Resting Energy Expenditure and Protein Balance in People with Epidermolysis Bullosa
Abstract
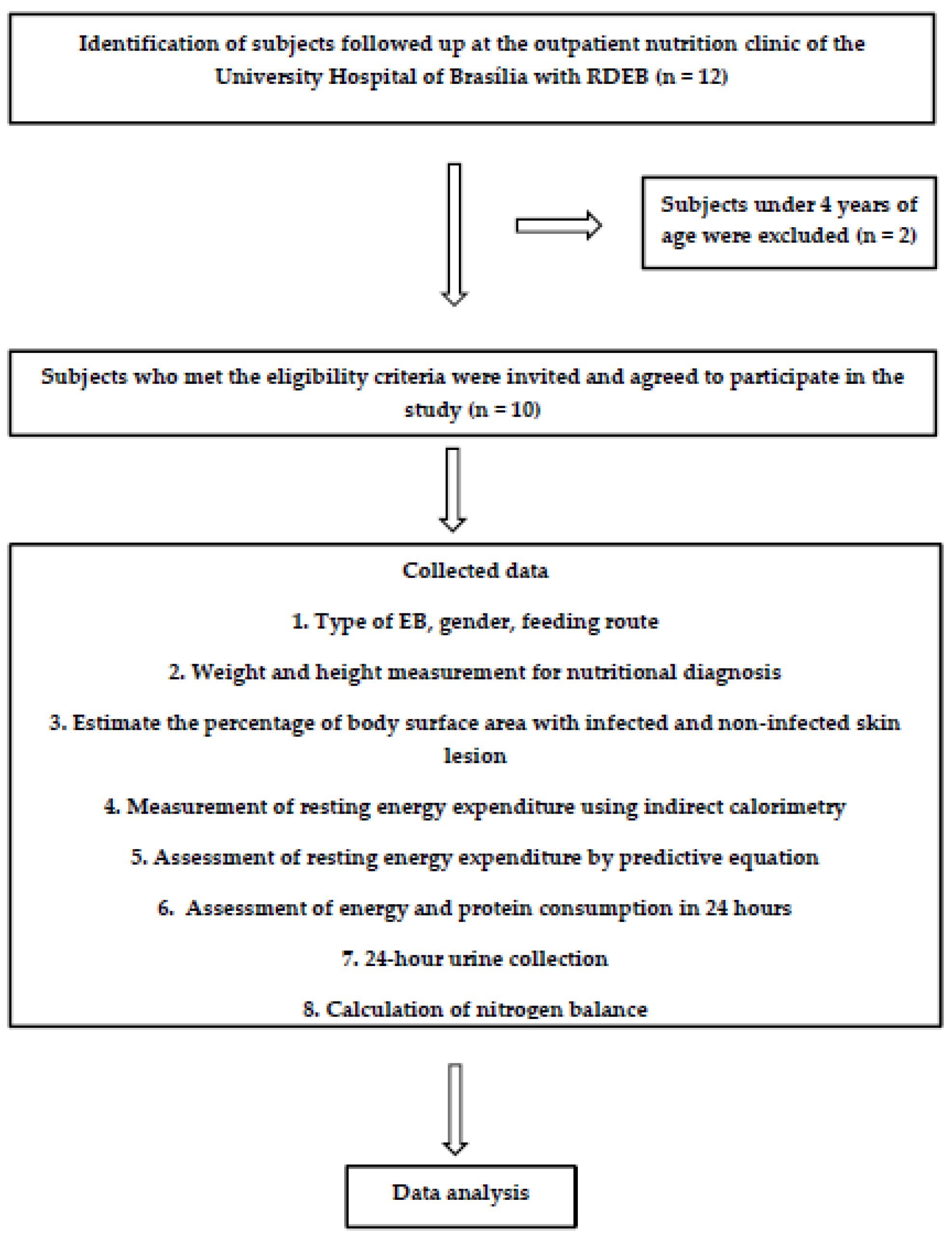
:1. Introduction
2. Materials and Methods
2.1. Type of Study and Subjects
2.2. Nutritional Status
2.3. Estimation of Percentage of Infected and Non-Infected Skin Lesions of Body Surface Area (BSA)
2.4. Resting Energy Expenditure Measured by Indirect Calorimetry
2.5. Resting Energy Expenditure Estimated by Predictive Equation
2.6. Energy and Protein Intake
2.7. Nitrogen Balance
(0.1 g × % BSA wounded/blistered)]}
2.8. Data Analysis
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Intong, L.R.A.; Murrell, D.F. Inherited epidermolysis bullosa: New diagnostic criteria and classification. Clin. Dermatol. 2012, 30, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.D.; Johnson, L.B.; Weiner, M.; Suchindran, C. Gastrointestinal complications of inherited epidermolysis bullosa: Cumulative experience of the National Epidermolysis Bullosa Registry. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.-D.; Bruckner-Tuderman, L.; Eady, R.A.; Bauer, E.A.; Bauer, J.W.; Has, C.; Heagerty, A.; Hintner, H.; Hovnanian, A.; Jonkman, M.F.; et al. Inherited epidermolysis bullosa: Updated recommendations on diagnosis and classification. J. Am. Acad. Dermatol. 2014, 70, 1103–1126. [Google Scholar] [CrossRef] [PubMed]
- Haynes, L. Nutrition for Children with Epidermolysis Bullosa. Dermatol. Clin. 2010, 28, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Zidorio, A.P.C.; Leão, D.O.D.; de Carvalho, K.M.B.; Dutra, E.S. Nutritional outcomes in children with epidermolysis bullosa: Long-term follow-up. Nutr. Hosp. 2018, 35, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.; Mellerio, J.E. Extracutaneous manifestations and complications of inherited epidermolysis bullosa: Part I. Epithelial associated tissues. J. Am. Acad. Dermatol. 2009, 61, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Lechner-Gruskay, D.; Honig, P.J.; Pereira, G.; McKinney, S. Nutritional and Metabolic Profile of Children with Epidermolysis Bullosa. Pediatr. Dermatol. 1988, 5, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Lechner-Gruskay, D. Nutritional Assessment of the Child with Junctional and Recessive Distrofic Epidermolysis Bullosa. Master’s Thesis, Drextel University, Philadelphia, PA, USA, 1986. [Google Scholar]
- Gamelli, R.L. Nutritional problems of the acute and chronic burn patient. Relevance to epidermolysis bullosa. Arch. Dermatol. 1988, 124, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; Mlcak, R.P.; Finnerty, C.C.; Norbury, W.B.; Gauglitz, G.G.; A Kulp, G.; Herndon, D.N. Burn size determines the inflammatory and hypermetabolic response. Crit. Care 2007, 11, R90. [Google Scholar] [CrossRef]
- Goran, M.I.; Peters, E.J.; Herndon, D.N.; Wolfe, R.R. Total energy expenditure in burned children using the doubly labeled water technique. Am. J. Physiol. Metab. 1990, 259, E576–E585. [Google Scholar] [CrossRef]
- Tancheva, D.; Arabadziev, J.; Gergov, G.; Lachev, N.; Todorova, S.; Hristova, A. Comparison of estimated energy requirements in severely burned patients with measurements by using indirect calorimetry. Ann. Burns Fire Disasters 2005, 18, 16. [Google Scholar] [PubMed]
- Tayek, J.A.; Blackburn, G.L. Goals of nutritional support in acute infections. Am. J. Med. 1984, 76, 81–90. [Google Scholar] [CrossRef]
- Haynes, L. Clinical Practice Guidelines for Nutrition Support in Children with Epidermolysis Bullosa Including Tool to Help Identify Nutritional Compromise (THINC) in EB. 2007. Available online: http://www.debra.org.uk/uploads/resources/EB_Guidelines_Final_for_web.pdf: (accessed on 13 August 2018).
- Zidorio, A.P.C.; Dutra, E.S.; Leão, D.O.D.; Costa, I.M.C. Nutritional aspects of children and adolescents with epidermolysis bullosa: Literature review. An. Bras. Dermatol. 2015, 90, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Birge, K. Nutrition management of patients with epidermolysis bullosa. J. Am. Diet. Assoc. 1995, 95, 575–579. [Google Scholar] [CrossRef]
- Bonada Sanjaume, A.; Azón, A.; Guillén Rey, N.; Llort Baiget, M.; Figueredo, R.; Salas-Salvadó, J. Estado nutricional y gasto energético en paciente con epidermiólisis ampollosa hereditaria distrófica. Nutr. Hosp. 2004, 19, 58. [Google Scholar]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Developments; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- World Health Organization. Physical status: The use and interpretation of anthropometry. Report of a WHO Expert Committee. In WHO Technical Report Series; No. 854; WHO: Geneva, Switzerland, 1995. [Google Scholar]
- Ministério da Saúde. Secretaria de Atenção à Saúde. Departamento de Atenção Básica. Orientações Para A Coleta e Análise de Dados Antropométricos Em Serviços de Saúde: Norma Técnica do SISTEMA de Vigilância Alimentar e Nutricional—Sisvan. Brasília. 2011. Available online: http://200.17.213.49/lib/exe/fetch.php/projetos:obesidade:sisvannormatecnica.pdf (accessed on 19 August 2018).
- Oshima, T.; Berger, M.M.; De Waele, E.; Guttormsen, A.B.; Heidegger, C.-P.; Hiesmayr, M.; Singer, P.; Wernerman, J.; Pichard, C. Indirect calorimetry in nutritional therapy. A position paper by the ICALIC study group. Clin. Nutr. 2017, 36, 651–662. [Google Scholar] [CrossRef]
- Haugen, H.A.; Chan, L.-N.; Li, F. Indirect calorimetry: A practical guide for clinicians. Nutr. Clin. Pract. 2007, 22, 377–388. [Google Scholar] [CrossRef]
- Suman, O.E.; Mlcak, R.P.; Chinkes, D.L.; Herndon, D.N. Resting energy expenditure in severely burned children: Analysis of agreement between indirect calorimetry and prediction equations using the Bland–Altman method. Burns 2006, 32, 335–342. [Google Scholar] [CrossRef]
- Henry, C.J.K. Basal metabolic rate studies in humans: Measurement and development of new equations. Public Health Nutr. 2005, 8, 1133–1152. [Google Scholar] [CrossRef]
- Schofield, W.N. Predicting basal metabolic rate, new standards and review of previous work. Hum. Nutr. Clin. Nutr. 1985, 39, 5–41. [Google Scholar]
- Costa, T.H.M. CalcNut: Plataforma Para Cálculo de Dieta. Departamento de Nutrição, Faculdade de Ciências da Saúde, Universidade de Brasília. Available online: https://fs.unb.br/nutricao/calcnut/?page_id=47 (accessed on 31 August 2018).
- Universidade Estadual de Campinas—UNICAMP. Tabela Brasileira de Composição de Alimentos—TACO. 4. ed. rev. e ampl; UNICAMP/NEPA: Campinas, Brazil, 2011; p. 161. Available online: http://www.unicamp.br/nepa/taco/tabela.php?ativo=tabela (accessed on 10 September 2018).
- Jacobs, S.C. Assessment of automated nitrogen analysis of biological fluids with reference to the Kjeldahl method. J. Clin. Pathol. 1968, 21, 218–219. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Harris, J.A.; Benedict, F.G. A biometric study of human basal metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine; National Research Council. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids (Macronutrients); National Academy Press: Washington, DC, USA, 2005; Available online: http://www.nap.edu/catalog/10490 (accessed on 16 March 2018).
- Fedeles, F.; Murphy, M.; Rothe, M.J.; Grant-Kels, J.M. Nutrition and bullous skin diseases. Clin. Dermatol. 2010, 28, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Bello, Y.M.; Falabella, A.F.; Schachner, L.A. Management of epidermolysis bullosa in infants and children. Clin. Dermatol. 2003, 21, 278–282. [Google Scholar] [CrossRef]
- Jotterand Chaparro, C.; Moullet, C.; Taffé, P.; Laure Depeyre, J.; Perez, M.; Longchamp, D.; Cotting, J. Estimation of Resting Energy Expenditure Using Predictive Equations in Critically Ill Children: Results of a Systematic Review. J. Parenter. Enter. Nutr. 2018, 42, 976–986. [Google Scholar] [CrossRef]
- Mc Clave, S.A.; Lowen, C.C.; Kleber, M.J.; McConnell, J.W.; Jung, L.Y.; Goldsmith, L.J. Clinical use of the respiratory quotient obtained from indirect calorimetry. J. Parenter. Enter. Nutr. 2003, 27, 21–26. [Google Scholar] [CrossRef]
- De Cosmi, V.; Milani, G.P.; Mazzocchi, A.; D’Oria, V.; Silano, M.; Calderini, E.; Agostoni, C.; D’Oria, V. The metabolic response to stress and infection in critically ill children: The opportunity of an individualized approach. Nutrients 2017, 9, 1032. [Google Scholar] [CrossRef]
- Dickerson, R.N. Using nitrogen balance in clinical practice. Hosp. Pharm. 2005, 40, 1081–1087. [Google Scholar] [CrossRef]
- World Health Organization. Protein and amino acid requirements in human nutrition. In WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2002; p. 935. [Google Scholar]
- Colomb, V.; Bourdon-Lannoy, E.; Lambe, C.; Sauvat, F.; Hadj-Rabia, S.; Teillac, D.; De Prost, Y.; Bodemer, C.; Bourdon-Lannoy, E. Nutritional outcome in children with severe generalized recessive dystrophic epidermolysis bullosa: A short-and long-term evaluation of gastrostomy and enteral feeding. Br. J. Dermatol. 2012, 166, 354–361. [Google Scholar] [CrossRef]


| Age | Female (Kcal/day) | Male (Kcal/day) |
|---|---|---|
| 3–10 years | 20.1 × Weight + 507 | 23.3 × Weight +514 |
| 10–18 years | 11.1 × Weight + 761 | 18.4 × Weight + 581 |
| 18–30 years | 13.1 × Weight + 558 | 16.0 × Weight + 545 |
| 30–59 years | 9.74 × Weight + 694 | 14.2 × Weight + 593 |
| Patient | Gender | Age | Corrected Age * | H/A (Percentile) | BMI (kg/m2) | Nutritional Status ** |
|---|---|---|---|---|---|---|
| 1 | F | 4 y 8 m | 3 y 3 m | 1–3° | 11.9 | severe thinness |
| 2 | F | 7 y 7 m | 6 y 3 m | 1–3° | 12.0 | thinness |
| 3 | M | 12 y 11 m | 7 y 11 m | <1° | 13.4 | thinness |
| 4 | F | 13 y | 9 y 9 m | <1° | 14.5 | thinness |
| 5 | F | 16 y 7 m | 11 y 1 m | <1° | 10.3 | severe thinness |
| 6 | F | 16 y 7 m | 12 y 10 m | 3–5° | 12.9 | severe thinness |
| 7 | M | 21 y | NA | NA | 17.6 | thinness |
| 8 | F | 22 y | NA | NA | 16.7 | severe thinness |
| 9 | M | 23 y | NA | NA | 12.2 | severe thinness |
| 10 | F | 33 y | NA | NA | 17.5 | thinness |
| Patient | Gender | Corrected Age * | % BSA with Non-Infected Lesions | % BSA with Infected Lesions | Respiratory Quotient | REE Predicted ** | REE Measured by IC | Energy Intake (kcal/kg) | Protein Intake | Urinary Urea Nitrogen (g/day) | Nitrogen Balance (g) | Nitrogen Balance Conclusion | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| kcal/day | kcal/kg | kcal/day | kcal/kg | g | g/kg Body Weight | ||||||||||
| 1 | F | 3 y 3 m | 15 | 0 | 0.89 | 726 | 67 | 747 | 68 | 75 | 44 | 4.01 | 2.91 | −1.91 | catabolic |
| 2 | F | 6 y 3 m | 13 | 3 | 0.82 | 816 | 53 | 973 | 63 | 95 | 81 | 5.24 | 2.78 | −2.52 | catabolic |
| 3 | M | 7 y 11 m | 20 | 0 | 0.89 | 989 | 48 | 1111 | 54 | 130 | 85 | 4.18 | 3.42 | 2.23 | anabolic |
| 4 | F | 9 y 9 m | 22 | 0 | 0.90 | 1032 | 39 | 1093 | 42 | 135 | 138 | 5.27 | 5.17 | 8.05 | anabolic |
| 5 a | F | 11 y 1 m | 20 | 0 | 0.82 | 1047 | 41 | 1000 | 39 | 130 | 149 | 5.79 | NA | NA | NA |
| 6 | F | 12 y 10 m | 27 | 0 | 0.82 | 1024 | 43 | 1023 | 43 | 57 | 29 | 1.22 | 3.27 | −9.42 | catabolic |
| 7 | M | 21 y | 17 | 0 | 0.96 | 1164 | 30 | 1508 | 39 | 45 | 59 | 1.49 | 1.83 | 0.62 | balance |
| 8 | F | 22 y | 28 | 4 | 0.93 | 1193 | 25 | 1484 | 31 | 59 | 109 | 2.25 | 8.63 | −5.57 | catabolic |
| 9 b | M | 23 y | 0 | 16 | 0.89 | 929 | 39 | 1233 | 51 | 74 | 99 | 4.12 | 4.71 | −1.70 | catabolic |
| 10 | F | 33 y | 23 | 7 | 0.83 | 1130 | 25 | 1402 | 31 | 43 | 70 | 1.56 | 4.68 | −8.27 | catabolic |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zidorio, A.P.; Togo, C.; Jones, R.; Dutra, E.; de Carvalho, K. Resting Energy Expenditure and Protein Balance in People with Epidermolysis Bullosa. Nutrients 2019, 11, 1257. https://doi.org/10.3390/nu11061257
Zidorio AP, Togo C, Jones R, Dutra E, de Carvalho K. Resting Energy Expenditure and Protein Balance in People with Epidermolysis Bullosa. Nutrients. 2019; 11(6):1257. https://doi.org/10.3390/nu11061257
Chicago/Turabian StyleZidorio, Ana Paula, Camille Togo, Rosie Jones, Eliane Dutra, and Kenia de Carvalho. 2019. "Resting Energy Expenditure and Protein Balance in People with Epidermolysis Bullosa" Nutrients 11, no. 6: 1257. https://doi.org/10.3390/nu11061257





