Composition and In Vitro Effects of Cultivars of Humulus lupulus L. Hops on Cholinesterase Activity and Microbial Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Extraction
2.3. Composition of Chlorophylls and Carotenoids
2.4. Content of Flavonols and Phenolic Acids
2.5. Chelating Activity
2.6. Antiradical Activity with DPPH and ABTS
2.7. Cholinesterase Inhibition
2.8. Antimicrobial Activity of Hop Cones with Respect to Potentially Pathogenic Microorganisms
2.9. Statistical Analysis
3. Results
3.1. Chlorophyll and Carotenoids
3.2. Phenolic Acids and Flavonols
3.3. Chelating Activity
3.4. Antioxidant Activity DPPH and ABTS
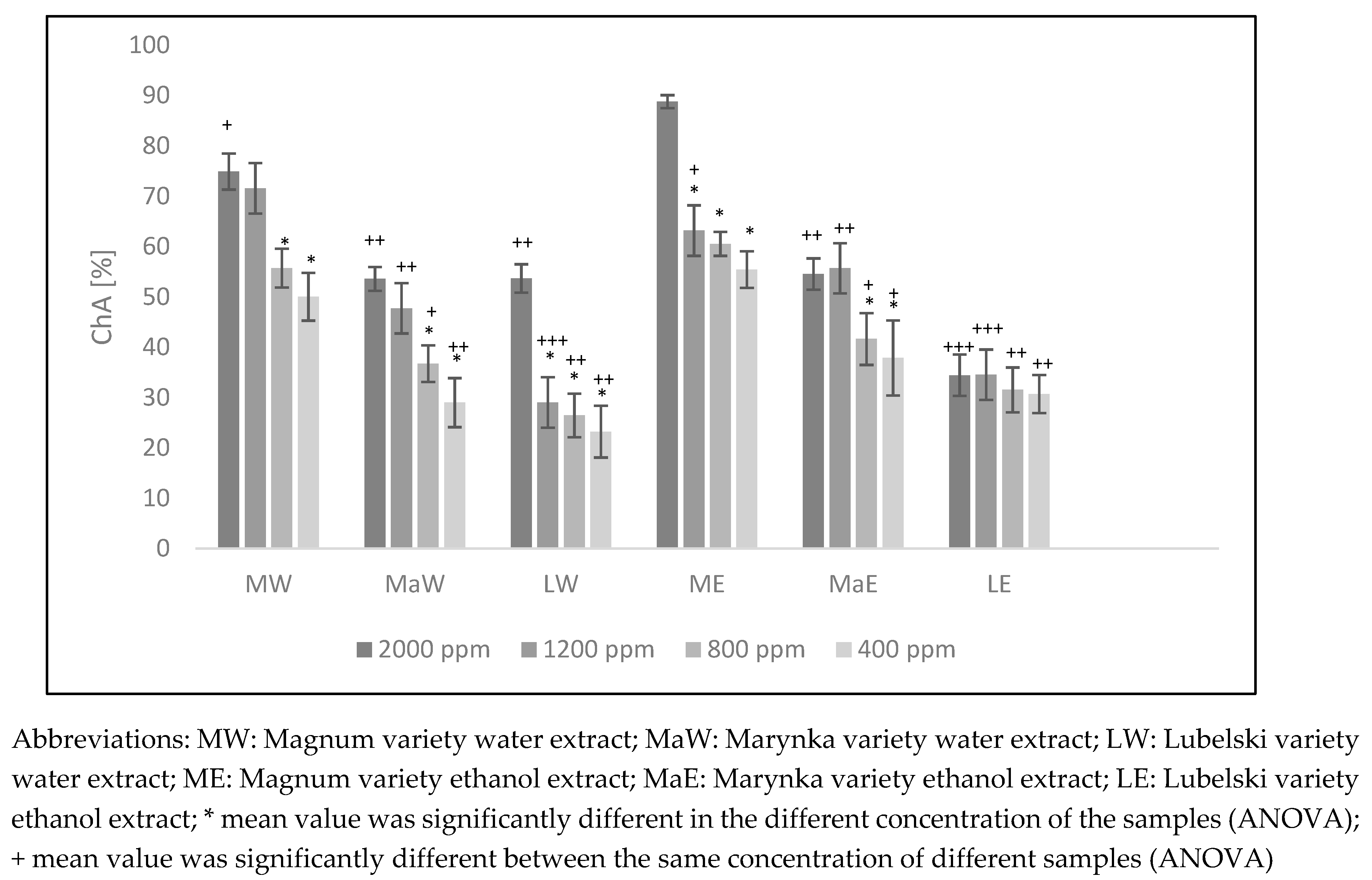
3.5. Cholinesterases
3.6. Antimicrobial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A story that begs to be told. A review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Abram, V.; Čeh, B.; Vidmar, M.; Hercezi, M.; Lazić, N.; Bucik, V.; Možina, S.S.; Košir, I.J.; Kač, M.; Demšar, L.; et al. A comparison of antioxidant and antimicrobial activity between hop leaves and hop cones. Ind. Crops Prod. 2015, 64, 124–134. [Google Scholar] [CrossRef]
- Zanoli, P.; Zavatti, M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Ocvirk, M.; Nečemer, M.; Košir, I.J. The determination of the geographic origins of hops (Humulus lupulus L.) by multi-elemental fingerprinting. Food Chem. 2019, 277, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Van Cleemput, M.; Cattoor, K.; De Bosscher, K.; Haegeman, G.; De Keukeleire, D.; Heyerick, A. Hop (Humulus lupulus)-Derived Bitter Acids as Multipotent Bioactive Compounds. J. Nat. Prod. 2009, 72, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Wong, Y.F.; Shellie, R.A.; Marriott, P.J.; Whittock, S.P.; Koutoulis, A. Assessment of the phytochemical profiles of novel hop (Humulus lupulus L.) cultivars: A potential route to beer crafting. Food Chem. 2019, 275, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Gołąbczak, J.; Gendaszewska-Darmach, E. Xanthohumol and other prenylated flavonoids of hop cones—Biological and technological aspects. Biotechnologia 2010, 1, 82–96. [Google Scholar]
- Wang, S.; Dunlap, T.L.; Howell, C.E.; Mbachu, O.C.; Rue, E.A.; Phansalkar, R.; Chen, S.-N.; Pauli, G.F.; Dietz, B.M.; Bolton, J.L. Hop (Humulus lupulus L.) Extract and 6-Prenylnaringenin Induce P450 1A1 Catalyzed Estrogen 2-Hydroxylation. Chem. Res. Toxicol. 2016, 29, 1142–1150. [Google Scholar] [CrossRef]
- Craig, W.J. The Therapeutic Use and Safety of Common Herbal Beverages BT. In Beverages in Nutrition and Health; Wilson, T., Temple, N.J., Eds.; Humana Press: Totowa, NJ, USA, 2004; pp. 187–201. ISBN 978-1-59259-415-3. [Google Scholar]
- Abou-Arab, A.E.; Abou-Arab, A.A.; Abu-Salem, M.F. Physico-chemical assessment of natural sweeteners steviosides produced from Stevia rebaudiana bertoni plant. Afr. J. Food Sci. 2010, 4, 269–281. [Google Scholar] [CrossRef]
- von Wettstein, D. Developmental Changes in Chloroplasts and Their Genetic Control. Developmental Cytology; Romnald Press & co.: New York, NY, USA, 1959; pp. 123–160. [Google Scholar]
- Kobus, J.; Flaczyk, E.; Siger, A.; Nogala-Kałucka, M.; Korczak, J.; Pegg, R.B. Phenolic compounds and antioxidant activity of extracts of Ginkgo leaves. Eur. J. Lipid Sci. Technol. 2009, 111, 1150–1160. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szymanowska, D.; Szczepaniak, O.M.; Gramza-Michałowska, A.; Kmiecik, D.; Kulczyński, B.; Szulc, P.; Górnaś, P. Composition of polyphenols of asparagus spears (Asparagus officinalis) and their antioxidant potential. Ciência Rural 2019, 49. [Google Scholar] [CrossRef]
- Amarowicz, R.; Zegarska, Z.; Pegg, R.B.; Karamac, M.; Kosinska, A. Antioxidant and radical scavenging activities of a barley crude extract and its fraction. Czech J. Food Sci. 2008, 25, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szymanowska, D.; Maciejewska, P.; Kmiecik, D.; Gramza-Michałowska, A.; Kulczyński, B.; Cielecka-Piontek, J. In vitro screening for acetylcholinesterase and butyrylcholinesterase inhibition and antimicrobial activity of chia seeds (Salvia hispanica). Electron. J. Biotechnol. 2019, 37, 1–10. [Google Scholar] [CrossRef]
- Nuutinen, T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur. J. Med. Chem. 2018, 157, 198–228. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Menghini, L.; Di Sotto, A.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L.; et al. Chromatographic analyses, in vitro biological activities, and cytotoxicity of cannabis sativa l. Essential oil: A multidisciplinary study. Molecules 2018, 23, 3266. [Google Scholar] [CrossRef] [PubMed]
- Fasakin, C.F.; Udenigwe, C.C.; Aluko, R.E. Antioxidant properties of chlorophyll-enriched and chlorophyll-depleted polyphenolic fractions from leaves of Vernonia amygdalina and Gongronema latifolium. Food Res. Int. 2011, 44, 2435–2441. [Google Scholar] [CrossRef]
- Sevik, H.; Guney, D.; Karakas, H.; Aktar, G. Change to Amount of Chlorophyll on Leaves Depend on Insolation in Some Landscape Plants. Int. J. Environ. Sci. 2012, 3, 1057–1064. [Google Scholar] [CrossRef]
- Kamble, P.; Giri, S.P.; Mane, R.S.; Tiwana, A. Estimation of Chlorophyll Content in Young and Adult Leaves of Some Selected Plants. Univers. J. Environ. Res. Technol. 2015, 5, 306–310. [Google Scholar]
- Devmalkar, V.S.; Murumkar, C.V.; Salunkhe, S.M.; Chavan, S.J. Studies on pigment chlorophyll isolation and estimation of different bryophytes for their biochemical properties. J. Nat. Prod. Plant Resour. 2014, 4, 56–61. [Google Scholar]
- Nahvi, A.; Daghighi, A.; Nazif, S. The environmental impact assessment of drainage systems: A case study of the Karun river sugarcane development project. Arch. Agron. Soil Sci. 2018, 64, 185–195. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, X.-H.; Tang, J.; Liu, K. Antioxidant Activities of Hops (Humulus lupulus) and Their Products. J. Am. Soc. Brew. Chem. 2007, 65, 116–121. [Google Scholar] [CrossRef]
- Rivière, C.; Krisa, S.; Péchamat, L.; Nassra, M.; Delaunay, J.-C.; Marchal, A.; Badoc, A.; Waffo-Téguo, P.; Mérillon, J.-M. Polyphenols from the stems of Morus alba and their inhibitory activity against nitric oxide production by lipopolysaccharide-activated microglia. Fitoterapia 2014, 97, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Zeković, Z.; Kaplan, M.; Pavlić, B.; Olgun, E.O.; Vladić, J.; Canlı, O.; Vidović, S. Chemical characterization of polyphenols and volatile fraction of coriander (Coriandrum sativum L.) extracts obtained by subcritical water extraction. Ind. Crops Prod. 2016, 87, 54–63. [Google Scholar] [CrossRef]
- Fu, Z.; Tu, Z.; Zhang, L.; Wang, H.; Wen, Q.; Huang, T. Antioxidant activities and polyphenols of sweet potato (Ipomoea batatas L.) leaves extracted with solvents of various polarities. Food Biosci. 2016, 15, 11–18. [Google Scholar] [CrossRef]
- Parus, A. Antioxidant and pharmacological properties of phenolic acids. Postępy Fitoter. 2013, 1, 48–53. [Google Scholar]
- Przeor, M.; Flaczyk, E. Antioxidant properties of paratha type flat bread enriched with white mulberry leaf extract. Indian J. Tradit. Knowl. 2016, 15, 237–244. [Google Scholar]
- Giusti, F.; Caprioli, G.; Ricciutelli, M.; Vittori, S.; Sagratini, G. Determination of fourteen polyphenols in pulses by high performance liquid chromatography-diode array detection (HPLC-DAD) and correlation study with antioxidant activity and colour. Food Chem. 2017, 221, 689–697. [Google Scholar] [CrossRef]
- Kusztal, D.; Mielczarek, C. Chelation of Iron Ions (II) as the Method for Testing of the antioxidant Properties. Bromatol. Chem. Toksykol. 2011, XLIV, 1097–1104. [Google Scholar]
- Sánchez-Vioque, R.; Polissiou, M.; Astraka, K.; De Los Mozos-Pascual, M.; Tarantilis, P.; Herraiz-Peñalver, D.; Santana-Méridas, O. Polyphenol composition and antioxidant and metal chelating activities of the solid residues from the essential oil industry. Ind. Crops Prod. 2013, 49, 150–159. [Google Scholar] [CrossRef]
- Inui, T.; Okumura, K.; Matsui, H.; Hosoya, T.; Kumazawa, S. Effect of harvest time on some in vitro functional properties of hop polyphenols. Food Chem. 2017, 225, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiang, L.; Wang, C.; Tang, C.; He, X. Antidiabetic and Antioxidant Effects and Phytochemicals of Mulberry Fruit (Morus alba L.) Polyphenol Enhanced Extract. PLoS ONE 2013, 8, e71144. [Google Scholar] [CrossRef] [PubMed]
- Nafis, A.; Kasrati, A.; Jamali, C.A.; Mezrioui, N.; Setzer, W.; Abbad, A.; Hassani, L. Antioxidant activity and evidence for synergism of Cannabis sativa (L.) essential oil with antimicrobial standards. Ind. Crops Prod. 2019, 137, 396–400. [Google Scholar] [CrossRef]
- Önder, F.C.; Ay, M.; Sarker, S.D. Comparative study of antioxidant properties and total phenolic content of the extracts of Humulus lupulus L. and quantification of bioactive components by LC–MS/MS and GC–MS. J. Agric. Food Chem. 2013, 61, 10498–10506. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Gaweł, M.; Potulska-Chromik, A. Neurodegenerative diseases: Alzheimer’s and Parkinson’s disease. Postępy Nauk Med. 2015, XVIII, 468–476. [Google Scholar]
- Zamzow, D.R.; Elias, V.; Legette, L.L.; Choi, J.; Stevens, J.F.; Magnusson, K.R. Xanthohumol improved cognitive flexibility in young mice. Behav. Brain Res. 2014, 275, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ożarowski, M.; Mikołajczak, P.Ł.; Bobkiewlcz-Kozłowska, T.; Kujawski, R.; Mrozikiewicz, P.M. Neuroactive compounds from medicinal plants of the Lamiaceae family showing potentially beneficial activity in treatment of Alzheimer’s disease. Herba Pol. 2009, 55, 148–163. [Google Scholar]
- Oberbauer, E.; Urmann, C.; Steffenhagen, C.; Bieler, L.; Brunner, D.; Furtner, T.; Humpel, C.; Bäumer, B.; Bandtlow, C.; Couillard-Despres, S. Chroman-like cyclic prenylflavonoids promote neuronal differentiation and neurite outgrowth and are neuroprotective. J. Nutr. Biochem. 2013, 24, 1953–1962. [Google Scholar] [CrossRef]
- Gonzalez-Munoz, M.J.; Meseguer, I.; Sanchez-Reus, M.I.; Schultz, A.; Olivero, R.; Benedí, J.; Sánchez-Muniz, F.J. Beer consumption reduces cerebral oxidation caused by aluminum toxicity by normalizing gene expression of tumor necrotic factor alpha and several antioxidant enzymes. Food Chem. Toxicol. 2008, 46, 1111–1118. [Google Scholar] [CrossRef]
- Ngoungoure, V.L.N.; Schluesener, J.; Moundipa, P.F.; Schluesener, H. Natural polyphenols binding to amyloid: A broad class of compounds to treat different human amyloid diseases. Mol. Nutr. Food Res. 2015, 59, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H. Biophenols pharmacology against the amyloidogenic activity in Alzheimer’s disease. Biomed. Pharmacother. 2017, 89, 396–413. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Taylor, A.W.; Nickerson, G.B.; Ivancic, M.; Henning, J.; Haunold, A.; Deinzer, M.L. Prenylflavonoid variation in Humulus lupulus: Distribution and taxonomic significance of xanthogalenol and 4′-O-methylxanthohumol. Phytochemistry 2000, 53, 759–775. [Google Scholar] [CrossRef]
- Stevens, J.F.; Ivancic, M.; Hsu, V.L.; Deinzer, M.L. Prenylflavonoids from Humulus lupulus. Phytochemistry 1997, 44, 1575–1585. [Google Scholar] [CrossRef]
- Stevens, J.F.; Page, J.E. Xanthohumol and related prenylflavonoids from hops and beer: To your good health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Mizobuchi, S.; Sato, Y. A new flavanone with antifungal activity isolated from hops. Agric. Biol. Chem. 1984, 48, 2771–2775. [Google Scholar]

| Extract | Chlorophyll α [mg/g dw] | Chlorophyll β [mg/g dw] | Total Chlorophyll [mg/g dw] | Carotenoids [mg/g dw] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MW | 0.44 b | ± | 0.02 | 0.10 a | ± | 0.03 | 0.54 a | ± | 0.03 | 0.59 b | ± | 0.03 |
| MaW | 0.38 a | ± | 0.02 | 0.11 a | ± | 0.02 | 0.49 a | ± | 0.06 | 0.46 a | ± | 0.01 |
| LW | 0.37 a | ± | 0.05 | 0.11 a | ± | 0.03 | 0.48 a | ± | 0.11 | 0.46 a | ± | 0.02 |
| ME | 1.32 d | ± | 0.01 | 0.56 b | ± | 0.01 | 1.88 b | ± | 0.09 | 1.11 c | ± | 0.05 |
| MaE | 1.33 d | ± | 0.03 | 0.66 b | ± | 0.01 | 1.99 b | ± | 0.08 | 1.31 d | ± | 0.09 |
| LE | 1.07 c | ± | 0.04 | 0.76 b | ± | 0.02 | 1.83 b | ± | 0.09 | 1.22 d | ± | 0.06 |
| Polyphenol [µg/g dw of Extract] | MW | MaW | LW | ME | MaE | LE |
|---|---|---|---|---|---|---|
| Chlorogenic acid | 903.3 b ± 9.8 | 126.6 a ± 1.1 | 191.4 a ± 1.4 | 1077.0 c ± 7.0 | 133.7 a ± 0.6 | 734.1 b ± 17.7 |
| Ferulic acid | 0.8 a ± 0.0 | 0.5 a ± 0.0 | 0.7 a ± 0.0 | 1.4 a ± 0.0 | 0.4 a ± 0.0 | 0.9 a ± 0.0 |
| Vanillic acid | 5.9 a ± 0.0 | 4.6 a ± 0.1 | 2.4 a ± 0.0 | 13.3 c ± 0.2 | 9.3 b ± 0.0 | 8.2 b ± 0.2 |
| Gallic acid | 17.3 b ± 0.2 | 10.6 a ± 0.3 | 15.4 b ± 0.2 | 30.6 c ± 0.4 | 18.7 b ± 0.1 | 18.8 b ± 0.5 |
| o-coumaric acid | 33.9 a ± 0.1 | 67.8 b ± 0.3 | 23.0 a ± 0.2 | 66.5 b ± 0.1 | 128.3 c ± 0.5 | 37.9 ab ± 0.4 |
| p-coumaric acid | 56.2 a ± 0.2 | 89.2 b ± 0.3 | 44.5 a ± 0.2 | 88.0 a ±0.1 | 256.5 c ± 1.0 | 69.4 a ± 0.4 |
| Cinnamic acid | 6.2 a ± 0.0 | 110.7 d ± 0.3 | 66.0 c ± 0.2 | 109.5 d ± 0.1 | 31.0 b ± 0.1 | 60.8 c ± 0.4 |
| Syringic acid | 8.4 a ± 0.0 | 149.8 d ± 0.4 | 29.8 b ± 0.1 | 49.4 b ± 0.0 | 42.0 b ± 0.2 | 82.3 c ± 0.6 |
| p-hydroxybenzoic acid | 72.8 b ± 0.0 | 49.9 b ± 0.1 | 89.3 b ± 0.3 | 148.1 c ± 0.1 | 14.0 a ± 0.1 | 27.4 a ± 0.2 |
| Caffeic acid | 0.2 a ± 0.0 | 3.1 b ± 0.0 | 2.3 b ± 0.0 | 12.3 d ± 0.0 | 1.2 a ± 0.0 | 7.4 e ± 0.0 |
| Catechin | 84.3 c ± 0.3 | 73.9 c ± 1.0 | 4.0 a ± 0.0 | 13.2 b ± 0.1 | 139.9 d ± 0,1 | 2.6 a ± 0.0 |
| Epicatechin | 1759.9 d ± 5.9 | 634.8 b ± 4.1 | 216.4a ± 0.8 | 2085.5 e ± 1.0 | 1344.9 c ± 1.4 | 343.5 a ± 0.5 |
| Quercetin | 625.7 c ± 0.2 | 639.1 c ± 9.0 | 354.5 b ± 0.1 | 395.1 b ± 0.1 | 309.4 b ± 0.7 | 222.9 a ± 0.1 |
| Rutin | 324.4 a ± 0.5 | 481.0 b ± 0.3 | 1085.4 d ± 2.6 | 799.7 c ± 0.8 | 644.8 c± 3.3 | 1764.7 e ± 31.7 |
| Kaempferitrin | 49.9 b ± 0.0 | 26.5 ab ± 0.0 | 441.3 d ± 0.2 | 13.7a ± 0.1 | 9.7 a ± 0.0 | 180.4 c ± 2.5 |
| Total [mg/ g dw extract] | 4049.2 c ± 13.3 | 2466.9 a ± 4.2 | 2566.4 c ± 4.6 | 4903.5 d ± 6.2 | 3083.9 b ± 5.3 | 3561.3 bc ± 31.4 |
| Compound | AChE Activity | BChE Activity | DPPH (mmol Tx/g dw) | ABTS (mmol Tx/g dw) | ChA (%) |
|---|---|---|---|---|---|
| Chlorogenic acid | 0.535 NS | 0.754 * | 0.225 NS | 0.811 * | 0.228 NS |
| Ferulic acid | 0.339 NS | 0.523 NS | 0.522 NS | 0.834NS | 0.757* |
| Vanillic acid | 0.621* | 0.258 NS | 0.432 NS | 0.354 NS | 0.554 NS |
| Gallic acid | 0.654 NS | 0.265 NS | 0.865 * | 0.743 * | 0.335 NS |
| o-coumaric acid | 0.883 * | 0.056 NS | 0.387 NS | 0.433 NS | 0.432 NS |
| p-coumaric acid | 0.255 NS | 0.322 NS | 0.115 NS | 0.522NS | 0.633 * |
| Cinnamic acid | 0.312 NS | 0.461 NS | 0.321 NS | 0.386 NS | 0.441 NS |
| Syringic acid | 0.308 NS | 0.225 NS | 0.532 NS | 0.276 NS | 0.654 NS |
| p-hydroxybenzoic acid | 0.297NS | 0.821 * | 0.835 N* | 0.532NS | 0.555 NS |
| Caffeic acid | 0.623 NS | 0.433 NS | 0.332 NS | 0.464 NS | 0.664 NS |
| Catechin | 0.411 NS | 0.609 NS | 0.376 NS | 0.742 * | 0.221 NS |
| Epicatechin | 0.854 * | 0.360 NS | 0.773 * | 0.228 NS | 0.667 * |
| Quercetin | 0.227 NS | 0.692 * | 0.321 NS | 0.799 * | 0.663 NS |
| Rutin | 0.471 NS | 0.783 * | 0.054 NS | 0.441 NS | 0.439 NS |
| Kaempferitrin | 0.694 * | 0.210 NS | 0.354 NS | 0.115 NS | 0.330 NS |
| Sample | DPPH (mmol Tx/g dw) | DPPH EC 50 mg extract/mL) | ABTS (mmol Tx/g dw) | ABTS EC 50 (mg extract/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MW | 4.75 e | ± | 0.09 | 0.44 b | ± | 0.12 | 1.32 a | ± | 0.06 | 1.23 b | ± | 0.10 |
| MaW | 4.37 d | ± | 0.10 | 0.38 a | ± | 0.08 | 1.43 a | ± | 0.02 | 1.25 b | ± | 0.03 |
| LW | 4.11 c | ± | 0.06 | 0.31 a | ± | 0.17 | 1.37 a | ± | 0.05 | 1.26 b | ± | 0.08 |
| ME | 4.12 c | ± | 0.11 | 0.56 c | ± | 0.06 | 2.33 b | ± | 0.06 | 0.92 a | ± | 0.08 |
| MaE | 3.50 a | ± | 0.09 | 0.87 d | ± | 0.12 | 2.43 b | ± | 0.09 | 0.99 a | ± | 0.04 |
| LE | 3.74 b | ± | 0.11 | 0.98 e | ± | 0.07 | 2.41 b | ± | 0.13 | 0.93 a | ± | 0.03 |
| Quercetin | 4.01 | ± | 0.07 | 0.61 c | ± | 0.03 | 1.65 | ± | 0.11 | 1.05 | ± | 0.06 |
| Chlorogenic acid | 4.65 | ± | 0.03 | 0.77 c | ± | 0.01 | 1.88 | ± | 0.07 | 1.16 | ± | 0.04 |
| Microorganism | MW | MaW | LW | ME | MaE | LE | |
|---|---|---|---|---|---|---|---|
| 1 | Propionibacterium acnes ATTC 11827 | 9.0 ± 1.0 | 7.0 ± 1.0 | 6.0 ± 0.0 | na | na | na |
| 2 | Propionibacterium acnes clinical isolates | 7.0 ± 1.0 | 5.0 ± 0.0 | 5.0 ± 0.0 | na | na | na |
| 3 | Enterococcus faecium ATCC 27270 | 4.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 | na | na | na |
| 4 | Enterococcus faecium clinical isolates | 2.0 ± 0.0 | 1.0 ± 0.0 | 2.0 ± 0.0 | na | na | na |
| 5 | Staphylococcus aureus ATCC 25923 | 39.0 ± 3.0 | 27.0 ± 2.0 | 18.0 ± 2.0 | 8.0 ± 1.0 | 4.0 ± 0.0 | 2.0 ± 0.0 |
| 6 | Staphylococcus aureus clinical isolates | 28.0 ± 2.0 | 22.0 ± 2.0 | 11.0 ± 1.0 | 4.0 ± 0.0 | 1.0 ± 0.0 | na |
| 7 | Staphylococcus epidermidis ATCC 12228 | 34.0 ± 2.0 | 31.0 ± 2.0 | 12.0 ± 1.0 | 6.0 ± 1.0 | 5.0 ± 0.0 | 2.0 ± 0.0 |
| 8 | Staphylococcus epidermidis clinical isolates | 25.0 ± 2.0 | 26.0 ± 2.0 | 8.0 ± 1.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 1.0 ± 1.0 |
| 9 | Streptococcus salivarius ATCC 13419 | 18.0 ± 2.0 | 15.0 ± 1.0 | 7.0 ± 1.0 | 5.0 ± 0.0 | 5.0 ± 0.0 | na |
| 10 | Streptococcus salivarius clinical isolates | 13.0 ± 2.0 | 11.0 ± 1.0 | 4.0 ± 0.0 | 2.0 ± 0.0 | 1.0 ± 0.0 | na |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobus-Cisowska, J.; Szymanowska-Powałowska, D.; Szczepaniak, O.; Kmiecik, D.; Przeor, M.; Gramza-Michałowska, A.; Cielecka-Piontek, J.; Smuga-Kogut, M.; Szulc, P. Composition and In Vitro Effects of Cultivars of Humulus lupulus L. Hops on Cholinesterase Activity and Microbial Growth. Nutrients 2019, 11, 1377. https://doi.org/10.3390/nu11061377
Kobus-Cisowska J, Szymanowska-Powałowska D, Szczepaniak O, Kmiecik D, Przeor M, Gramza-Michałowska A, Cielecka-Piontek J, Smuga-Kogut M, Szulc P. Composition and In Vitro Effects of Cultivars of Humulus lupulus L. Hops on Cholinesterase Activity and Microbial Growth. Nutrients. 2019; 11(6):1377. https://doi.org/10.3390/nu11061377
Chicago/Turabian StyleKobus-Cisowska, Joanna, Daria Szymanowska-Powałowska, Oskar Szczepaniak, Dominik Kmiecik, Monika Przeor, Anna Gramza-Michałowska, Judyta Cielecka-Piontek, Małgorzata Smuga-Kogut, and Piotr Szulc. 2019. "Composition and In Vitro Effects of Cultivars of Humulus lupulus L. Hops on Cholinesterase Activity and Microbial Growth" Nutrients 11, no. 6: 1377. https://doi.org/10.3390/nu11061377
APA StyleKobus-Cisowska, J., Szymanowska-Powałowska, D., Szczepaniak, O., Kmiecik, D., Przeor, M., Gramza-Michałowska, A., Cielecka-Piontek, J., Smuga-Kogut, M., & Szulc, P. (2019). Composition and In Vitro Effects of Cultivars of Humulus lupulus L. Hops on Cholinesterase Activity and Microbial Growth. Nutrients, 11(6), 1377. https://doi.org/10.3390/nu11061377












