Aging Clin. Exp. Res. 2016, 28, 1047–1060. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Gonzalez, M.C.; Lu, J.; Jia, G.; Zheng, J. Skeletal muscle mass and quality: Evolution of modern measurement concepts in the context of sarcopenia. Proc. Nutr. Soc. 2015, 74, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Doherty, J.F.; Adam, E.J.; Griffin, G.E.; Golden, M.H. Ultrasonographic assessment of the extent of hepatic steatosis in severe malnutrition. Arch. Dis. Child. 1992, 67, 1348–1352. [Google Scholar] [CrossRef]
- Whitehead, R.G.; Coward, W.A.; Lunn, P.G. Serum-albumin concentration and the onset of kwashiorkor. Lancet 1973, 7794, 63–66. [Google Scholar] [CrossRef]
- Gabr, M.; el-Hawary, M.F.; el-Dali, M.J. Serum transferrin in kwashiorkor. J. Trop. Med. Hyg. 1971, 74, 216–221. [Google Scholar]
- Ingenbleek, Y.; De Visscher, M.; De Nayer, P. Measurement of prealbumin as index of protein-calorie malnutrition. Lancet 1972, 7768, 106–109. [Google Scholar] [CrossRef]
- Ingenbleek, Y.; Van den Schrieck, H.G.; De Nayer, P.; De Visscher, M. The role of retinol-binding protein in protein-calorie malnutrition. Metabolism 1975, 24, 633–641. [Google Scholar] [CrossRef]
- Sullivan, D.H.; Carter, W.J. Insulin-like growth factor 1 as an indicator of protein-energy undernutrition among metabolically stable hospitalized elderly. J. Am. Coll. Nutr. 1994, 13, 184–191. [Google Scholar] [CrossRef]
- Duque, E.; Bolaños, O.; Lotero, H.; Mayoral, L.G. Enteropathy in adult protein malnutrition: Light microscopic findings. Am. J. Clin. Nutr. 1975, 28, 901–913. [Google Scholar] [CrossRef]
- Ibrahim, M.K.; Zambruni, M.; Melby, C.L.; Melby, P.C. Impact of childhood malnutrition on host defense and infection. Clin. Microbiol. Rev. 2017, 30, 919–971. [Google Scholar]
- Cahill, G.F., Jr. Starvation in man. N. Engl. J. Med. 1970, 282, 668–675. [Google Scholar] [CrossRef]
- Van Loon, H.; Saverijs, V.; Vuysteke, J.P.; Vlietinck, R.F.; Van den Berghe, H. Screening for marasmus: A discriminant analysis as a guide to choose the anthropometric variables. Am. J. Clin. Nutr. 1987, 45, 488–493. [Google Scholar] [CrossRef]
- Ingenbleek, Y.; Van den Schrieck, H.G.; De Nayer, P.; De Visscher, M. Albumin, transferrin and the thyroxine-binding prealbumin/retinol-binding protein (TBPA-RBP) complex in assessment of malnutrition. Clin. Chim. Acta 1975, 63, 61–67. [Google Scholar] [CrossRef]
- Cohn, S.H.; Gartenhaus, W.; Sawitsky, A.; Rai, K.; Zanzi, I.; Vaswani, A.; Ellis, K.J.; Yasumura, S.; Cortes, E.; Vartsky, D. Compartmental body composition of cancer patients by measurement of total body nitrogen, potassium, and water. Metabolism 1981, 30, 222–229. [Google Scholar] [CrossRef]
- Kotler, D.P.; Tierney, A.R.; Wang, J.; Pierson, R.N., Jr. Magnitude of body-cell-mass depletion and the timing of death from wasting in AIDS. Am. J. Clin. Nutr. 1989, 50, 444–447. [Google Scholar] [CrossRef]
- Young, V.R.; Yu, Y.M.; Fukagawa, N.K. Energy and protein turnover. In Energy Metabolism: Tissue Determinants and Cellular Corollaries; Kinney, J.M., Tucker, H.N., Eds.; Raven Press: New York, NY, USA, 1992; pp. 439–466. [Google Scholar]
- Picou, D.; Phillips, M. Urea metabolism in malnourished and recovered children receiving a high or low protein diet. Am. J. Clin. Nutr. 1972, 25, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Bessey, P.Q.; Jiang, Z.M.; Johnson, D.J.; Smith, R.J.; Wilmore, D.W. Posttraumatic skeletal muscle proteolysis: The role of the hormonal environment. World J. Surg. 1989, 13, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Plank, L.D.; Connolly, A.B.; Hill, G.L. Sequential changes in the metabolic response in severely septic patients during the first 23 days after the onset of peritonitis. Ann. Surg. 1998, 228, 146–158. [Google Scholar] [CrossRef]
- Hart, D.W.; Wolf, S.E.; Chinkes, D.L.; Gore, D.C.; Mlcak, R.P.; Beauford, R.B.; Obeng, M.K.; Lal, S.; Gold, W.F.; Wolfe, R.R.; Herndon, D.N. Determinants of skeletal muscle catabolism after severe burn. Ann. Surg. 2000, 232, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.; Campbell, I.T.; Samuels, T.A.; Devlin, J.C.; Green, C.J.; Hipkin, L.J.; MacDonald, I.A.; Scrimgeour, C.M.; Smith, K.; Rennie, M.J. Increased whole body protein breakdown predominates over increased whole body synthesis in multiple organ failure. Clin. Sci. (Lond) 1993, 84, 655–661. [Google Scholar] [CrossRef]
- Wolfe, RR. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef]
- Virgili, F.; Maiani, G.; Zahoor, Z.H.; Ciarapica, D.; Raguzzini, A.; Ferro-Luzzi, A. Relationship between fat-free mass and urinary excretion of creatinine and 3-methylhistidine in adult humans. J. Appl. Physiol. 1994, 76, 1946–1950. [Google Scholar] [CrossRef]
- Carlotti, A.P.; Bohn, D.; Matsuno, A.K.; Pasti, D.M.; Gowrishankar, M.; Halperin, M.L. Indicators of lean body mass catabolism: Emphasis on the creatinine excretion rate. QJM 2008, 101, 197–205. [Google Scholar] [CrossRef][Green Version]
- Heymsfield, S.B.; Arteaga, C.; McManus, C.; Smith, J.; Moffitt, S. Measurement of muscle mass in man: Validity of the 24-hour urinary creatinine method. Am. J. Clin. Nutr. 1983, 37, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Arroyave, G.; Wilson, D.; Béhar, M.; Scrimshaw, N.S. Serum and urinary creatinine in children with severe protein malnutrition. Am. J. Clin. Nutr. 1961, 9, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Tzankoff, S.P.; Norris, A.H. Longitudinal changes in basal metabolism in man. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1978, 45, 536–539. [Google Scholar] [CrossRef]
- Rowe, J.W.; Andres, R.; Tobin, J.D.; Norris, A.H.; Shock, N.W. The effect of age on creatinine clearance in man: A cross-sectional and longitudinal study. J. Gerontol. 1976, 31, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Lykken, G.I.; Jacob, R.A.; Munoz, J.M.; Sandstead, H.H. A mathematical model of creatine metabolism in normal males—comparison between theory and experiment. Am. J. Clin. Nutr. 1980, 33, 2674–2685. [Google Scholar] [CrossRef] [PubMed]
- Huszar, G.; Golenwsky, G.; Maiocco, J.; Davis, E. Urinary 5-methylhistidine excretion in man: The role of protein-bound and soluble 3-methylhistidine. Brit. J. Nutr. 1983, 49, 287–294. [Google Scholar] [CrossRef]
- Tallan, H.H.; Stein, W.H.; Moore, S. 3-methylhistidine, a new amino acid from human urine. J. Biol. Chem. 1953, 200, 825–834. [Google Scholar]
- Nagabhushan, V.S.; Narasinga Rao, B.S. Studies on 3-methylhistidine metabolism in children with protein-energy malnutrition. Am. J. Clin. Nutr. 1978, 31, 1322–1327. [Google Scholar] [CrossRef]
- Long, C.L.; Haverberg, L.N.; Young, V.R.; Kinney, J.M.; Munro, H.N.; Geiger, J.W. Metabolism of 3-methylhistidine in man. Metabolism 1975, 24, 929–935. [Google Scholar] [CrossRef]
- Lukaski, H.; Mendez, J. Relationship between fat-free weight and urinary 3-methylhistidine excretion in man. Metabolism 1980, 29, 758–761. [Google Scholar] [CrossRef]
- Elia, M.; Carter, A.; Bacon, S.; Smith, R. The effect of 3-methylhistidine in food on its urinary excretion in man. Clin. Sci (Lond) 1980, 59, 509–511. [Google Scholar] [CrossRef]
- Long, C.L.; Dillard, D.R.; Bodzin, J.H.; Geiger, J.W.; Blakemore, W.S. Validity of 3- methylhistidine excretion as an indicator of skeletal muscle protein breakdown in humans. Metabolism 1988, 37, 844–849. [Google Scholar] [CrossRef]
- Garrow, J.S.; Webster, J. Quetelet’s index (W/H2) as a mesure of fatness. Int. J. Obes. 1985, 9, 147–153. [Google Scholar]
- Deurenberg, P.; Weststrate, J.A.; Seidell, J.C. Body mass index as a measure of body fatness: Age- and sex-specific prediction formulas. Br. J. Nutr. 1991, 65, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.V.; Ferro-Luzzi, A. Use of body mass index in adults in assessing individual and community nutritional status. Bull. World Health Organ. 1995, 73, 673–680. [Google Scholar] [PubMed]
- Vernarelli, J.A.; Mitchell, D.C.; Rolls, B.J.; Hartman, T.J. Dietary energy density is associated with obesity and other biomarkers of chronic disease in US adults. Eur. J. Nutr. 2015, 54, 59–65. [Google Scholar] [CrossRef]
- Porter Starr, K.N.; Bales, C.W. Excessive body weight in older adults. Clin. Geriatr. Med. 2015, 31, 311–326. [Google Scholar] [CrossRef]
- James, W.P. The epidemiology of obesity: The size of the problem. J. Intern. Med. 2008, 263, 336–352. [Google Scholar] [CrossRef]
- Prentice, A.M. The emerging epidemic of obesity in developing countries. Int. J. Epidemiol. 2006, 35, 93–99. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and managing the global epidemic; WHO Tech. Rep. Series No. 894; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Levin, B.E. Synergy of nature and nurture in the development of childhood obesity. Int. J. Obes. (Lond) 2009, 33, S53–S56. [Google Scholar] [CrossRef][Green Version]
- Lobstein, T.; Jackson-Leach, R.; Moodie, M.L.; Hall, K.D.; Gortmaker, S.L.; Swinburn, B.A.; James, W.P.; Wang, Y.; McPherson, K. Child and adolescent obesity: Part of a bigger picture. Lancet 2015, 9986, 2510–2520. [Google Scholar] [CrossRef]
- Abdullah, A. The double burden of undernutrition and overnutrition in developing countries: An update. Curr. Obes. Rep. 2015, 3, 337–349. [Google Scholar] [CrossRef]
- Min, J.; Zhao, Y.; Slivka, L.; Wang, Y. Double burden of diseases worldwide: Coexistence of undernutrition and overnutrition-related non-communicable chronic diseases. Obes. Rev. 2018, 19, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Quetelet, A. Sur l’Homme et le Développement de ses Facultés, Essai de Physique Sociale; Bachelier: Paris, France, 1835. [Google Scholar]
- Heymsfield, S.B.; Peterson, C.M.; Thomas, D.M.; Heo, M.; Schuna, J.M., Jr. Why are there race/ethnic differences in adult body mass index-adiposity relationships? A quantitative critical review. Obes. Rev. 2016, 17, 262–275. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneidert, S.M.; et al. Diagnostic criteria for malnutrition-An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef]
- Wada, T.; Kunisaki, C.; Ono, H.A.; Makino, H.; Akiyama, H.; Endo, I. Implications of BMI for the prognosis of gastric cancer among the Japanese population. Dig. Surg. 2015, 32, 480–486. [Google Scholar] [CrossRef]
- Eschbach, D.; Kirchbichler, T.; Oberkircher, L.; Knobe, M.; Juenemann, M.; Ruchholtz, S.; Buecking, B. Management of malnutrition in geriatric trauma patients: Results of a nationwide survey. Eur. J. Trauma Emerg. Surg. 2016, 42, 553–558. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Soldati, L.; Sarlo, F.; Calvani, M.; Di Lorenzo, N.; Di Renzo, L. New obesity classification criteria as a tool for bariatric surgery indication. World J. Gastroenterol. 2016, 22, 681–703. [Google Scholar] [CrossRef]
- Delgado, C.; Chertow, G.M.; Kaysen, G.A.; Dalrymple, L.S.; Komak, J.; Grimes, B.; Johansen, K.L. Associations of body mass index and body fat with markers of inflammation and nutrition among patients receiving hemodialysis. Am. J. Kidney Dis. 2017, 70, 817–825. [Google Scholar] [CrossRef]
- Han, S.S.; Kim, K.W.; Kim, K.I.; Na, K.Y.; Chae, D.W.; Kim, S.; Chin, H.J. Lean mass index: A better predictor of mortality than body mass index in elderly Asians. J. Am. Geriatr. Soc. 2010, 58, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Cederholm, T.; Muscaritoli, M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: From sarcopenic obesity to cachexia. Clin. Nutr. 2014, 33, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.C.; Correia, M.I.T.D.; Heymsfield, S.B. A requiem for BMI in the clinical setting. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 314–321. [Google Scholar] [CrossRef]
- Kim, K.M.; Jang, H.C.; Lim, S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J. Intern. Med. 2016, 31, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M. Sarcopenia and sarcopenic obesity. Korean J. Intern. Med. 2016, 31, 1054–1060. [Google Scholar] [CrossRef]
- Oppenheimer, J.H.; Surks, M.I.; Bernstein, G.; Smith, J.C. Metabolism of iodine-131-labeled thyroxine-binding prealbumin in man. Science 1965, 149, 748–750. [Google Scholar] [CrossRef]
- Ingenbleek, Y.; Young, V.R. Transthyretin (prealbumin) in health and disease: Nutritional implications. Annu. Rev. Nutr. 1994, 14, 495–533. [Google Scholar] [CrossRef] [PubMed]
- Bienvenu, J.; Jeppson, J.O.; Ingenbleek, Y. Transthyretin & retinol-binding protein. In Serum Proteins in Clinical Medicine, Chapter 9; Ritchie, R.F., Navolotskaya, O., Eds.; Foundation for Blood Research: Scarborough, ME, USA, 1996; pp. 11–18. [Google Scholar]
- Ingenbleek, Y.; Bernstein, L.H. Plasma transthyretin as a biomarker of lean body mass and catabolic states. Adv. Nutr. 2015, 6, 572–580. [Google Scholar] [CrossRef]
- Vahlquist, A.; Rask, L.; Peterson, P.A.; Berg, T. The concentrations of retinol-binding protein, prealbumin, and transferrin in the sera of newly delivered mothers and children of various ages. Scand. J. Clin. Lab. Invest. 1975, 35, 569–575. [Google Scholar] [CrossRef]
- Veldhuis, J.D.; Roemmich, J.N.; Richmond, E.J.; Rogol, A.D.; Lovejoy, J.C.; Sheffield-Moore, M.; Mauras, N.; Bowers, C.Y. Endocrine control of body composition in infancy, childhood and puberty. Endocr. Rev. 2005, 26, 114–146. [Google Scholar] [PubMed]
- Ingenbleek, Y. The nutritional relationship linking sulfur to nitrogen in living organisms. J. Nutr. 2006, 136, S1642–S1651. [Google Scholar] [CrossRef]
- Vanacore, D.; Messina, G.; Lama, S.; Bitti, G.; Ambrosio, P.; Tenore, G.; Messina, A.; Monda, V.; Zappavigna, S.; Bocellino, M.; et al. Effect of restriction vegan diet’s on muscle mass, oxidative status, and myocytes differentiation: A pilot study. J. Cell Physiol. 2018, 233, 9345–9353. [Google Scholar] [CrossRef]
- de Jong, F.A.; Schreiber, G. Messenger RNA levels of plasma proteins in rat liver during protein depletion and refeeding. J. Nutr. 1987, 117, 1795–1800. [Google Scholar] [CrossRef] [PubMed]
- Straus, D.S.; Marten, N.W.; Hayden, J.M.; Burke, E.J. Protein restriction specifically decreases the abundance of serum albumin and transthyretin nuclear transcripts in rat liver. J. Nutr. 1994, 124, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Ingenbleek, Y.; McCully, K.S. Vegetarianism produces subclinical malnutrition, hyperhomocysteinemia and atherogenesis. Nutrition 2012, 28, 148–153. [Google Scholar] [CrossRef]
- Kim, D.H.; Sarbassov, D.D.; Ali, S.M.; King, J.E.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 2002, 110, 163–175. [Google Scholar] [CrossRef]
- Averous, J.; Lambert-Langlais, S.; Mesclon, F.; Carraro, V.; Parry, L.; Jousse, C.; Bruhat, A.; Maurin, A.C.; Pierre, P.; Proud, C.G.; et al. GCN2 contributes to mTORC1 inhibition by leucine deprivation through an ATF4 independent mechanism. Sci. Rep. 2016, 6, 27698. [Google Scholar] [CrossRef] [PubMed]
- Rudland, P.S.; Clark, B.F.C. Polypeptide chain initiation and the role of methionine tRNA. In In the Mechanism of Protein Synthesis and its Regulation; Bosch, L., Ed.; North-Holland Publishing Co.: Amsterdam, Netherlands, 1972; pp. 55–131. [Google Scholar]
- Ingenbleek, Y.; Kimura, H. Nutritional essentiality of sulfur in health and disease. Nutr. Rev. 2013, 71, 413–432. [Google Scholar] [CrossRef]
- Gilsing, A.M.; Crowe, F.L.; Lloyd-Wright, Z.; Sanders, T.A.; Appleby, P.N.; Allen, N.E.; Key, T.J. Serum concentrations of vitamin B12 and folate in British male omnivores, vegetarians, and vegans: Results from a cross-sectional analysis of the EPIC-Oxford cohort study. Eur. J. Clin. Nutr. 2010, 64, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Stabler, S.P.; Allen, R.H. Vitamin B12 deficiency as a worldwide problem. Annu. Rev. Nutr 2004, 24, 299–326. [Google Scholar] [CrossRef] [PubMed]
- Škovierová, H.; Vidomanová, E.; Mahmood, S.; Sopková, J.; Drgová, A.; Červenová, T.; Halašová, E.; Lehotský, J. The molecular and cellular effect of homocysteine metabolism imbalance on human health. Int. J. Mol. Sci. 2016, 17, 1733–1750. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.J.; Huang, P.C.; Lu, S.C.; Li, Y.H.; Huang, H.B.; Lin, B.F.; Chang, S.J.; Chou, H.F. Plasma homocysteine levels in Taiwanese vegetarians are higher than those of omnivores. J. Nutr. 2002, 132, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Yajnik, C.S.; Lubree, H.G.; Thuse, N.V.; Ramdas, L.V.; Deshpande, S.S.; Deshpande, V.U.; Uradey, B.S.; Ganpule, A.A.; Naik, S.S.; Joshi, N.P.; et al. Oral vitamin B12 supplementation reduces plasma total homocysteine concentration in women in India. Asia Pac. J. Clin. Nutr. 2007, 16, 103–109. [Google Scholar] [PubMed]
- Ingenbleek, Y. Hyperhomocysteinemia is a biomarker of sulfur-deficiency in human morbidities. Open Clin. Chem. J. 2009, 2, 49–60. [Google Scholar] [CrossRef]
- Campbell, W.W.; Trappe, T.A.; Wolfe, R.R.; Evans, W.J. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M373–M380. [Google Scholar] [CrossRef] [PubMed]
- Lamar, K.M.; McNally, E.M. Genetic modifiers for neuromuscular diseases. J. Neuromuscul. Dis. 2014, 1, 3–13. [Google Scholar]
- Su, J.; Ekman, C.; Oskolko, N.; Lahti, L.; Ström, K.; Brazma, A.; Groop, L.; Rung, J.; Hansson, O. A novel atlas of gene expression in human skeletal muscle reveals molecular changes associated with aging. Skelet. Muscle 2015, 5, 35–47. [Google Scholar] [CrossRef]
- Morley, J.E. Hormones and sarcopenia. Curr. Pharm. Des. 2017, 23, 4484–4492. [Google Scholar] [CrossRef]
- Brown, J.C.; Haray, M.O.; Harhay, M.N. Physical activity, diet quality, and mortality among sarcopenic older adults. Aging Clin. Exp. Res. 2017, 29, 257–263. [Google Scholar] [CrossRef]
- Morley, J.E.; von Haehling, S.; Anker, S.D. Are we closer to having drugs to treat muscle wasting disease? J. Cachexia, Sarcopenia, Muscle 2014, 5, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I. Evolution of sarcopenia research. Appl. Physiol. Nutr. Metab. 2010, 35, 707–712. [Google Scholar] [CrossRef]
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength: A quantitative review. Front. Physiol. 2012. [CrossRef]
- Morley, J.E.; Anker, S.D.; von Haehling, S. Prevalence, incidence, and clinical impact of sarcopenia: Facts, numbers, and epidemiology-update. J. Cachexia, Sarcopenia, Muscle 2014, 5, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi-Sucich, M.; Prestwood, K.M.; Kenny, A.M. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M772–M777. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Genton, L.; Hans, D.; Karsegard, V.L.; Michel, J.P.; Slosman, D.O.; Pichard, C. Total body mass, fat mass, fat-free mass, and skeletal muscle in older people: Cross sectional differences in 60-year-old pesons. J. Am. Geriatr. Soc. 2001, 49, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Wikby, A.; Nilsson, O.; Forsey, R.; Thompson, J.; Strindhall, J.; Löfgren, S.; Ernerudh, J.; Pawelec, G.; Ferguson, F.; Johansson, B. The immune risk phenotype is associated with IL-6 in the terminal decline stage: Findings from the Swedish NONA immune longitudinal study of very late life functioning. Mech. Ageing Dev. 2006, 127, 695–704. [Google Scholar] [CrossRef]
- Beyer, I.; Mets, T.; Bautmans, I. Chronic low-grade inflammation and age-related sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Castell, J.V.; Gómez-Lechón, M.J.; David, M.; Andus, T.; Geiger, T.; Trullenque, R.; Fabra, R.; Heinrich, P.C. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989, 242, 237–239. [Google Scholar] [CrossRef]
- Wirth, K.; Klenk, J.; Brefka, S.; Dallmeier, D.; Faehling, K.; Roqué, I.; Figuls, M.; Tully, M.A.; Giné-Garriga, M.; Caserotti, P.; et al. Biomarkers associated with sedentary behaviour in older adults: A systematic review. Ageing Res. Rev. 2017, 35, 87–111. [Google Scholar] [CrossRef] [PubMed]
- Schaap, L.A.; Pluijm, S.M.; Deeg, D.J.; Visser, M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am. J. Med. 2006, 119, e9–e17. [Google Scholar] [CrossRef] [PubMed]
- Lustgarten, M.S.; Fielding, R.A. Metabolites associated with circulating interleukin-6 in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1277–1283. [Google Scholar] [CrossRef]
- Murakami, T.; Ohnishi, S.; Nishiguchi, S.; Maeda, S.; Araki, S.; Shimada, K. Acute phase response for mRNAs for serum amyloid P component, C-reactive protein and prealbumin (transthyretin) in mouse liver. Biochem. Biophys. Res. Commun. 1988, 155, 554–560. [Google Scholar] [CrossRef]
- Banks, R.E.; Forbes, M.A.; Storr, M.; Higginson, J.; Thompson, D.; Raynes, J.; Illingworth, J.M.; Perren, T.J.; Selby, P.J.; Whicher, J.T. The acute phase protein response in patients receiving subcutaneous IL-6. Clin. Exp. Immunol. 1995, 102, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Breitzkreuz, R.; Holm, S.; Pittack, N.; Beichert, M.; Babylon, A.; Yodoi, J.; Dröge, W. Massive loss of sulfur in HIV infection. AIDS Res. Hum. Retroviruses 2000, 16, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.P. The distribution of nitrogen and sulphur in the urine during conditions of increased catabolism. Biochem. J. 1931, 25, 236–244. [Google Scholar] [CrossRef]
- Vary, T.C.; Jurasinski, C.; Kimball, S.R. Reduced 40S initiation complex formation in skeletal muscle during sepsis. Mol. Cell Biochem. 1998, 178, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Malmezat, T.; Breuillé, D.; Pouyet, C.; Buffière, C.; Denis, P.; Mirand, P.P.; Obled, C. Methionine transsulfuration is increased during sepsis in rats. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E1391–E1399. [Google Scholar] [CrossRef]
- Malmezat, T.; Breuillé, D.; Capitan, P.; Mirand, P.P.; Obled, C. Glutathione turnover is increased during the acute phase of sepsis in rats. J. Nutr. 2000, 130, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Malmezat, T.; Breuillé, D.; Pouyet, C.; Mirand, P.P.; Obled, C. Metabolism of cysteine is modified during the acute phase of sepsis in rats. J. Nutr. 1998, 128, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Schindler, K.; Zauner, C.; Buchmayer, H.; Födinger, M.; Wölfl, G.; Bieglmayer, C.; Heinz, G.; Wilfing, A.; Hörl, W.H.; Sunder-Plassmann, G. High prevalence of hyperhomocysteinemia in critically ill patients. Crit. Care Med. 2000, 28, 991–995. [Google Scholar] [CrossRef]
- McKeever, M.P.; Weir, D.G.; Molloy, A.; Scott, J.M. Betaine—homocysteine methyltransferase: Organ distribution in man, pig and rat and subcellular distribution in the rat. Clin. Sci. (Lond) 1991, 81, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Ingenbleek, Y. Lean body mass harbors sensing mechanisms that allow safeguarding of methionine homeostasis. Nutrients 2017. [Google Scholar] [CrossRef] [PubMed]
- Strakova, J.; Williams, K.T.; Gupta, S.; Schalinske, K.L.; Kruger, W.D.; Rozen, R.; Jiracek, J.; Li, L.; Garrow, T.A. Dietary intake of S-(alpha-carboxybutyl)-DL-homocysteine induces hyperhomocysteinemia in rats. Nutr. Res. 2010, 30, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Stead, L.M.; Brosnan, M.E.; Brosnan, J.T. Characterization of homocysteine metabolism in the rat liver. Biochem. J. 2000, 350, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Gori, A.M.; Corsi, A.M.; Fedi, S.; Gazzini, A.; Sofi, F.; Bartali, B.; Bandinelli, S.; Gensini, G.F.; Abbate, R.; Ferrucci, L. A proinflammatory state is associated with hyperhomocysteinemia in the elderly. Am. J. Clin. Nutr. 2005, 82, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.J.; Mansoor, M.A.; van der Pols, J.; Prentice, A.; Cole, T.J.; Finch, S. Plasma total homocysteine in a representative sample of 927 British men and women aged 65 and over. Eur. J. Clin. Nutr. 1997, 51, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Landi, F.; Topinková, E.; Michel, J.P. Understanding sarcopenia as a geriatric syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznaric, Z.; Nair, S.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef]
- Rymarz, A.; Bartoszewicz, Z.; Szamotulska, K.; Niemczyk, S. The associations between body cell mass and nutritional and inflammatory markers in patients with chronic kidney disease and in subjects without kidney diseases. J. Ren. Nutr. 2016, 26, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Sergi, G.; Coin, A.; Enzi, G.; Volpato, S.; Inelmen, E.M.; Buttarello, M.; Peloso, M.; Mulone, S.; Marine, S.; Bonometto, P. Role of visceral proteins in detecting malnutrition in the elderly. Eur. J. Clin. Nutr. 2006, 60, 203–209. [Google Scholar] [CrossRef]
- Dong, J.; Li, Y.J.; Lu, X.H.; Gan, H.P.; Zuo, L.; Wang, H.Y. Correlations of lean body mass with nutritional indicators and mortality in patients on peritoneal dialysis. Kidney Int. 2008, 73, 334–340. [Google Scholar] [CrossRef]
- Lee, K.H.; Cho, J.H.; Kwon, O.; Kim, S.U.; Kim, R.H.; Cho, Y.W.; Jung, H.Y.; Choi, J.Y.; Jim, C.D.; Kim, Y.L.; et al. Low prealbumin levels are independently associated with higher mortality in patients on peritoneal dialysis. Kidney Res. Clin. Pract. 2016, 35, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Rambod, M.; Kovesdy, C.P.; Bross, R.; Kopple, J.D.; Kalantar-Zadeh, K. Association of serum prealbumin and its changes over time with clinical outcomes and survival in patients receiving hemodialysis. Am. J. Clin. Nutr. 2008, 88, 1485–1494. [Google Scholar] [CrossRef]
- Salvetti, D.J.; Tempel, Z.J.; Goldschmidt, E.; Colwell, N.A.; Angriman, F.; Panczykowski, D.; Agarwal, N.; Kanter, A.S.; Okonkwo, D.O. Low preoperative serum prealbumin levels and the postoperative surgical site infection risk in elective spine surgery: A consecutive series. J. Neurosurg. Spine 2018, 27, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.J.; Cassiere, H.A.; Dellis, S.L.; Manetta, F.; Kohn, N.; Hartman, A.R. Impact of preoperative prealbumin on outcomes after cardiac surgery. J. Parenter. Enter. Nutr. 2015, 39, 870–874. [Google Scholar] [CrossRef]
- Han, W.X.; Chen, Z.M.; Wei, Z.J.; Xu, A.M. Preoperative pre-albumin predicts prognosis of patients after gastrectomy for adenocarcinoma of esophagogastric junction. World J. Surg. Oncol. 2016. [CrossRef]
- Devakonda, A.; George, L.; Raoof, S.; Esan, A.; Saleh, A.; Bernstein, L.H. Transthyretin as a marker to predict outcome in critically ill patients. Clin. Biochem. 2008, 41, 1126–1130. [Google Scholar] [CrossRef] [PubMed]
- Perez Valdivieso, J.R.; Bes-Rastrollo, M.; Monedoro, P.; de Irala, J.; Lavilla, F.J. Impact of prealbumin levels in patients with acute kidney injury: An observational cohort study. J. Ren. Nutr. 2008, 18, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pan, Y.; Tang, X.; Hao, G.; Xie, Y.; Ma, S.; Luo, J.; Guo, D.; Ding, F. Serum prealbumin and its changes over time are associated with mortality in acute kidney injury. Sci. Rep. 2017. [Google Scholar] [CrossRef] [PubMed]
- Isono, N.; Imamura, Y.; Ohmura, K.; Ueda, N.; Kawabata, S.; Furuse, M.; Kuroiwa, T. Transthyretin concentrations in acute stroke patients predict convalescent rehabilitation. J. Stroke Cerebrovasc. Dis. 2017, 26, 1375–1382. [Google Scholar] [CrossRef]
- Ho, S.Y.; Guo, H.R.; Chen, H.H.; Peng, C.J. Nutritional predictors of survival in terminally ill cancer patients. J. Formos. Med. Assoc. 2003, 102, 544–550. [Google Scholar] [PubMed]
- Vieira, M.; Saraiva, M.J. Transthyretin: A multifaceted protein. Biomol. Concepts 2014, 5, 45–54. [Google Scholar] [CrossRef]
- Dellière, S.; Cynober, L. Is transthyretin a good marker of nutritional status? Clin. Nutr. 2017, 36, 364–370. [Google Scholar] [CrossRef]
- Shenkin, A. Serum prealbumin: Is it a marker of nutritional status or of risk of malnutrition? Clin. Chem. 2006, 52, 2177–2179. [Google Scholar] [CrossRef] [PubMed]
- Myron Johnson, A.; Merlini, G.; Sheldon, J.; Ichihara, K. Clinical indications for plasma protein assays: Transthyretin (prealbumin) in inflammation and malnutrition. IFCC Scientific Division Committee on Plasma Proteins (C-PP). Clin. Chem. Lab. Med. 2007, 45, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Ingenbleek, Y. The retinol circulating complex releases hormonal ligands in acute stress disorders. Front. Endocrinol. (Lausanne) 2018. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ingenbleek, Y. Plasma Transthyretin as A Biomarker of Sarcopenia in Elderly Subjects. Nutrients 2019, 11, 895. https://doi.org/10.3390/nu11040895
Ingenbleek Y. Plasma Transthyretin as A Biomarker of Sarcopenia in Elderly Subjects. Nutrients. 2019; 11(4):895. https://doi.org/10.3390/nu11040895
Chicago/Turabian StyleIngenbleek, Yves. 2019. "Plasma Transthyretin as A Biomarker of Sarcopenia in Elderly Subjects" Nutrients 11, no. 4: 895. https://doi.org/10.3390/nu11040895
APA StyleIngenbleek, Y. (2019). Plasma Transthyretin as A Biomarker of Sarcopenia in Elderly Subjects. Nutrients, 11(4), 895. https://doi.org/10.3390/nu11040895




