Identifying Dietary Strategies to Improve Nutrient Adequacy among Ethiopian Infants and Young Children Using Linear Modelling
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Optifood Analysis
2.2.1. Data Preparation
2.2.2. Analysis Using Linear Programming
2.2.3. Analysis of Alternative Options Using Linear Programming
2.3. Analysis of Inadequate and Excess Intakes
2.4. Other Analyses
3. Results
3.1. Overview of Foods Consumed
3.2. Problem Nutrients
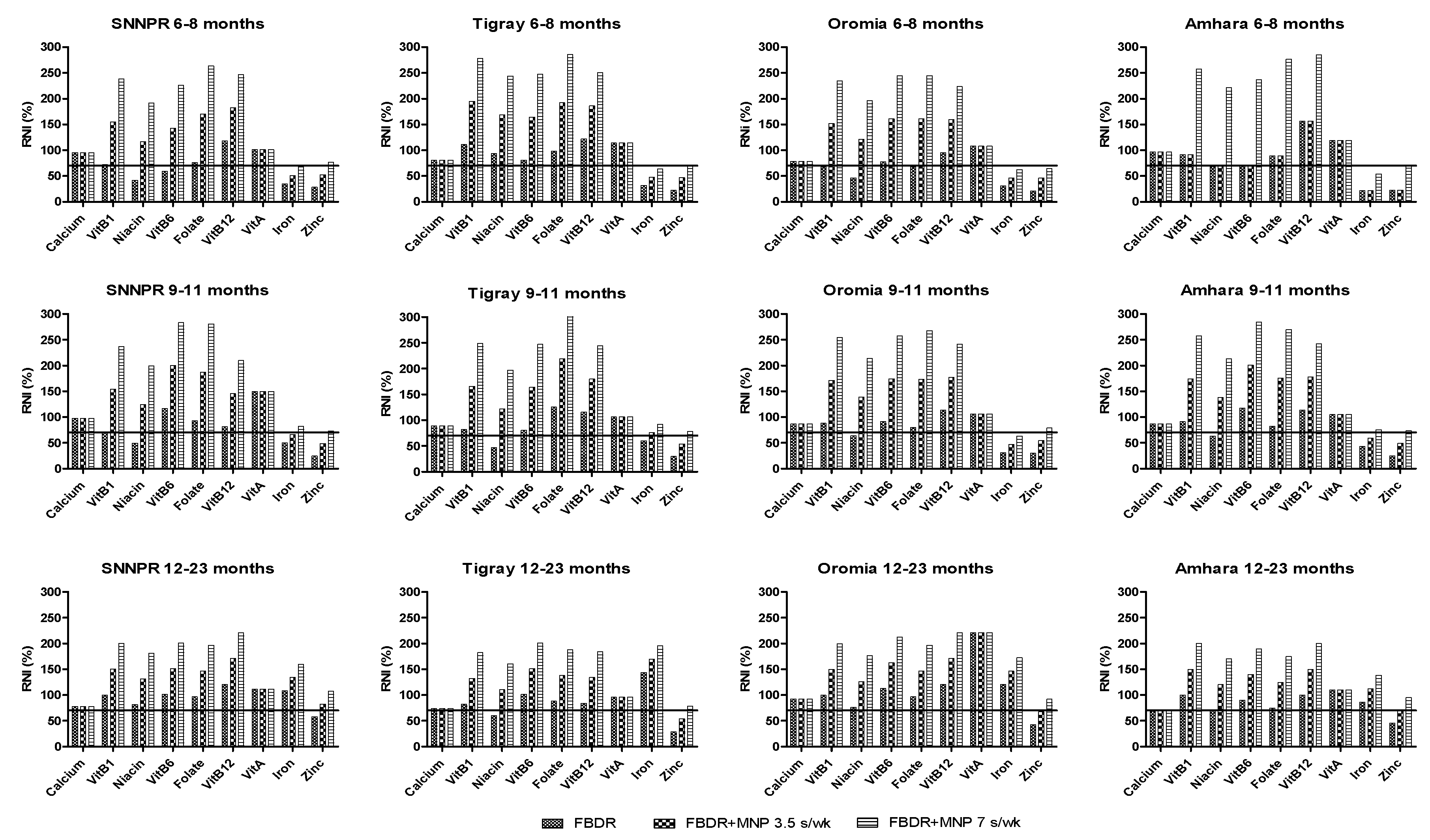
3.3. Food-Based Dietary Recommendations
3.4. FBDR Combined with Local Complementary Food Products and Supplementation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bhutta, Z.A.; Das, J.K.; Rizvi, A.; Gaffey, M.F.; Walker, N.; Horton, S.; Webb, P.; Lartey, A.; Black, R.E. Evidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet 2013, 382, 452–477. [Google Scholar] [CrossRef]
- WHO. Indicators for Assessing Infant and Young Child Feeding Practices; Part 1 Definitions; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- WHO. Global Strategy for Infant and Young Child Feeding; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Chastre, C.; Duffield, A.; Kindness, H.; LeJeune, S.; Taylor, A. The Minimum Cost of a Healthy Diet, Findings from Piloting a New Methodology in Four Study Locations; Save the Children: London, UK, 2007; p. 27. [Google Scholar]
- FAO. Ethiopia Nutrition Profile. Nutrition and Consumer Protection Division; Food and Agriculture Organization: Rome, Italy, 2008. [Google Scholar]
- FMOH. National Nutrition Program I, Program Implementation Manual for NNP; Federal Ministry of Health: Addis Ababa, Ethiopia, 2008. [Google Scholar]
- Ijarotimi, O.S. Determinants of childhood malnutrition and consequences in developing countries. Curr. Nutr. Rep. 2013, 2, 129–133. [Google Scholar] [CrossRef]
- CSA. Ethiopia Demographic and Health Survey 2016; Central Statistical Agency and ICF International: Addis Ababa, Ethiopia; Rockville, MD, USA, 2016. [Google Scholar]
- EHNRI. The National Nutrition Baseline Survey Report; Ethiopian Health and Nutrition Research Institute: Addis Ababa, Ethiopia, 2010. [Google Scholar]
- EPHI. Ethiopia National Food Consumption Survey; Ethiopian Public Health Institute: Addis Ababa, Ethiopia, 2013. [Google Scholar]
- AAU. Rapid Assessment of Community-Based Production of Complementary Food in Tigray, Amhara, Oromia and SNNP Regions; Addis Ababa University: Addis Ababa, Ethiopia, 2010. [Google Scholar]
- USAID. Focusing on Improving Complementary Feeding in Ethiopia: Trials of Improved Practices in an Urban Area, USAID’s Infant and Young Child Nutrition project; United States Agency for International Development: Washington, DC, USA, 2011. [Google Scholar]
- Kang, Y.; Kim, S.; Sinamo, S.; Christian, P. Effectiveness of a community-based nutrition programme to improve child growth in rural Ethiopia: A cluster randomized trial. Matern. Child Nutr. 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Earle, M. Diet planning in the third world by linear and goal programming. J. Oper. Res. Soc. 1983, 34, 9–16. [Google Scholar] [CrossRef]
- Briend, A.; Darmon, N.; Ferguson, E.; Erhardt, J.G. Linear programming: A mathematical tool for analyzing and optimizing children’s diets during the complementary feeding period. J. Pediatr. Gastroenterol. Nutr. 2003, 36, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Daelmans, B.; Ferguson, E.; Lutter, C.K.; Singh, N.; Pachon, H.; Creed-Kanashiro, H.; Woldt, M.; Mangasaryan, N.; Cheung, E.; Mir, R.; et al. Designing appropriate complementary feeding recommendations: Tools for programmatic action. Matern. Child Nutr. 2013, 9, 116–130. [Google Scholar] [CrossRef]
- Ferguson, E.L.; Darmon, N.; Fahmida, U.; Fitriyanti, S.; Harper, T.B.; Premachandra, I.M. Design of optimal food-based complementary feeding recommendations and identification of key “problem nutrients” using goal programming. J. Nutr. 2006, 136, 2399–2404. [Google Scholar] [CrossRef]
- Santika, O.; Fahmida, U.; Ferguson, E. Development of food-based complementary feeding recommendations for 9-to 11-month-old peri-urban Indonesian infants using linear programming. J. Nutr. 2009, 139, 135–141. [Google Scholar] [CrossRef]
- Darmon, N.; Ferguson, E.; Briend, A. Linear and nonlinear programming to optimize the nutrient density of a population’s diet: An example based on diets of preschool children in rural Malawi. Am. J. Clin. Nutr. 2002, 75, 245–253. [Google Scholar] [CrossRef]
- Maillot, M.; Vieux, F.; Amiot, M.J.; Darmon, N. Individual diet modeling translates nutrient recommendations into realistic and individual-specific food choices. Am. J. Clin. Nutr. 2010, 91, 421–430. [Google Scholar] [CrossRef]
- Vossenaar, M.; Hernandez, L.; Campos, R.; Solomons, N.W. Several ‘problem nutrients’ are identified in complementary feeding of Guatemalan infants with continued breastfeeding using the concept of ‘critical nutrient density’. Eur. J. Clin. Nutr. 2013, 67, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Dewey, K.G.; Brown, K.H. Update on technical issues concerning complementary feeding of young children in developing countries and implications for intervention programs. Food Nutr. Bull. 2003, 24, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Vitta, B.; Dewey, K. Identifying Micronutrient Gaps in The Diets of Breastfed 6-11-Month-Old Infants in Bangladesh, Ethiopia and Viet Nam Using Linear Programming; Alive and Thrive: Washington, DC, USA, 2012. [Google Scholar]
- Zlotkin, S.; Arthur, P.; Antwi, K.Y.; Yeung, G. Treatment of anemia with microencapsulated ferrous fumarate plus ascorbic acid supplied as sprinkles to complementary (weaning) foods. Am. J. Clin. Nutr. 2001, 74, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, L.L.; Dulience, S.J.L.; Green, J.; Joseph, S.; François, J.; Anténor, M.-L.; Lesorogol, C.; Mounce, J.; Nickerson, N.M. Linear growth increased in young children in an urban slum of Haiti: A randomized controlled trial of a lipid-based nutrient supplement. Am. J. Clin. Nutr. 2014, 99, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Nestel, P.; Briend, A.; De Benoist, B.; Decker, E.; Ferguson, E.; Fontaine, O.; Micardi, A.; Nalubola, R. Complementary food supplements to achieve micronutrient adequacy for infants and young children. J. Pediatr. Gastroenterol. Nutr. 2003, 36, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Ayana, G.; Moges, T.; Samuel, A.; Asefa, T.; Eshetu, S.; Kebede, A. Dietary zinc intake and its determinants among Ethiopian children 6–35 months of age. BMC Nutr. 2018, 4, 30. [Google Scholar] [CrossRef]
- MI. Formative Research Report for UNICEF Local Complementary Food Production Project; Micronutrient Initiative: Addis Ababa, Ethiopia, 2014. [Google Scholar]
- Skau, J.K.; Bunthang, T.; Chamnan, C.; Wieringa, F.T.; Dijkhuizen, M.A.; Roos, N.; Ferguson, E.L. The use of linear programming to determine whether a formulated complementary food product can ensure adequate nutrients for 6- to 11-month-old Cambodian infants. Am. J. Clin. Nutr. 2013, 99, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, E.; Chege, P.; Kimiywe, J.; Wiesmann, D.; Hotz, C. Zinc, iron and calcium are major limiting nutrients in the complementary diets of rural Kenyan children. Matern. Child Nutr. 2015, 11, 6–20. [Google Scholar] [CrossRef] [PubMed]
- WHO. Child Growth Standards. Length/height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. (NLM Classification: WS 103); World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- FAO. Human Energy Requirements, Report of a Joint FAO/WHO/UNU Expert Consultation; FAO/WHO/UNU: Rome, Italy, 2004. [Google Scholar]
- EHNRI. Food Composition Table for Use in Ethiopia Part III; Ethiopian Health and Nutrition Research Institute: Addis Ababa, Ethiopia, 1997. [Google Scholar]
- EHNRI. Food Composition Table for Use in Ethiopia Part IV; Ethiopian Health and Nutrition Research Institute: Addis Ababa, Ethiopia, 1998. [Google Scholar]
- FAO. Human Vitamin and Mineral Requirements, Report of a Joint FAO/WHO Expert Consultation; WHO/FAO: Bangkok, Thailand, 2004. [Google Scholar]
- FAO/WHO/UNU. Protein and Amino Acid Requirements in Human Nutrition, Report of a Joint FAO/WHO/UNU Expert Consultation; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- FAO. Fats and Fatty Acids in Human Nutrition, Report of an Expert Consultation; FAO: Rome, Italy, 2010. [Google Scholar]
- Alive&Thrive. IYCF Practices, Beliefs and Influences in Tigray Region Ethiopia; Alive & Thrive: Addis Ababa, Ethiopia, 2010. [Google Scholar]
- Aemro, M.; Mesele, M.; Birhanu, Z.; Atenafu, A. Dietary diversity and meal frequency practices among infant and young children aged 6–23 months in Ethiopia: A secondary analysis of Ethiopian demographic and health survey 2011. J. Nutr. Metab. 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lutter, C.K.; Dewey, K.G. Proposed nutrient composition for fortified complementary foods. J. Nutr. 2003, 133, 3011S–3020S. [Google Scholar] [CrossRef]
- Samuel, A.; Brouwer, I.; Feskens, E.; Adish, A.; Kebede, A.; De-Regil, L.; Osendarp, S. Effectiveness of a program intervention with reduced-iron multiple micronutrient powders on iron status, morbidity and growth in young children in Ethiopia. Nutrients 2018, 10, 1508. [Google Scholar] [CrossRef] [PubMed]
- DSM. Mix ME Vitamin Mineral Powder; DSM: Isando, South Africa, 2014; Available online: http://phsl.co/our-products/mix-metm-vitamin-mineral-powder?tmpl=component&print=1&page= (accessed on 15 January 2019).
- Herrador, Z.; Sordo, L.; Gadisa, E.; Moreno, J.; Nieto, J.; Benito, A.; Aseffa, A.; Cañavate, C.; Custodio, E. Cross-sectional study of malnutrition and associated factors among school aged children in rural and urban settings of Fogera and Libo Kemkem districts, Ethiopia. PLoS ONE 2014, 9, e105880. [Google Scholar] [CrossRef] [PubMed]
- WHO/UNICEF. Complementary Feeding of Young Children in Developing Countries: A Review of Current Scientific Knowledge (WHO/NUT/98.1); World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- EPHI. Ethiopian National Micronutrient Survey Report; Ethiopian Public Health Institute: Addis Ababa, Ethiopia, 2016. [Google Scholar]
- Nusser, S.M.; Carriquiry, A.L.; Dodd, K.W.; Fuller, W.A. A semiparametric transformation approach to estimating usual daily intake distributions. J. Am. Stat. Assoc. 1996, 91, 1440–1449. [Google Scholar] [CrossRef]
- Dodd, K.W.; Guenther, P.M.; Freedman, L.S.; Subar, A.F.; Kipnis, V.; Midthune, D.; Tooze, J.A.; Krebs-Smith, S.M. Statistical methods for estimating usual intake of nutrients and foods: A review of the theory. J. Am. Diet. Assoc. 2006, 106, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Vorster, H.H. Methods for using nutrient intake values (NIVs) to assess or plan nutrient intakes. Food Nutr. Bull. 2007, 28, S51–S60. [Google Scholar] [CrossRef]
- Gibson, R.S. Principles of Nutritonal Assessment, 2nd ed.; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- IOM. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Institute of Medicine; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- WHO/FAO. Guidelines on Food Fortification with Micronutrients. UNSCN Org; World Health Organization/Food and Agriculture Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Hotz, C.; Brown, K.H. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 2004, 25, 130–156. [Google Scholar]
- WHO. WHO Anthro (Version 3.2. 2 January 2011) and Macros (2011); World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Hess, S.Y.; Brown, K.H. Impact of zinc fortification on zinc nutrition. Food Nutr. Bull. 2009, 30, S79–S107. [Google Scholar] [CrossRef]
- Vossenaar, M.; Knight, F.A.; Tumilowicz, A.; Hotz, C.; Chege, P.; Ferguson, E.L. Context-specific complementary feeding recommendations developed using Optifood could improve the diets of breast-fed infants and young children from diverse livelihood groups in northern Kenya. In Public Health Nutrition; Cambridge University Press: Cambridge, UK, 2016; pp. 1–13. [Google Scholar]
- Rah, J.H.; dePee, S.; Kraemer, K.; Steiger, G.; Bloem, M.W.; Spiegel, P.; Wilkinson, C.; Bilukha, O. Program experience with micronutrient powders and current evidence. J. Nutr. 2012, 142, 191S–196S. [Google Scholar] [CrossRef]
- Chaparro, C.M.; Dewey, K.G. Use of lipid-based nutrient supplements (LNS) to improve the nutrient adequacy of general food distribution rations for vulnerable sub-groups in emergency settings. Matern. Child Nutr. 2010, 6, 1–69. [Google Scholar] [CrossRef]
- Soofi, S.; Ahmed, S.; Fox, M.P.; MacLeod, W.B.; Thea, D.M.; Qazi, S.A.; Bhutta, Z.A. Effectiveness of community case management of severe pneumonia with oral amoxicillin in children aged 2–59 months in Matiari district, rural Pakistan: A cluster-randomised controlled trial. Lancet 2012, 379, 729–737. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Chassard, C.; Rohner, F.; N’Goran, E.K.; Nindjin, C.; Dostal, A.; Utzinger, J.; Ghattas, H.; Lacroix, C.; Hurrell, R.F. The effects of iron fortification on the gut microbiota in African children: A randomized controlled trial in Côte d’Ivoire. Am. J. Clin. Nutr. 2010, 92, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Abebe, Z.; Haki, G.D.; Baye, K. Simulated effects of home fortification of complementary foods with micronutrient powders on risk of inadequate and excessive intakes in west Gojjam, Ethiopia. Mater. Child Nutr. 2018, 14, e12443. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.R. Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am. J. Clin. Nutr. 2003, 78, 633S–639S. [Google Scholar] [CrossRef] [PubMed]
- Krebs, N.F.; Long, J.; Mondal, P.; Westcott, J.; Miller, L.; Islam, M.; Ahmed, M.; Mahfuz, M.; Ahmed, T. Zinc (Zn) absorption from micronutrient powders (MNP) is low in Bangladeshi toddlers with environmental enteric dysfunction (EED). FASEB J. 2017, 31, 786.4. [Google Scholar]
- Seleshe, S.; Jo, C.; Lee, M. Meat consumption culture in Ethiopia. Korean J. Food Sci. Anim. Res. 2014, 34, 7. [Google Scholar] [CrossRef] [PubMed]
- Hirvonen, K.; Hoddinott, J. Agricultural production and children’s diets: Evidence from rural Ethiopia. Agric. Econ. 2016, 48, 469–480. [Google Scholar] [CrossRef]
- Wessells, K.; Young, R.; Ferguson, E.; Ouédraogo, C.; Faye, M.; Hess, S. Assessment of Dietary Intake and Nutrient Gaps, and Development of Food-Based Recommendations, among Pregnant and Lactating Women in Zinder, Niger: An Optifood Linear Programming Analysis. Nutrients 2019, 11, 72. [Google Scholar] [CrossRef]
| CF * Tigray | CF * Amhara | CF * Oromia | CF * SNNPR | MNP ** | Sq-LNS *** | |
|---|---|---|---|---|---|---|
| 100 g | 100 g | 100 g | 100 g | 1 g | 20 g | |
| Energy (kcal) | 351 | 357 | 371 | 373 | 0 | 118 |
| Protein (g) | 13.4 | 13.1 | 10.7 | 11.2 | 0 | 2.6 |
| Water (g) | 9.8 | 9 | 8.0 | 7.5 | 0 | 4 |
| Fat (g) | 1.7 | 2.1 | 2.8 | 2.9 | 0 | 9.6 |
| Carbohydrate (g) | 73.2 | 73.9 | 76.4 | 76.2 | 0 | 5.3 |
| Calcium (mg) | 57.5 | 42.8 | 38.8 | 27.7 | 0 | 280 |
| Iron (mg) | 11.9 | 7.8 | 10.8 | 4.7 | 6 | 6 |
| Zinc (mg) | 1.6 | 1.7 | 1.2 | 1.5 | 4.1 | 8 |
| Vitamin C (mg) | 0.5 | 1.4 | 2.6 | 3.2 | 30 | 30 |
| Thiamin (mg) | 0.3 | 0.3 | 0.3 | 0.3 | 0.5 | 0.3 |
| Riboflavin (mg) | 0.2 | 0.2 | 0.1 | 0.1 | 0.5 | 0.4 |
| Niacin (mg) | 3.3 | 2.9 | 1.5 | 1.6 | 6 | 4 |
| Vitamin B6 (mg) | 0.3 | 0.3 | 0.3 | 0.2 | 0.5 | 0.3 |
| Folate (µg dietary eq.) | 138.7 | 130 | 107.2 | 103.8 | 150 | 80 |
| Vitamin B12 (mg) | 0 | 0 | 0 | 0 | 0.9 | 0.5 |
| Vitamin A (mg) | 0.8 | 1.5 | 1.5 | 1.9 | 400 | 400 |
| Characteristics | Tigray | Amhara | Oromia | SNNPR |
|---|---|---|---|---|
| Total number | 472 | 659 | 675 | 692 |
| 6–8 months | 89 | 122 ‡ | 135 | 151 |
| 9–11 months | 86 | 120 | 129 | 129 |
| 12–23 months | 297 | 417 ‡ | 411 ‡ | 412 |
| Sex-male n (%) | ||||
| 6–8 months | 39 (43.8) | 68 (56.2) | 73 (54.1) | 73 (48.3) |
| 9–11 months | 38 (44.2) | 57 (47.5) | 77 (59.7) | 67 (51.9) |
| 12–23 months | 135 (45.5) | 216 (51.9) | 236 (57.3) | 227 (55.1) |
| Place of residence n (%) | ||||
| Urban | 91 (19.3) | 90 (13.7) | 70 (10.4) | 71 (10.3) |
| Rural | 381 (80.7) | 569 (86.3) | 605 (89.6) | 621 (89.7) |
| Nutritional status | ||||
| HAZ * (mean ± SD) | −1.73 ± 1.39 | −1.61 ± 1.79 | −1.21 ± 2.00 | −1.37 ± 1.72 |
| Stunting n (%) | 201 (42.6) | 267 (40.8) ‡‡ | 227 (33.7) | 241 (34.8) |
| WAZ ** (mean ± SD) | −1.45 ± 1.09 | −1.33 ± 1.24 | −1.16 ± 1.40 | −1.03 ± 1.34 |
| Underweight n (%) | 145 (30.8) | 185 (28.2) ‡‡ | 172 (25.5) | 144 (20.8) |
| WHZ *** (mean ± SD) | −0.73 ± 1.10 | −0.66 ± 1.27 | −0.69 ± 1.31 | −0.40 ± 1.21 |
| Wasting n (%) | 54 (11.5) | 76 (11.7) ‡‡ | 97 (14.4) | 59 (8.5) |
| Calcium | Thiamin | Niacin | Vit.B6 | Folate | Vit.B12 | Vit.A | Iron | Zinc | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Tigray | 6–8 mo. | |||||||||
| 9–11 mo. | ||||||||||
| 12–23 mo. | ||||||||||
| Amhara | 6–8 mo. | |||||||||
| 9–11 mo. | ||||||||||
| 12–23 mo. | ||||||||||
| Oromia | 6–8 mo. | |||||||||
| 9–11 mo. | ||||||||||
| 12–23 mo. | ||||||||||
| SNNPR | 6–8 mo. | |||||||||
| 9–11 mo. | ||||||||||
| 12–23 mo. | ||||||||||
| * | Nutrient requirements that can be met but require changes consistent with FBDR | |||||||||
| ** | Nutrient requirements cannot be met by any combination of local foods | |||||||||
| Food Group | Foods 2 | Age Group | |||
|---|---|---|---|---|---|
| 6 to 8 mo. 1 | 9 to 11 mo. 1 | 12 to 23 mo. 1 | |||
| s/wk. 3 | s/wk. 3 | s/wk. 3 | |||
| Tigray | Dairy | Milk | 7 | 7 | 7 |
| FICFP 4 | 7 | - | - | ||
| Grains | Wheat, teff | 4 | 7 | 14 | |
| Vegetables | Vitamin C rich vegetables | - | 7 | 3 to 4 | |
| Legumes | Broad beans, vetch, (chick) peas | - | 14 | 14 | |
| Eggs | - | - | 7 | ||
| Amhara | Dairy | Milk | 7 | 7 | 7 |
| FICFP | 7 | - | - | ||
| Grains | Wheat, teff | - | 14 | 14 | |
| Vegetables 5 | Tomato, onions | - | - | 14 | |
| Legumes | Broad beans, lentils | 7 | 7 | 21 | |
| Starchy Roots | Potato | - | 7 | 7 | |
| Oromia | Dairy | Milk | 3 to 4 | 3 to 4 | 3 to 4 |
| Grains | Wheat, teff | 7 | 14 | 14 | |
| Vegetables 5 | Tomato, onion | - | 14 | 14 | |
| Legumes | Broad beans, lentils | 7 | 3 to 4 | 14 | |
| Starchy Roots | Potato | 7 | - | - | |
| Eggs | - | - | 7 | ||
| SNNPR | Dairy | (butter) milk | 3 to 4 | 3 to 4 | 7 |
| Grains | Barley, millet, tef | 14 | 35 | 21 | |
| DGLV 6 | Kale | - | 7 | - | |
| Legumes | Chickpeas, kidney beans | 21 | 14 | 21 | |
| Starchy roots | Potato | - | 3 to 4 | - | |
| Eggs | - | - | 7 | ||
| Nutrient *(EAR) | Age Group 6–8 m (n = 495) | Age Group 9–11 m (n = 465) | Age Group 12–23 m (n = 1544) | |||
|---|---|---|---|---|---|---|
| Inadequate% | Excess% | Inadequate% | Excess% | Inadequate% | Excess% | |
| Iron (10%) ** | ||||||
| Usual diet | 77.7 | 4.6 | 67.1 | 3.7 | 40.1 | 18.7 |
| +1/2MNP/d | 67.3 | 5.3 | 52.8 | 4.1 | 26.2 | 20.5 |
| +1MNP/d | 39.8 | 6.1 | 26.6 | 4.5 | 10.4 | 22.2 |
| Iron (5%) ** | ||||||
| Usual diet | 86.5 | 4.6 | 81.6 | 3.7 | 52.9 | 18.7 |
| +1/2MNP/d | 82.3 | 5.3 | 75.3 | 4.1 | 46.4 | 20.5 |
| +1MNP/d | 75.8 | 6.1 | 66.5 | 4.5 | 35.5 | 22.2 |
| Zinc (moderate bioavailability) ∞ (WHO cut-off) | ||||||
| Usual diet | 92.7 | 0 | 92.3 | 0 | 68.6 | 0.1 |
| +1/2MNP/d | 0 | 0 | 0 | 0 | 8.2 | 0.1 |
| +1MNP/d | 0 | 0.2 | 0 | 0 | 0 | 0.1 |
| Zinc (low bioavailability) ∞ (WHO cut-off) | ||||||
| Usual diet | 98.6 | 0 | 100 | 0 | 96.1 | 0.1 |
| +1/2MNP/d | 96.0 | 0 | 97.2 | 0 | 87.3 | 0.1 |
| +1MNP/d | 0 | 0.2 | 0 | 0 | 53.9 | 0.1 |
| Zinc ≡ (IZiNCG cut-off) | ||||||
| Usual diet | 0.4 | 0 | 2.4 | |||
| +1/2MNP/d | 1.6 | 0.4 | 6.9 | |||
| +1MNP/d | 21.2 | 51.0 | 22.4 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samuel, A.; Osendarp, S.J.M.; Ferguson, E.; Borgonjen, K.; Alvarado, B.M.; Neufeld, L.M.; Adish, A.; Kebede, A.; Brouwer, I.D. Identifying Dietary Strategies to Improve Nutrient Adequacy among Ethiopian Infants and Young Children Using Linear Modelling. Nutrients 2019, 11, 1416. https://doi.org/10.3390/nu11061416
Samuel A, Osendarp SJM, Ferguson E, Borgonjen K, Alvarado BM, Neufeld LM, Adish A, Kebede A, Brouwer ID. Identifying Dietary Strategies to Improve Nutrient Adequacy among Ethiopian Infants and Young Children Using Linear Modelling. Nutrients. 2019; 11(6):1416. https://doi.org/10.3390/nu11061416
Chicago/Turabian StyleSamuel, Aregash, Saskia J. M. Osendarp, Elaine Ferguson, Karin Borgonjen, Brenda M. Alvarado, Lynnette M. Neufeld, Abdulaziz Adish, Amha Kebede, and Inge D. Brouwer. 2019. "Identifying Dietary Strategies to Improve Nutrient Adequacy among Ethiopian Infants and Young Children Using Linear Modelling" Nutrients 11, no. 6: 1416. https://doi.org/10.3390/nu11061416
APA StyleSamuel, A., Osendarp, S. J. M., Ferguson, E., Borgonjen, K., Alvarado, B. M., Neufeld, L. M., Adish, A., Kebede, A., & Brouwer, I. D. (2019). Identifying Dietary Strategies to Improve Nutrient Adequacy among Ethiopian Infants and Young Children Using Linear Modelling. Nutrients, 11(6), 1416. https://doi.org/10.3390/nu11061416





