Developmental Vitamin D Deficiency Produces Behavioral Phenotypes of Relevance to Autism in an Animal Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Postnatal Growth and Developmental Milestones
2.3. Behavioral Tests and Procedures
2.3.1. Righting Reflex
2.3.2. Ultrasonic Vocalizations and Pup Retrieval
2.3.3. Negative Geotaxis
2.3.4. Olfactory Discrimination
2.3.5. Social Play Behavior
2.3.6. Three-Chambered Social Interaction Test
2.3.7. Marble Burying
2.3.8. Elevated Plus Maze
2.3.9. Purkinje Cell Number
2.3.10. Purkinje Cell Dendritic Complexity
2.3.11. Statistical Analysis
3. Results
3.1. Weight Gain and Developmental Milestones
3.2. Righting Reflex
3.3. USVs
3.4. Pup Retrieval
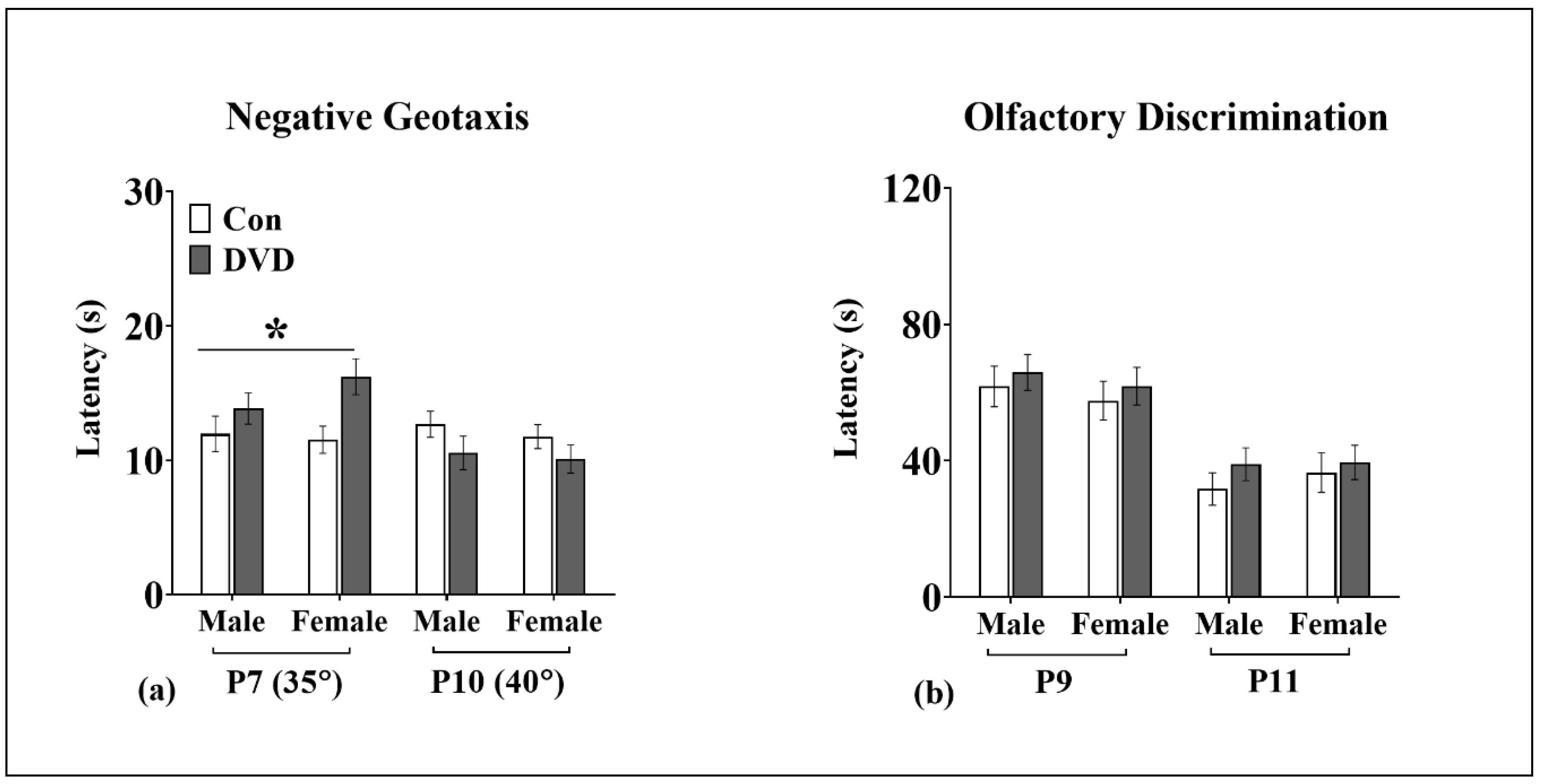
3.5. Negative Geotaxis
3.6. Olfactory Discrimination
3.7. Social Play Behavior
3.8. Three-Chambered Social Interaction Assay
3.9. Marble Burying
3.10. EPM
3.11. Estimation of Purkinje Cell Number
3.12. Dendritic Architecture of Purkinje Cells
4. Discussion
4.1. DVD-Deficiency Induced Delays in Motor Development
4.2. DVD-Deficiency Altered Vocal Communication in Pups
4.3. No Effect of DVD-Deficiency on Pup Retrieval Behavior
4.4. DVD-Deficiency Altered Marble-Burying/Stereotyped Repetitive Behavior in Adolescent and Adult Rats
4.5. DVD-Deficiency Reduced Social Interaction in Adolescent Rats But Not in Adults
4.6. DVD-Deficiency Induced Hyperlocomotion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Volkmar, F.; Chawarska, K.; Klin, A. Autism in infancy and early childhood. Ann. Rev. Psychol. 2005, 56, 315–336. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Provost, B.; Lopez, B.R.; Heimerl, S. A comparison of motor delays in young children: Autism spectrum disorder, developmental delay, and developmental concerns. J. Autism Dev. Disord. 2007, 37, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, M.; MacDonald, M.; Lord, C. Motor Skills of Toddlers with Autism Spectrum Disorders. Autism Int. J. Res. Pract. 2013, 17, 133–146. [Google Scholar] [CrossRef]
- Tonacci, A.; Billeci, L.; Tartarisco, G.; Ruta, L.; Muratori, F.; Pioggia, G.; Gangemi, S. Olfaction in autism spectrum disorders: A systematic review. Child Neuropsychol. 2017, 23, 1–25. [Google Scholar] [CrossRef]
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Robinson Rosenberg, C.; White, T.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill. Summ. 2018, 67, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Vinkhuyzen, A.A.; Eyles, D.W.; Burne, T.H.; Blanken, L.M.; Kruithof, C.J.; Verhulst, F.; Jaddoe, V.W.; Tiemeier, H.; McGrath, J.J. Gestational vitamin D deficiency and autism-related traits: The Generation R Study. Mol. Psychiatry 2018, 23, 240–246. [Google Scholar] [CrossRef]
- Vinkhuyzen, A.A.E.; Eyles, D.W.; Burne, T.H.J.; Blanken, L.M.E.; Kruithof, C.J.; Verhulst, F.; White, T.; Jaddoe, V.W.; Tiemeier, H.; McGrath, J.J. Gestational vitamin D deficiency and autism spectrum disorder. Br. J. Psychiatry Open 2017, 3, 85–90. [Google Scholar] [CrossRef]
- Whitehouse, A.J.; Holt, B.J.; Serralha, M.; Holt, P.G.; Kusel, M.M.; Hart, P.H. Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics 2012, 129, 485–493. [Google Scholar] [CrossRef]
- Muir, S.W.; Montero-Odasso, M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: A systematic review and meta-analysis. J. Am. Geriatr. Soc. 2011, 59, 2291–2300. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Suzuki, T.; Yoshida, H.; Kim, H.; Yoshida, Y.; Iwasa, H. Concomitant lower serum albumin and vitamin D levels are associated with decreased objective physical performance among Japanese community-dwelling elderly. Gerontology 2007, 53, 322–328. [Google Scholar] [CrossRef]
- García-Serna, A.M.; Morales, E. Neurodevelopmental effects of prenatal vitamin D in humans: Systematic review and meta-analysis. Mol. Psychiatry 2019. [Google Scholar] [CrossRef]
- Eyles, D.W.; Burne, T.H.J.; McGrath, J.J. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front. Neuroendocrinol. 2013, 34, 47–64. [Google Scholar] [CrossRef]
- Eyles, D.; Brown, J.; Mackay-Sim, A.; McGrath, J.; Feron, F. Vitamin D3 and brain development. Neuroscience 2003, 118, 641–653. [Google Scholar] [CrossRef]
- Du, L.; Zhao, G.; Duan, Z.; Li, F. Behavioral improvements in a valproic acid rat model of autism following vitamin D supplementation. Psychiatry Res. 2017, 253, 28–32. [Google Scholar] [CrossRef]
- Main, S.L.; Kulesza, R.J. Repeated prenatal exposure to valproic acid results in cerebellar hypoplasia and ataxia. Neuroscience 2017, 340, 34–47. [Google Scholar] [CrossRef]
- Schneider, T.; Przewlocki, R. Behavioral alterations in rats prenatally exposed to valproic acid: Animal model of autism. Neuropsychopharmacology 2005, 30, 80–89. [Google Scholar] [CrossRef]
- O’Loan, J.; Eyles, D.W.; Kesby, J.; Ko, P.; McGrath, J.J.; Burne, T.H. Vitamin D deficiency during various stages of pregnancy in the rat; its impact on development and behaviour in adult offspring. Psychoneuroendocrinology 2007, 32, 227–234. [Google Scholar] [CrossRef]
- Eyles, D.W.; Burne, T.H.; Alexander, S.; Cui, X.; McGrath, J.J. The Developmental Vitamin D (DVD) Model of Schizophrenia. In Animal Models of Schizophrenia and Related Disorders; O’Donnell, P., Ed.; Humana Press: New York, NY, USA, 2011; Volume 59, pp. 115–118. [Google Scholar]
- Foley, K.A.; Ossenkopp, K.-P.; Kavaliers, M.; MacFabe, D.F. Pre- and Neonatal Exposure to Lipopolysaccharide or the Enteric Metabolite, Propionic Acid, Alters Development and Behavior in Adolescent Rats in a Sexually Dimorphic Manner. PLoS ONE 2014, 9, e87072. [Google Scholar] [CrossRef]
- Curley, J.P.; Jensen, C.L.; Franks, B.; Champagne, F.A. Variation in maternal and anxiety-like behavior associated with discrete patterns of oxytocin and vasopressin 1a receptor density in the lateral septum. Horm. Behav. 2012, 61, 454–461. [Google Scholar] [CrossRef] [Green Version]
- St. Omer, V.E.; Ali, S.F.; Holson, R.R.; Duhart, H.M.; Scalzo, F.M.; Slikker, W., Jr. Behavioral and neurochemical effects of prenatal methylenedioxymethamphetamine (MDMA) exposure in rats. Neurotoxicol. Teratol. 1991, 13, 13–20. [Google Scholar] [CrossRef]
- Van Kerkhof, L.W.; Damsteegt, R.; Trezza, V.; Voorn, P.; Vanderschuren, L.J. Social play behavior in adolescent rats is mediated by functional activity in medial prefrontal cortex and striatum. Neuropsychopharmacology 2013, 38, 1899–1909. [Google Scholar] [CrossRef]
- Crawley, J.N. Translational animal models of autism and neurodevelopmental disorders. Dialogues Clin. Neurosci. 2012, 14, 293–305. [Google Scholar]
- Yang, M.; Silverman, J.L.; Crawley, J.N. Automated Three-Chambered Social Approach Task for Mice. Curr. Protoc. Neurosci. 2011, 56, 8–26. [Google Scholar]
- Deacon, R.M. Digging and marble burying in mice: Simple methods for in vivo identification of biological impacts. Nat. Protoc. 2006, 1, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.G.; de Jong, D.; Kanatsou, S.; Krugers, H.; Knapman, A.; Heinzmann, J.-M.; Holsboer, F.; Landgraf, R.; Joëls, M.; Touma, C. Dendritic Morphology of Hippocampal and Amygdalar Neurons in Adolescent Mice Is Resilient to Genetic Differences in Stress Reactivity. PLoS ONE 2012, 7, e38971. [Google Scholar] [CrossRef] [PubMed]
- Fournier, K.A.; Hass, C.J.; Naik, S.K.; Lodha, N.; Cauraugh, J.H. Motor coordination in autism spectrum disorders: A synthesis and meta-analysis. J. Autism Dev. Disord. 2010, 40, 1227–1240. [Google Scholar] [CrossRef]
- Darling, A.L.; Rayman, M.P.; Steer, C.D.; Golding, J.; Lanham-New, S.A.; Bath, S.C. Association between maternal vitamin D status in pregnancy and neurodevelopmental outcomes in childhood: Results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Br. J. Nutr. 2017, 117, 1682–1692. [Google Scholar] [CrossRef]
- Salinger, W.L.; Ladrow, P.; Wheeler, C. Behavioral phenotype of the reeler mutant mouse: Effects of RELN gene dosage and social isolation. Behav. Neurosci. 2003, 117, 1257–1275. [Google Scholar] [CrossRef]
- Al Sagheer, T.; Haida, O.; Balbous, A.; Francheteau, M.; Matas, E.; Fernagut, P.-O.; Jaber, M. Motor Impairments Correlate with Social Deficits and Restricted Neuronal Loss in an Environmental Model of Autism. Int. J. Neuropsychopharmacol. 2018, 21, 871–882. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Chung, S.K.; Chow, B.K.C. The Knockout of Secretin in Cerebellar Purkinje Cells Impairs Mouse Motor Coordination and Motor Learning. Neuropsychopharmacology 2014, 39, 1460–1468. [Google Scholar] [CrossRef]
- Groszer, M.; Keays, D.A.; Deacon, R.M.J.; de Bono, J.P.; Prasad-Mulcare, S.; Gaub, S.; Baum, M.G.; French, C.A.; Nicod, J.; Coventry, J.A.; et al. Impaired Synaptic Plasticity and Motor Learning in Mice with a Point Mutation Implicated in Human Speech Deficits. Curr. Biol. 2008, 18, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Inoue, A.; Iwasaki, S.; Ushio, M.; Chihara, Y.; Fujimoto, C.; Egami, N.; Yamasoba, T. Effect of vestibular dysfunction on the development of gross motor function in children with profound hearing loss. Audiol. Neuro-Otol. 2013, 18, 143–151. [Google Scholar] [CrossRef]
- Kaga, K.; Shinjo, Y.; Jin, Y.; Takegoshi, H. Vestibular failure in children with congenital deafness. Int. J. Audiol. 2008, 47, 590–599. [Google Scholar] [CrossRef]
- Vidal, P.P.; Degallaix, L.; Josset, P.; Gasc, J.P.; Cullen, K.E. Postural and locomotor control in normal and vestibularly deficient mice. J. Physiol. 2004, 559, 625–638. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, D.; Raveendran, N.N.; Pondugula, S.R.; Kampalli, S.B.; Sanneman, J.D.; Harbidge, D.G.; Marcus, D.C. Vitamin D upregulates expression of ECaC1 mRNA in semicircular canal. Biochem. Biophys. Res. Commun. 2005, 331, 1353–1357. [Google Scholar] [CrossRef]
- Zou, J.; Minasyan, A.; Keisala, T.; Zhang, Y.; Wang, J.H.; Lou, Y.R.; Kalueff, A.; Pyykko, I.; Tuohimaa, P. Progressive hearing loss in mice with a mutated vitamin D receptor gene. Audiol. Neuro-Otol. 2008, 13, 219–230. [Google Scholar] [CrossRef]
- Minasyan, A.; Keisala, T.; Zou, J.; Zhang, Y.; Toppila, E.; Syvälä, H.; Lou, Y.-R.; Kalueff, A.V.; Pyykkö, I.; Tuohimaa, P. Vestibular dysfunction in vitamin D receptor mutant mice. J. Steroid Biochem. Mol. Biol. 2009, 114, 161–166. [Google Scholar] [CrossRef]
- Norrelgen, F.; Fernell, E.; Eriksson, M.; Hedvall, A.; Persson, C.; Sjolin, M.; Gillberg, C.; Kjellmer, L. Children with autism spectrum disorders who do not develop phrase speech in the preschool years. Autism 2015, 19, 934–943. [Google Scholar] [CrossRef]
- Kas, M.J.; Glennon, J.C.; Buitelaar, J.; Ey, E.; Biemans, B.; Crawley, J.; Ring, R.H.; Lajonchere, C.; Esclassan, F.; Talpos, J.; et al. Assessing behavioural and cognitive domains of autism spectrum disorders in rodents: Current status and future perspectives. Psychopharmacology 2014, 231, 1125–1146. [Google Scholar] [CrossRef]
- Yates, N.J.; Tesic, D.; Feindel, K.W.; Smith, J.T.; Clarke, M.W.; Wale, C.; Crew, R.C.; Wharfe, M.D.; Whitehouse, A.J.O.; Wyrwoll, C.S. Vitamin D is crucial for maternal care and offspring social behaviour in rats. J. Endocrinol. 2018, 237, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Burne, T.H.; O’Loan, J.; Splatt, K.; Alexander, S.; McGrath, J.J.; Eyles, D.W. Developmental vitamin D (DVD) deficiency alters pup-retrieval but not isolation-induced pup ultrasonic vocalizations in the rat. Physiol. Behav. 2011, 102, 201–204. [Google Scholar] [CrossRef]
- Scattoni, M.L.; Crawley, J.; Ricceri, L. Ultrasonic vocalizations: A tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci. Biobehav. Rev. 2009, 33, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Wöhr, M. Ultrasonic vocalizations in Shank mouse models for autism spectrum disorders: Detailed spectrographic analyses and developmental profiles. Neurosci. Biobehav. Rev. 2014, 43, 199–212. [Google Scholar] [CrossRef]
- Nicolini, C.; Fahnestock, M. The valproic acid-induced rodent model of autism. Exp. Neurol. 2018, 299, 217–227. [Google Scholar] [CrossRef]
- Picker, J.D.; Yang, R.; Ricceri, L.; Berger-Sweeney, J. An altered neonatal behavioral phenotype in Mecp2 mutant mice. Neuroreport 2006, 17, 541–544. [Google Scholar] [CrossRef]
- Young, D.M.; Schenk, A.K.; Yang, S.B.; Jan, Y.N.; Jan, L.Y. Altered ultrasonic vocalizations in a tuberous sclerosis mouse model of autism. Proc. Natl. Acad. Sci. USA 2010, 107, 11074–11079. [Google Scholar] [CrossRef] [Green Version]
- Schwartzer, J.J.; Careaga, M.; Onore, C.E.; Rushakoff, J.A.; Berman, R.F.; Ashwood, P. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transl. Psychiatry 2013, 3, e240. [Google Scholar] [CrossRef]
- Choi, G.B.; Yim, Y.S.; Wong, H.; Kim, S.; Kim, H.; Kim, S.V.; Hoeffer, C.A.; Littman, D.R.; Huh, J.R. The maternal interleukin-17a pathway in mice promotes autismlike phenotypes in offspring. Science 2016, 351, 933–939. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.; Yim, Y.S.; Ha, S.; Atarashi, K.; Tan, T.G.; Longman, R.S.; Honda, K.; Littman, D.R.; Choi, G.B.; et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 2017, 549, 528. [Google Scholar] [CrossRef]
- Gandal, M.J.; Edgar, J.C.; Ehrlichman, R.S.; Mehta, M.; Roberts, T.P.L.; Siegel, S.J. Validating γ Oscillations and Delayed Auditory Responses as Translational Biomarkers of Autism. Biol. Psychiatry 2010, 68, 1100–1106. [Google Scholar] [CrossRef]
- Curry, T.; Egeto, P.; Wang, H.; Podnos, A.; Wasserman, D.; Yeomans, J. Dopamine receptor D2 deficiency reduces mouse pup ultrasonic vocalizations and maternal responsiveness. Genes Brain Behav. 2013, 12, 397–404. [Google Scholar] [CrossRef]
- Williams, S.N.; Undieh, A.S. Dopamine-sensitive signaling mediators modulate psychostimulant-induced ultrasonic vocalization behavior in rats. Behav. Brain Res. 2016, 296, 1–6. [Google Scholar] [CrossRef]
- Cui, X.; Pelekanos, M.; Burne, T.H.; McGrath, J.J.; Eyles, D.W. Maternal vitamin D deficiency alters the expression of genes involved in dopamine specification in the developing rat mesencephalon. Neurosci. Lett. 2010, 486, 220–223. [Google Scholar] [CrossRef]
- Luan, W.; Hammond, L.A.; Cotter, E.; Osborne, G.W.; Alexander, S.A.; Nink, V.; Cui, X.; Eyles, D.W. Developmental Vitamin D (DVD) Deficiency Reduces Nurr1 and TH Expression in Post-mitotic Dopamine Neurons in Rat Mesencephalon. Mol. Neurobiol. 2017. [Google Scholar] [CrossRef]
- Insel, T.R.; Hill, J.L.; Mayor, R.B. Rat pup ultrasonic isolation calls: Possible mediation by the benzodiazepine receptor complex. Pharmacol. Biochem. Behav. 1986, 24, 1263–1267. [Google Scholar] [CrossRef]
- Takahashi, A.; Yap, J.J.; Bohager, D.Z.; Faccidomo, S.; Clayton, T.; Cook, J.M.; Miczek, K.A. Glutamatergic and GABAergic modulations of ultrasonic vocalizations during maternal separation distress in mouse pups. Psychopharmacology 2009, 204, 61–71. [Google Scholar] [CrossRef]
- Eyles, D.W.; Rogers, F.; Buller, K.; McGrath, J.J.; Ko, P.; French, K.; Burne, T.H. Developmental vitamin D (DVD) deficiency in the rat alters adult behaviour independently of HPA function. Psychoneuroendocrinology 2006, 31, 958–964. [Google Scholar] [CrossRef]
- Tesic, D.; Hawes, J.E.; Zosky, G.R.; Wyrwoll, C.S. Vitamin D Deficiency in BALB/c Mouse Pregnancy Increases Placental Transfer of Glucocorticoids. Endocrinology 2015, 156, 3673–3679. [Google Scholar] [CrossRef] [Green Version]
- Brunelli, S.A.; Shair, H.N.; Hofer, M.A. Hypothermic vocalizations of rat pups (Rattus norvegicus) elicit and direct maternal search behavior. J. Comp. Psychol. 1994, 108, 298–303. [Google Scholar] [CrossRef]
- Wöhr, M.; Scattoni, M.L. Behavioural methods used in rodent models of autism spectrum disorders: Current standards and new developments. Behav. Brain Res. 2013, 251, 5–17. [Google Scholar] [CrossRef]
- Ghanizadeh, A. Clinical Approach to Motor Stereotypies in Autistic Children. Iran. J. Pediatr. 2010, 20, 149–159. [Google Scholar]
- Thomas, A.; Burant, A.; Bui, N.; Graham, D.; Yuva-Paylor, L.A.; Paylor, R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology 2009, 204, 361–373. [Google Scholar] [CrossRef] [Green Version]
- Sungur, A.Ö.; Vörckel, K.J.; Schwarting, R.K.W.; Wöhr, M. Repetitive behaviors in the Shank1 knockout mouse model for autism spectrum disorder: Developmental aspects and effects of social context. J. Neurosci. Methods 2014, 234, 92–100. [Google Scholar] [CrossRef]
- Kouser, M.; Speed, H.E.; Dewey, C.M.; Reimers, J.M.; Widman, A.J.; Gupta, N.; Liu, S.; Jaramillo, T.C.; Bangash, M.; Xiao, B.; et al. Loss of predominant Shank3 isoforms results in hippocampus-dependent impairments in behavior and synaptic transmission. J. Neurosci. 2013, 33, 18448–18468. [Google Scholar] [CrossRef]
- Mosienko, V.; Bert, B.; Beis, D.; Matthes, S.; Fink, H.; Bader, M.; Alenina, N. Exaggerated aggression and decreased anxiety in mice deficient in brain serotonin. Transl. Psychiatry 2012, 2, e122. [Google Scholar] [CrossRef]
- Hedlund, P.B.; Sutcliffe, J.G. The 5-HT7 receptor influences stereotypic behavior in a model of obsessive-compulsive disorder. Neurosci. Lett. 2007, 414, 247–251. [Google Scholar] [CrossRef] [Green Version]
- Lugo, J.N.; Smith, G.D.; Arbuckle, E.P.; White, J.; Holley, A.J.; Floruta, C.M.; Ahmed, N.; Gomez, M.C.; Okonkwo, O. Deletion of PTEN produces autism-like behavioral deficits and alterations in synaptic proteins. Front. Mol. Neurosci. 2014, 7, 27. [Google Scholar] [CrossRef]
- Vuillermot, S.; Luan, W.; Meyer, U.; Eyles, D. Vitamin D treatment during pregnancy prevents autism-related phenotypes in a mouse model of maternal immune activation. Mol. Autism 2017, 8, 9. [Google Scholar] [CrossRef]
- Kesby, J.P.; Turner, K.M.; Alexander, S.; Eyles, D.W.; McGrath, J.J.; Burne, T.H.J. Developmental vitamin D deficiency alters multiple neurotransmitter systems in the neonatal rat brain. Int. J. Dev. Neurosci. 2017, 62, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Patrick, R.P.; Ames, B.N. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: Relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J. 2015, 29, 2207–2222. [Google Scholar] [CrossRef]
- Patrick, R.P.; Ames, B.N. Vitamin D hormone regulates serotonin synthesis. Part 1: Relevance for autism. FASEB J. 2014, 28, 2398–2413. [Google Scholar] [CrossRef] [PubMed]
- Beatty, W.W.; Costello, K.B.; Berry, S.L. Suppression of play fighting by amphetamine: Effects of catecholamine antagonists, agonists and synthesis inhibitors. Pharmacol. Biochem. Behav. 1984, 20, 747–755. [Google Scholar] [CrossRef]
- Holloway, W.R., Jr.; Thor, D.H. Interactive effects of caffeine, 2-chloroadenosine and haloperidol on activity, social investigation and play fighting of juvenile rats. Pharmacol. Biochem. Behav. 1985, 22, 421–426. [Google Scholar] [CrossRef]
- Siviy, S.M.; Fleischhauer, A.E.; Kerrigan, L.A.; Kuhlman, S.J. D2 dopamine receptor involvement in the rough-and-tumble play behavior of juvenile rats. Behav. Neurosci. 1996, 110, 1168–1176. [Google Scholar] [CrossRef]
- Achterberg, E.J.; Trezza, V.; Siviy, S.M.; Schrama, L.; Schoffelmeer, A.N.; Vanderschuren, L.J. Amphetamine and cocaine suppress social play behavior in rats through distinct mechanisms. Psychopharmacology 2014, 231, 1503–1515. [Google Scholar] [CrossRef]
- Pertile, R.A.; Cui, X.; Eyles, D.W. Vitamin D signaling and the differentiation of developing dopamine systems. Neuroscience 2016, 333, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Kesby, J.P.; Cui, X.; Ko, P.; McGrath, J.J.; Burne, T.H.; Eyles, D.W. Developmental vitamin D deficiency alters dopamine turnover in neonatal rat forebrain. Neurosci. Lett. 2009, 461, 155–158. [Google Scholar] [CrossRef]
- Byrne, J.H.; Voogt, M.; Turner, K.M.; Eyles, D.W.; McGrath, J.J.; Burne, T.H.J. The Impact of Adult Vitamin D Deficiency on Behaviour and Brain Function in Male Sprague-Dawley Rats. PLoS ONE 2013, 8, e71593. [Google Scholar] [CrossRef]
- Kesby, J.P.; Burne, T.H.; McGrath, J.J.; Eyles, D.W. Developmental vitamin D deficiency alters MK 801-induced hyperlocomotion in the adult rat: An animal model of schizophrenia. Biol. Psychiatry 2006, 60, 591–596. [Google Scholar] [CrossRef]
- Harms, L.R.; Eyles, D.W.; McGrath, J.J.; Mackay-Sim, A.; Burne, T.H. Developmental vitamin D deficiency alters adult behaviour in 129/SvJ and C57BL/6J mice. Behav. Brain Res. 2008, 187, 343–350. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Vasileva, S.; Langguth, M.; Alexander, S.; Cui, X.; Whitehouse, A.; McGrath, J.J.; Eyles, D. Developmental Vitamin D Deficiency Produces Behavioral Phenotypes of Relevance to Autism in an Animal Model. Nutrients 2019, 11, 1187. https://doi.org/10.3390/nu11051187
Ali A, Vasileva S, Langguth M, Alexander S, Cui X, Whitehouse A, McGrath JJ, Eyles D. Developmental Vitamin D Deficiency Produces Behavioral Phenotypes of Relevance to Autism in an Animal Model. Nutrients. 2019; 11(5):1187. https://doi.org/10.3390/nu11051187
Chicago/Turabian StyleAli, Asad, Svetlina Vasileva, Mia Langguth, Suzanne Alexander, Xiaoying Cui, Andrew Whitehouse, John J. McGrath, and Darryl Eyles. 2019. "Developmental Vitamin D Deficiency Produces Behavioral Phenotypes of Relevance to Autism in an Animal Model" Nutrients 11, no. 5: 1187. https://doi.org/10.3390/nu11051187
APA StyleAli, A., Vasileva, S., Langguth, M., Alexander, S., Cui, X., Whitehouse, A., McGrath, J. J., & Eyles, D. (2019). Developmental Vitamin D Deficiency Produces Behavioral Phenotypes of Relevance to Autism in an Animal Model. Nutrients, 11(5), 1187. https://doi.org/10.3390/nu11051187




