Glutamine, but not Branched-Chain Amino Acids, Restores Intestinal Barrier Function during Activity-Based Anorexia
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Procedure
2.2. Body Composition
2.3. Evaluation of Intestinal Permeability
2.4. Protein Extraction and Western Blotting
2.5. Protein Synthesis Analysis by the SUnSET Method
2.6. RT-qPCR
2.7. Adiponectin and Leptin Plasma Concentrations
2.8. Colonic Intracellular Signaling Pathways
2.9. Statistical Analysis
3. Results
3.1. Body Weight and Body Composition
3.2. Food Intake and Hypothalamic Neuropeptides
3.3. Colonic Permeability and Tight Junction mRNA
3.4. Mucin-2 mRNA and Colonic Protein Synthesis
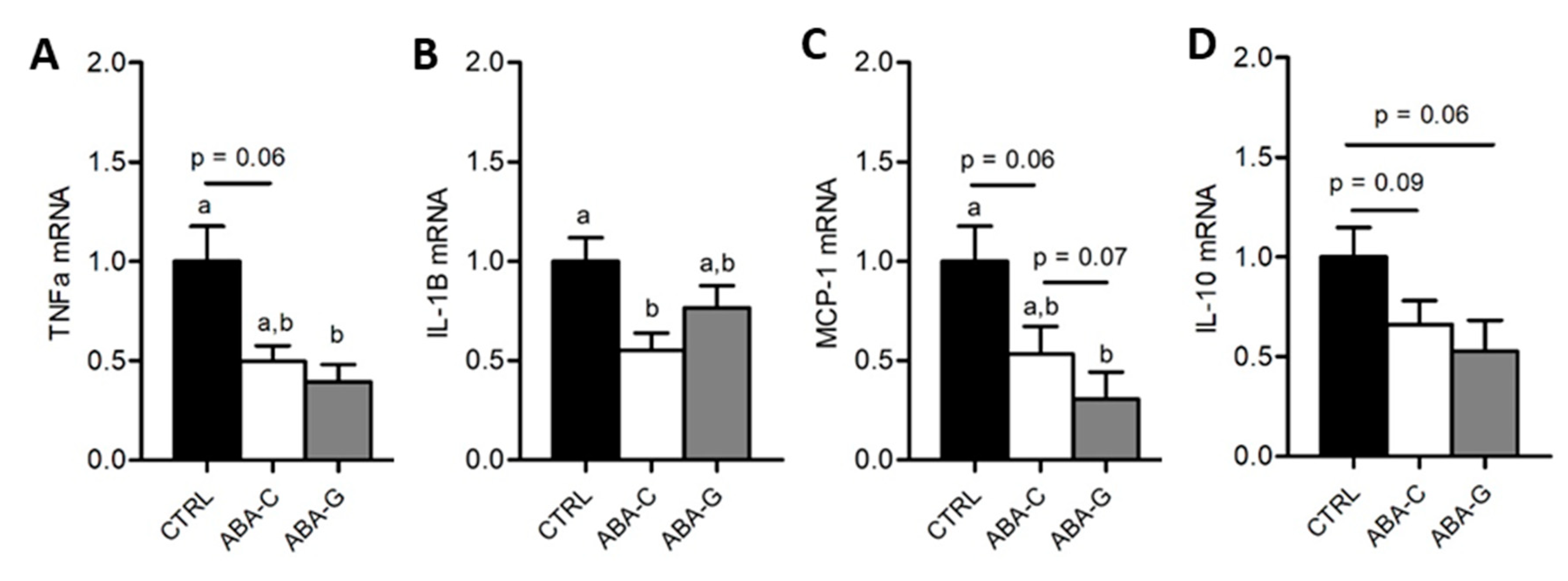
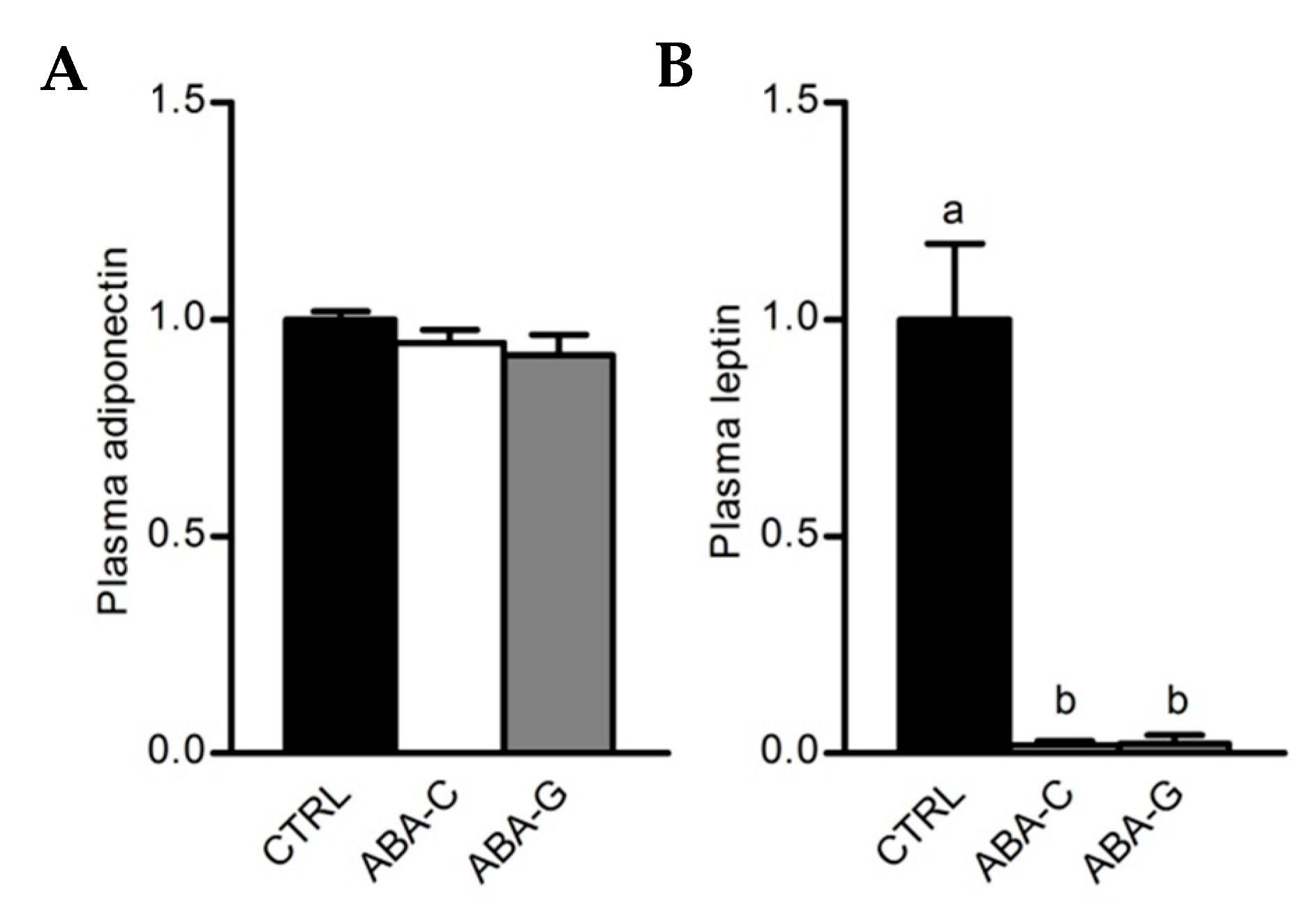
3.5. Colonic Inflammation and Plasmatic Adipose Tissue Markers
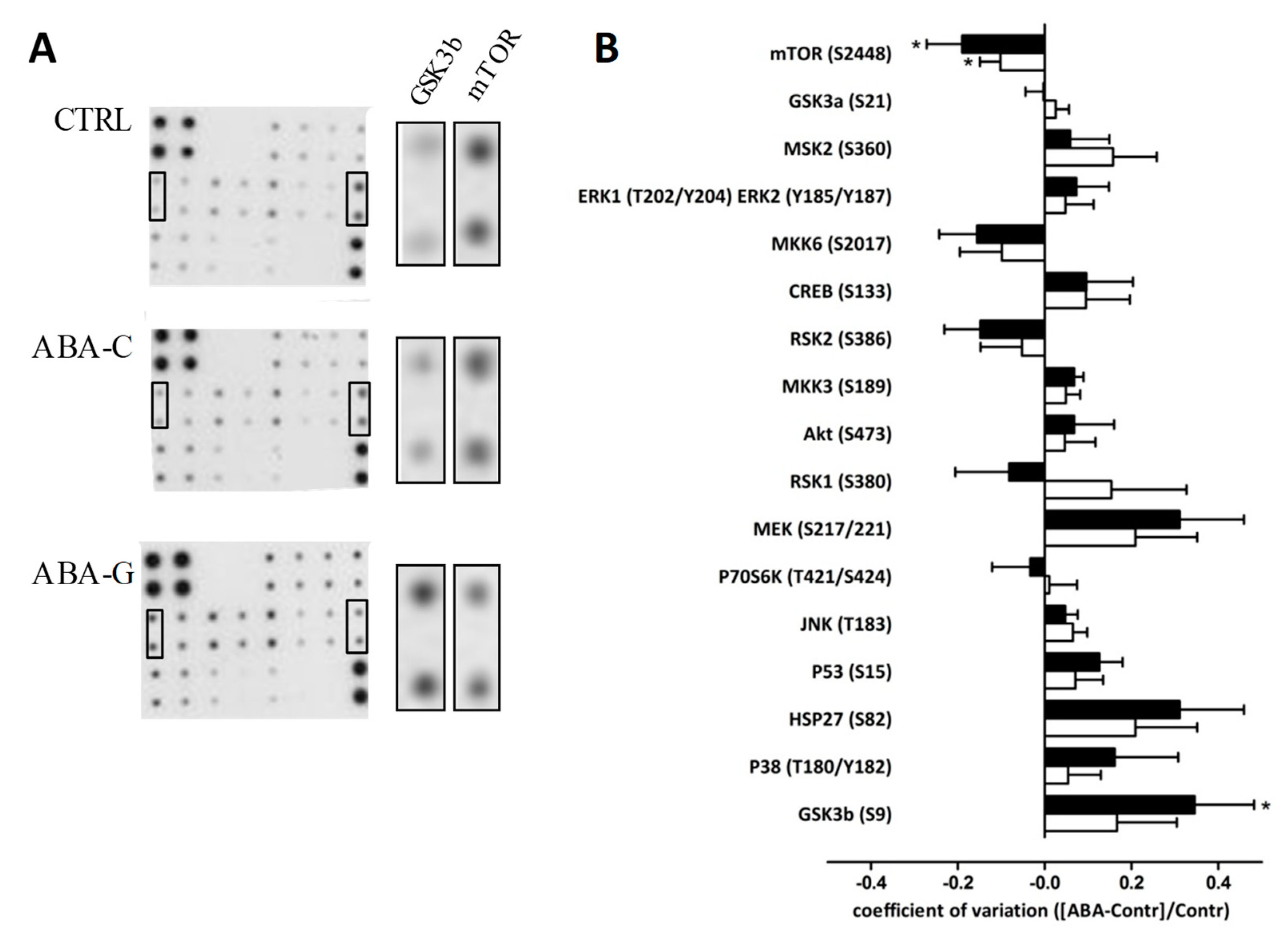
3.6. Signaling Pathways
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013; ISBN 978-0-89042-554-1. [Google Scholar]
- Andreeva, V.A.; Tavolacci, M.-P.; Galan, P.; Ladner, J.; Buscail, C.; Péneau, S.; Galmiche, M.; Hercberg, S.; Déchelotte, P.; Julia, C. Sociodemographic correlates of eating disorder subtypes among men and women in France, with a focus on age. J. Epidemiol. Community Health 2019, 73, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Rigaud, D.; Pennacchio, H.; Bizeul, C.; Reveillard, V.; Vergès, B. Outcome in AN adult patients: A 13-year follow-up in 484 patients. Diabetes Metab. 2011, 37, 305–311. [Google Scholar] [CrossRef]
- Lulé, D.; Schulze, U.M.E.; Bauer, K.; Schöll, F.; Müller, S.; Fladung, A.-K.; Uttner, I. Anorexia nervosa and its relation to depression, anxiety, alexithymia and emotional processing deficits. Eat. Weight Disord. Stud. Anorex. Bulim. Obes. 2014, 19, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Achamrah, N.; Coëffier, M.; Déchelotte, P. Physical activity in patients with anorexia nervosa. Nutr. Rev. 2016, 74, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Meczekalski, B.; Podfigurna-Stopa, A.; Katulski, K. Long-term consequences of anorexia nervosa. Maturitas 2013, 75, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Stheneur, C.; Bergeron, S.J.; Frappier, J.-Y.; Jamoulle, O.; Taddeo, D.; Sznajder, M.; Lapeyraque, A.-L. Renal injury in pediatric anorexia nervosa: A retrospective study. Eat. Weight Disord. Stud. Anorex. Bulim. Obes. 2019, 24, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Legroux-Gerot, I.; Vignau, J.; Collier, F.; Cortet, B. Bone loss associated with anorexia nervosa. Jt. Bone Spine 2005, 72, 489–495. [Google Scholar] [CrossRef]
- Norris, M.L.; Harrison, M.E.; Isserlin, L.; Robinson, A.; Feder, S.; Sampson, M. Gastrointestinal complications associated with anorexia nervosa: A systematic review: GASTROINTESTINAL COMPLICATIONS IN ANOREXIA NERVOSA. Int. J. Eat. Disord. 2016, 49, 216–237. [Google Scholar] [CrossRef]
- Schwensen, H.F.; Kan, C.; Treasure, J.; Høiby, N.; Sjögren, M. A systematic review of studies on the faecal microbiota in anorexia nervosa: Future research may need to include microbiota from the small intestine. Eat. Weight Disord. Stud. Anorex. Bulim. Obes. 2018, 23, 399–418. [Google Scholar] [CrossRef]
- Breton, J.; Déchelotte, P.; Ribet, D. Intestinal microbiota and Anorexia Nervosa. Clin. Nutr. Exp. 2019. [Google Scholar] [CrossRef]
- Monteleone, P.; Carratù, R.; Cartenì, M.; Generoso, M.; Lamberti, M.; Magistris, L.D.; Brambilla, F.; Colurcio, B.; Secondulfo, M.; Maj, M. Intestinal permeability is decreased in anorexia nervosa. Mol. Psychiatry 2004, 9, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Mörkl, S.; Lackner, S.; Meinitzer, A.; Mangge, H.; Lehofer, M.; Halwachs, B.; Gorkiewicz, G.; Kashofer, K.; Painold, A.; Holl, A.K.; et al. Gut microbiota, dietary intakes and intestinal permeability reflected by serum zonulin in women. Eur. J. Nutr. 2018, 57, 2985–2997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jésus, P.; Ouelaa, W.; François, M.; Riachy, L.; Guérin, C.; Aziz, M.; Do Rego, J.-C.; Déchelotte, P.; Fetissov, S.O.; Coëffier, M. Alteration of intestinal barrier function during activity-based anorexia in mice. Clin. Nutr. 2014, 33, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Bargiacchi, A.; Clarke, J.; Paulsen, A.; Leger, J. Refeeding in anorexia nervosa. Eur. J. Pediatrics 2019, 178, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.; Soyka, L.A.; Miller, K.K.; Grinspoon, S.; Levitsky, L.L.; Klibanski, A. Regional body composition in adolescents with anorexia nervosa and changes with weight recovery. Am. J. Clin. Nutr. 2003, 77, 1361–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mack, I.; Cuntz, U.; Grämer, C.; Niedermaier, S.; Pohl, C.; Schwiertz, A.; Zimmermann, K.; Zipfel, S.; Enck, P.; Penders, J. Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. Sci. Rep. 2016, 6, 26752. [Google Scholar] [CrossRef] [PubMed]
- Kaye, W.H.; Bailer, U.F.; Frank, G.K.; Wagner, A.; Henry, S.E. Brain imaging of serotonin after recovery from anorexia and bulimia nervosa. Physiol. Behav. 2005, 86, 15–17. [Google Scholar] [CrossRef]
- Achamrah, N.; Déchelotte, P.; Coëffier, M. New therapeutic approaches to target gut-brain axis dysfunction during anorexia nervosa. Clin. Nutr. Exp. 2019. [Google Scholar] [CrossRef]
- Belmonte, L.; Achamrah, N.; Nobis, S.; Guérin, C.; Riou, G.; Bôle-Feysot, C.; Boyer, O.; Richard, V.; Rego, J.C.D.; Déchelotte, P.; et al. A role for intestinal TLR4-driven inflammatory response during activity-based anorexia. Sci. Rep. 2016, 6, 35813. [Google Scholar] [CrossRef]
- Nobis, S.; Achamrah, N.; Goichon, A.; L’Huillier, C.; Morin, A.; Guérin, C.; Chan, P.; Rego, J.L.; Rego, J.C.; Vaudry, D.; et al. Colonic Mucosal Proteome Signature Reveals Reduced Energy Metabolism and Protein Synthesis but Activated Autophagy during Anorexia-Induced Malnutrition in Mice. Proteomics 2018, 18, 1700395. [Google Scholar] [CrossRef]
- Lacey, J.M.; Wilmore, D.W. Is Glutamine a Conditionally Essential Amino Acid? Nutr. Rev. 2009, 48, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, G.; Zhou, Z.; Dai, Z.; Sun, Y.; Ji, Y.; Li, W.; Wang, W.; Liu, C.; Han, F.; et al. Glutamine and intestinal barrier function. Amino Acids 2015, 47, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Achamrah, N.; Déchelotte, P.; Coëffier, M. Glutamine and the regulation of intestinal permeability: From bench to bedside. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Coëffier, M. Modulating effect of glutamine on IL-1β-induced cytokine production by human gut. Clin. Nutr. 2003, 22, 407–413. [Google Scholar] [CrossRef]
- Bertrand, J.; Marion-Letellier, R.; Azhar, S.; Chan, P.; Legrand, R.; Goichon, A.; Ghouzali, I.; Aziz, M.; Vaudry, D.; Savoye, G.; et al. Glutamine enema regulates colonic ubiquitinated proteins but not proteasome activities during TNBS-induced colitis leading to increased mitochondrial activity. Proteomics 2015, 15, 2198–2210. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, J.; Goichon, A.; Déchelotte, P.; Coëffier, M. Regulation of intestinal protein metabolism by amino acids. Amino Acids 2013, 45, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pierre, J.F.; Heneghan, A.F.; Busch, R.; Kudsk, K.A. Glutamine improves innate immunity and prevents bacterial enteroinvasion during parenteral nutrition. J. Parenter. Enter. Nutr. 2015, 39, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Verne, M.L.; Fields, J.Z.; Lefante, J.J.; Basra, S.; Salameh, H.; Verne, G.N. Randomised placebo-controlled trial of dietary glutamine supplements for postinfectious irritable bowel syndrome. Gut 2019, 68, 996–1002. [Google Scholar]
- Kim, D.-H.; Kim, S.-H.; Jeong, W.-S.; Lee, H.-Y. Effect of BCAA intake during endurance exercises on fatigue substances, muscle damage substances, and energy metabolism substances. J. Exerc. Nutr. Biochem. 2013, 17, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, M.H.; Shab-Bidar, S.; Mollahosseini, M.; Djafarian, K. Branched-chain amino acid supplementation and exercise-induced muscle damage in exercise recovery: A meta-analysis of randomized clinical trials. Nutrition 2017, 42, 30–36. [Google Scholar] [CrossRef]
- Fujita, S.; Volpi, E. Amino Acids and Muscle Loss with Aging. J. Nutr. 2006, 136, 277S–280S. [Google Scholar] [CrossRef] [PubMed]
- Fouré, A.; Bendahan, D. Is Branched-Chain Amino Acids Supplementation an Efficient Nutritional Strategy to Alleviate Skeletal Muscle Damage? A Systematic Review. Nutrients 2017, 9, 1047. [Google Scholar] [CrossRef] [PubMed]
- Platt, K.M.; Charnigo, R.J.; Shertzer, H.G.; Pearson, K.J. Branched-Chain Amino Acid Supplementation in Combination with Voluntary Running Improves Body Composition in Female C57BL/6 Mice. J. Diet. Suppl. 2016, 13, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Ghouzali, I.; Lemaitre, C.; Bahlouli, W.; Azhar, S.; Bôle-Feysot, C.; Meleine, M.; Ducrotté, P.; Déchelotte, P.; Coëffier, M. Targeting immunoproteasome and glutamine supplementation prevent intestinal hyperpermeability. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 3278–3288. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, H.; Sukegawa, E.; Takami, T.; Yoshida, T.; Muto, Y. Effects of Supplementation with Branched-Chain Amino Acids on Protein-Nutritional Status in Rats Treated by Carbon Tetrachloride. Available online: https://europepmc.org/abstract/med/2585790 (accessed on 18 April 2019).
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Schmidt, E.K.; Clavarino, G.; Ceppi, M.; Pierre, P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat. Methods 2009, 6, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Coëffier, M.; Miralles-Barrachina, O.; Le Pessot, F.; Lalaude, O.; Daveau, M.; Lavoinne, A.; Lerebours, E.; Déchelotte, P. Influence of Glutamine on Cytokine Production by Human Gut in vitro. Cytokine 2001, 13, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Hubert-Buron, A.; Leblond, J.; Jacquot, A.; Ducrotté, P.; Déchelotte, P.; Coëffier, M. Glutamine Pretreatment Reduces IL-8 Production in Human Intestinal Epithelial Cells by Limiting IκBα Ubiquitination. J. Nutr. 2006, 136, 1461–1465. [Google Scholar] [CrossRef]
- Kretzmann, N.A.; Fillmann, H.; Mauriz, J.L.; Marroni, C.A.; Marroni, N.; González-Gallego, J.; Tuñón, M.J. Effects of glutamine on proinflammatory gene expression and activation of nuclear factor kappa B and signal transducers and activators of transcription in TNBS-induced colitis. Inflamm. Bowel Dis. 2008, 14, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Khalsa, S.S.; Portnoff, L.C.; McCurdy-McKinnon, D.; Feusner, J.D. What happens after treatment? A systematic review of relapse, remission, and recovery in anorexia nervosa. J. Eat. Disord. 2017, 5, 20. [Google Scholar] [CrossRef]
- Pereira, M.G.; Silva, M.T.; da Cunha, F.M.; Moriscot, A.S.; Aoki, M.S.; Miyabara, E.H. Leucine supplementation improves regeneration of skeletal muscles from old rats. Exp. Gerontol. 2015, 72, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Rieu, I.; Balage, M.; Sornet, C.; Giraudet, C.; Pujos, E.; Grizard, J.; Mosoni, L.; Dardevet, D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J. Physiol. 2006, 575, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, S.; Vanschoonbeek, K.; Verdijk, L.B.; Koopman, R.; Wodzig, W.K.; Dendale, P.; Loon, V.; Jc, L. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am. J. Clin. Nutr. 2009, 89, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, M.; Hashimoto, K.; Schmidt, U.; Tchanturia, K.; Campbell, I.C.; Collier, D.A.; Iyo, M.; Treasure, J. Serum glutamine, set-shifting ability and anorexia nervosa. Ann. Gen. Psychiatry 2010, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Achamrah, N.; Nobis, S.; Breton, J.; Jésus, P.; Belmonte, L.; Maurer, B.; Legrand, R.; Bôle-Feysot, C.; Rego, J.L.; Goichon, A.; et al. Maintaining physical activity during refeeding improves body composition, intestinal hyperpermeability and behavior in anorectic mice. Sci. Rep. 2016, 6, 21887. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.; Feenstra, M.; Swain, N.; Cuesta, M.; Bandsma, R. Starved Guts. J. Pediatric Gastroenterol. Nutr. 2017, 65, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Genton, L.; Cani, P.D.; Schrenzel, J. Alterations of gut barrier and gut microbiota in food restriction, food deprivation and protein-energy wasting. Clin. Nutr. 2015, 34, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Beutheu, S.; Ouelaa, W.; Guérin, C.; Belmonte, L.; Aziz, M.; Tennoune, N.; Bôle-Feysot, C.; Galas, L.; Déchelotte, P.; Coëffier, M. Glutamine supplementation, but not combined glutamine and arginine supplementation, improves gut barrier function during chemotherapy-induced intestinal mucositis in rats. Clin. Nutr. 2014, 33, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, Z.; Liu, F.; Tan, L.; Wu, B.; Li, J. Oral Glutamine Ameliorates Chemotherapy-induced Changes of Intestinal Permeability and Does not Interfere with the Antitumor Effect of Chemotherapy in Patients with Breast Cancer: A Prospective Randomized Trial. Tumori J. 2006, 92, 396–401. [Google Scholar] [CrossRef]
- Choi, K.; Lee, S.S.; Oh, S.J.; Lim, S.Y.; Lim, S.Y.; Jeon, W.K.; Oh, T.Y.; Kim, J.W. The effect of oral glutamine on 5-fluorouracil/leucovorin-induced mucositis/stomatitis assessed by intestinal permeability test. Clin. Nutr. 2007, 26, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-E.; Wu, D.; Zheng, L.-W.; Shi, Y.; Wang, C.; Chen, Z.-H.; Peng, X. Effects of glutamine on intestinal mucus barrier after burn injury. Am. J. Transl. Res. 2018, 10, 3833–3846. [Google Scholar] [PubMed]
- Mao, X.; Hu, H.; Tang, J.; Chen, D.; Yu, B. Leucine increases mucin 2 and occludin production in LS174T cells partially via PI3K-Akt-mTOR pathway. Anim. Nutr. 2016, 2, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Momcilovic, M.; Bailey, S.T.; Lee, J.T.; Fishbein, M.C.; Braas, D.; Go, J.; Graeber, T.G.; Parlati, F.; Demo, S.; Li, R.; et al. The GSK3 Signaling Axis Regulates Adaptive Glutamine Metabolism in Lung Squamous Cell Carcinoma. Cancer Cell 2018, 33, 905–921. [Google Scholar] [CrossRef] [PubMed]
- Ban, H.; Shigemitsu, K.; Yamatsuji, T.; Haisa, M.; Nakajo, T.; Takaoka, M.; Nobuhisa, T.; Gunduz, M.; Tanaka, N.; Naomoto, Y. Arginine and Leucine regulate p70 S6 kinase and 4E-BP1 in intestinal epithelial cells. Int. J. Mol. Med. 2004, 13, 537–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coëffier, M.; Claeyssens, S.; Bensifi, M.; Lecleire, S.; Boukhettala, N.; Maurer, B.; Donnadieu, N.; Lavoinne, A.; Cailleux, A.-F.; Déchelotte, P. Influence of leucine on protein metabolism, phosphokinase expression, and cell proliferation in human duodenum. Am. J. Clin. Nutr. 2011, 93, 1255–1262. [Google Scholar] [CrossRef]
- Kamal, N.; Chami, T.; Andersen, A.; Rosell, F.A.; Schuster, M.M.; Whitehead, W.E. Delayed gastrointestinal transit times in anorexia nervosa and bulimia nervosa. Gastroenterology 1991, 101, 1320–1324. [Google Scholar] [CrossRef]
- Nobis, S.; Morin, A.; Achamrah, N.; Belmonte, L.; Legrand, R.; Chan, P.; Rego, J.-L.; Vaudry, D.; Gourcerol, G.; Déchelotte, P.; et al. Delayed gastric emptying and altered antrum protein metabolism during activity-based anorexia. Neurogastroenterol. Motil. 2018, 30, e13305. [Google Scholar] [CrossRef]
- Nobis, S.; Goichon, A.; Achamrah, N.; Guérin, C.; Azhar, S.; Chan, P.; Morin, A.; Bôle-Feysot, C.; do Rego, J.C.; Vaudry, D.; et al. Alterations of proteome, mitochondrial dynamic and autophagy in the hypothalamus during activity-based anorexia. Sci. Rep. 2018, 8, 7233. [Google Scholar] [CrossRef] [Green Version]
- Routtenberg, A.; Kuznesof, A.W. Self-starvation of rats living in activity wheels on a restricted feeding schedule. J. Comp. Physiol. Psychol. 1967, 64, 414–421. [Google Scholar] [CrossRef]
- Hillebrand, J.J.G.; Kas, M.J.H.; Adan, R.A.H. a-MSH enhances activity-based anorexia. Peptides 2005, 26, 1690–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méquinion, M.; Le Thuc, O.; Zgheib, S.; Alexandre, D.; Chartrel, N.; Rovère, C.; Hardouin, P.; Viltart, O.; Chauveau, C. Long-Term Energy Deficit in Mice Causes Long-Lasting Hypothalamic Alterations after Recovery. Neuroendocrinology 2017, 105, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Frintrop, L.; Trinh, S.; Liesbrock, J.; Paulukat, L.; Kas, M.J.; Tolba, R.; Konrad, K.; Herpertz-Dahlmann, B.; Beyer, C.; Seitz, J. Establishment of a chronic activity-based anorexia rat model. J. Neurosci. Methods 2018, 293, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-J.; Sun, X.; Du, M. AMPK in regulation of apical junctions and barrier function of intestinal epithelium. Tissue Barriers 2018, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, Q.; Rogers, C.J.; Du, M.; Zhu, M.-J. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ. 2017, 24, 819–831. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
L’Huillier, C.; Jarbeau, M.; Achamrah, N.; Belmonte, L.; Amamou, A.; Nobis, S.; Goichon, A.; Salameh, E.; Bahlouli, W.; do Rego, J.-L.; et al. Glutamine, but not Branched-Chain Amino Acids, Restores Intestinal Barrier Function during Activity-Based Anorexia. Nutrients 2019, 11, 1348. https://doi.org/10.3390/nu11061348
L’Huillier C, Jarbeau M, Achamrah N, Belmonte L, Amamou A, Nobis S, Goichon A, Salameh E, Bahlouli W, do Rego J-L, et al. Glutamine, but not Branched-Chain Amino Acids, Restores Intestinal Barrier Function during Activity-Based Anorexia. Nutrients. 2019; 11(6):1348. https://doi.org/10.3390/nu11061348
Chicago/Turabian StyleL’Huillier, Clément, Marine Jarbeau, Najate Achamrah, Liliana Belmonte, Asma Amamou, Séverine Nobis, Alexis Goichon, Emmeline Salameh, Wafa Bahlouli, Jean-Luc do Rego, and et al. 2019. "Glutamine, but not Branched-Chain Amino Acids, Restores Intestinal Barrier Function during Activity-Based Anorexia" Nutrients 11, no. 6: 1348. https://doi.org/10.3390/nu11061348
APA StyleL’Huillier, C., Jarbeau, M., Achamrah, N., Belmonte, L., Amamou, A., Nobis, S., Goichon, A., Salameh, E., Bahlouli, W., do Rego, J.-L., Déchelotte, P., & Coëffier, M. (2019). Glutamine, but not Branched-Chain Amino Acids, Restores Intestinal Barrier Function during Activity-Based Anorexia. Nutrients, 11(6), 1348. https://doi.org/10.3390/nu11061348




