The Impact of Pectin Supplementation on Intestinal Barrier Function in Healthy Young Adults and Healthy Elderly
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Gut Permeability Test
2.4. Mucosal Defense Parameters
2.5. Gastrointestinal Tolerance
2.6. Using Chamber Experiment
2.7. Gene Transcription of Relevant Proteins
2.8. Immunofluorescence Staining of TJP1 and Occludin
2.9. Statistical Analyses
3. Results
3.1. Study Subjects
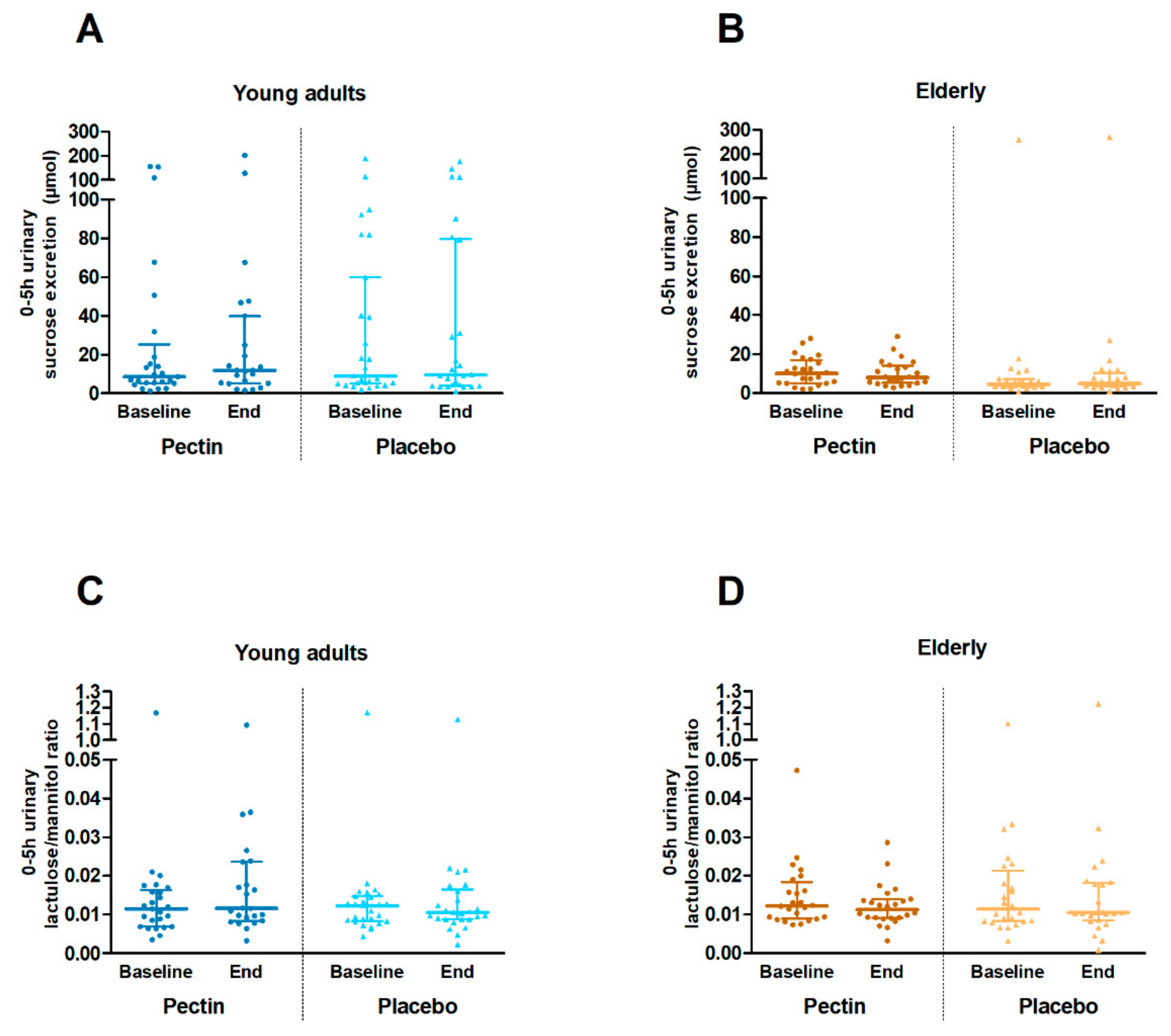
3.2. Intestinal Permeability In Vivo
3.3. Mucosal Defense Parameters
3.4. Gastrointestinal Tolerance
3.5. Intestinal Permeability Ex Vivo
3.6. Gene Transcription of Barrier-Related Genes
3.7. Immunofluorescence Staining of TJP1 and Occludin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.-J. Human Intestinal Barrier Function in Health and Disease. Clin. Trans. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Hollander, D.; Dadufalza, V.; Krugliak, P. Effect of aging and caloric restriction on intestinal permeability. Exp. Gerontol. 1992, 27, 321–333. [Google Scholar] [CrossRef]
- Tran, L.; Meerveld, B.G.-V. Age-Associated Remodeling of the Intestinal Epithelial Barrier. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.C.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P.; et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 2017, 21, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Siró, I.; Kápolna, E.; Kápolna, B.; Lugasi, A. Functional food. Product development, marketing and consumer acceptance—A review. Appetite 2008, 51, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F.; Theuwissen, E.; Adam, A.; Bell, M.; Berger, A.; Mensink, R.P. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. Eur. J. Clin. Nutr. 2012, 66, 591–599. [Google Scholar] [CrossRef]
- Tian, L.; Scholte, J.; Borewicz, K.; van den Bogert, B.; Smidt, H.; Scheurink, A.J.W.; Gruppen, H.; Schols, H.A. Effects of pectin supplementation on the fermentation patterns of different structural carbohydrates in rats. Mol. Nutr. Food Res. 2016, 60, 2256–2266. [Google Scholar] [CrossRef]
- Thakur, B.R.; Singh, R.K.; Handa, A.K.; Rao, M.A. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef]
- Garthoff, J.A.; Heemskerk, S.; Hempenius, R.A.; Lina, B.A.R.; Krul, C.A.M.; Koeman, J.H.; Speijers, G.J.A. Safety evaluation of pectin-derived acidic oligosaccharides (pAOS): Genotoxicity and sub-chronic studies. Regul. Toxicol. Pharmacol. 2010, 57, 31–42. [Google Scholar] [CrossRef]
- Tian, L.; Bruggeman, G.; van den Berg, M.; Borewicz, K.; Scheurink, A.J.W.; Bruininx, E.; de Vos, P.; Smidt, H.; Schols, H.A.; Gruppen, H. Effects of pectin on fermentation characteristics, carbohydrate utilization, and microbial community composition in the gastrointestinal tract of weaning pigs. Mol. Nutr. Food Res. 2017, 61, 1600186. [Google Scholar] [CrossRef] [PubMed]
- Vogt, L.M.; Sahasrabudhe, N.M.; Ramasamy, U.; Meyer, D.; Pullens, G.; Faas, M.M.; Venema, K.; Schols, H.A.; de Vos, P. The impact of lemon pectin characteristics on TLR activation and T84 intestinal epithelial cell barrier function. J. Funct. Foods 2016, 22, 398–407. [Google Scholar] [CrossRef]
- Sahasrabudhe, N.M.; Beukema, M.; Tian, L.; Troost, B.; Scholte, J.; Bruininx, E.; Bruggeman, G.; van den Berg, M.; Scheurink, A.; Schols, H.A.; et al. Dietary Fiber Pectin Directly Blocks Toll-Like Receptor 2–1 and Prevents Doxorubicin-Induced Ileitis. Front. Immunol. 2018, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Kim, C.Y.; Kaur, A.; Lamothe, L.; Shaikh, M.; Keshavarzian, A.; Hamaker, B.R. Dietary fibre-based SCFA mixtures promote both protection and repair of intestinal epithelial barrier function in a Caco-2 cell model. Food Funct. 2017, 8, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Hardy, H.; Harris, J.; Lyon, E.; Beal, J.; Foey, A.D. Probiotics, Prebiotics and Immunomodulation of Gut Mucosal Defences: Homeostasis and Immunopathology. Nutrients 2013, 5, 1869–1912. [Google Scholar] [CrossRef]
- Rabbani, G.H.; Teka, T.; Saha, S.K.; Zaman, B.; Majid, N.; Khatun, M.; Wahed, M.A.; Fuchs, G.J. Green banana and pectin improve small intestinal permeability and reduce fluid loss in Bangladeshi children with persistent diarrhea. Dig. Dis. Sci. 2004, 49, 475–484. [Google Scholar] [CrossRef]
- Shiau, S.-Y.; Chang, G.W. Effects of Certain Dietary Fibers on Apparent Permeability of the Rat Intestine. J. Nutr. 1986, 116, 223–232. [Google Scholar] [CrossRef]
- Jiang, T.; Gao, X.; Wu, C.; Tian, F.; Lei, Q.; Bi, J.; Xie, B.; Wang, H.Y.; Chen, S.; Wang, X. Apple-Derived Pectin Modulates Gut Microbiota, Improves Gut Barrier Function, and Attenuates Metabolic Endotoxemia in Rats with Diet-Induced Obesity. Nutrients 2016, 8, 126. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Caterina, R.D.; Görman, U.; Allayee, H.; Kohlmeier, M.; Prasad, C.; Choi, M.S.; Curi, R.; de Luis, D.A.; Gil, Á.; et al. Guide and Position of the International Society of Nutrigenetics/Nutrigenomics on Personalised Nutrition: Part 1—Fields of Precision Nutrition. J. Nutrigenet. Nutrigenomics 2016, 9, 12–27. [Google Scholar] [CrossRef]
- Marteau, P.; Seksik, P. Tolerance of Probiotics and Prebiotics. J. Clin. Gastroenterol. 2004, 38. [Google Scholar] [CrossRef]
- Schwab, U.; Louheranta, A.; Törrönen, A.; Uusitupa, M. Impact of sugar beet pectin and polydextrose on fasting and postprandial glycemia and fasting concentrations of serum total and lipoprotein lipids in middle-aged subjects with abnormal glucose metabolism. Eur. J. Clin. Nutr. 2006, 60, 1073–1080. [Google Scholar] [CrossRef]
- François, I.E.J.A.; Lescroart, O.; Veraverbeke, W.S.; Marzorati, M.; Possemiers, S.; Evenepoel, P.; Hamer, H.; Houben, E.; Windey, K.; Welling, G.W.; et al. Effects of a wheat bran extract containing arabinoxylan oligosaccharides on gastrointestinal health parameters in healthy adult human volunteers: A double-blind, randomised, placebo-controlled, cross-over trial. Br. J. Nutr. 2012, 108, 2229–2242. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Caporaso, J.G.; Hooda, S.; Brulc, J.M.; Fahey, G.C.; Swanson, K.S. Fiber supplementation influences phylogenetic structure and functional capacity of the human intestinal microbiome: follow-up of a randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 55–64. [Google Scholar] [CrossRef]
- Van Wijck, K.; van Eijk, H.M.H.; Buurman, W.A.; Dejong, C.H.C.; Lenaerts, K. Novel analytical approach to a multi-sugar whole gut permeability assay. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 2794–2801. [Google Scholar] [CrossRef] [PubMed]
- Van Wijck, K.; Verlinden, T.J.M.; van Eijk, H.M.H.; Dekker, J.; Buurman, W.A.; Dejong, C.H.C.; Lenaerts, K. Novel multi-sugar assay for site-specific gastrointestinal permeability analysis: A randomized controlled crossover trial. Clin. Nutr. 2013, 32, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Svedlund, J.; Sjödin, I.; Dotevall, G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig. Dis. Sci. 1988, 33, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Rinsma, N.F.; Farré, R.; Troost, F.J.; Elizalde, M.; Keszthelyi, D.; Helyes, Z.; Masclee, A.A.; Conchillo, J.M. Exploration of the Esophageal Mucosal Barrier in Non-Erosive Reflux Disease. Int. J. Mol. Sci. 2017, 18, 1091. [Google Scholar] [CrossRef]
- Pijls, K.E.; Jonkers, D.M.A.E.; Elizalde, M.; Drittij-Reijnders, M.-J.; Haenen, G.R.; Bast, A.; Masclee, A.A.M.; Koek, G.H. Is intestinal oxidative stress involved in patients with compensated liver cirrhosis? Ann. Hepatol. 2016, 15, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Elamin, E.; Masclee, A.; Troost, F.; Pieters, H.-J.; Keszthelyi, D.; Aleksa, K.; Dekker, J.; Jonkers, D. Ethanol Impairs Intestinal Barrier Function in Humans through Mitogen Activated Protein Kinase Signaling: A Combined In Vivo and In Vitro Approach. PLoS ONE 2014, 9, e107421. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.; Linsalata, M.; Clemente, C.; Chiloiro, M.; Orlando, A.; Marconi, E.; Chimienti, G.; Riezzo, G. Inulin-enriched pasta improves intestinal permeability and modifies the circulating levels of zonulin and glucagon-like peptide 2 in healthy young volunteers. Nutr. Res. 2012, 32, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Salden, B.N.; Troost, F.J.; Wilms, E.; Truchado, P.; Vilchez-Vargas, R.; Pieper, D.H.; Jáuregui, R.; Marzorati, M.; van de Wiele, T.; Possemiers, S.; et al. Reinforcement of intestinal epithelial barrier by arabinoxylans in overweight and obese subjects: A randomized controlled trial: Arabinoxylans in gut barrier. Clin. Nutr. 2018, 37, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Krumbeck, J.A.; Rasmussen, H.E.; Hutkins, R.W.; Clarke, J.; Shawron, K.; Keshavarzian, A.; Walter, J. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 2018, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Ganda Mall, J.P.; Löfvendahl, L.; Lindqvist, C.M.; Brummer, R.J.; Keita, Å.V.; Schoultz, I. Differential effects of dietary fibres on colonic barrier function in elderly individuals with gastrointestinal symptoms. Sci. Rep. 2018, 8, 13404. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; He, Y.; Wang, F.; Zhang, H.; de Vos, P.; Sun, J. Low-methoxyl lemon pectin attenuates inflammatory responses and improves intestinal barrier integrity in caerulein-induced experimental acute pancreatitis. Mol. Nutr. Food Res. 2017, 61, 1600885. [Google Scholar] [CrossRef] [PubMed]
- Woof, J.M.; Kerr, M.A. The function of immunoglobulin A in immunity. J. Pathol. 2006, 208, 270–282. [Google Scholar] [CrossRef]
- Lecerf, J.-M.; Dépeint, F.; Clerc, E.; Dugenet, Y.; Niamba, C.N.; Rhazi, L.; Cayzeele, A.; Abdelnour, G.; Jaruga, A.; Younes, H.; et al. Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br. J. Nutr. 2012, 108, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Walton, G.E.; Lu, C.; Trogh, I.; Arnaut, F.; Gibson, G.R. A randomised, double-blind, placebo controlled cross-over study to determine the gastrointestinal effects of consumption of arabinoxylan-oligosaccharides enriched bread in healthy volunteers. Nutr. J. 2012, 11, 36. [Google Scholar] [CrossRef]
- Vulevic, J.; Juric, A.; Tzortzis, G.; Gibson, G.R. A Mixture of trans-Galactooligosaccharides Reduces Markers of Metabolic Syndrome and Modulates the Fecal Microbiota and Immune Function of Overweight Adults. J. Nutr. 2013, 143, 324–331. [Google Scholar] [CrossRef]
- Kato, T.; Fukuda, S.; Fujiwara, A.; Suda, W.; Hattori, M.; Kikuchi, J.; Ohno, H. Multiple Omics Uncovers Host–Gut Microbial Mutualism During Prebiotic Fructooligosaccharide Supplementation. DNA Res. 2014, 21, 469–480. [Google Scholar] [CrossRef]
- Lim, B.O.; Yamada, K.; Nonaka, M.; Kuramoto, Y.; Hung, P.; Sugano, M. Dietary Fibers Modulate Indices of Intestinal Immune Function in Rats. J. Nutr. 1997, 127, 663–667. [Google Scholar] [CrossRef]
- Holscher, H.D.; Doligale, J.L.; Bauer, L.L.; Gourineni, V.; Pelkman, C.L.; Fahey, G.C.; Swanson, K.S. Gastrointestinal tolerance and utilization of agave inulin by healthy adults. Food Funct. 2014, 5, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Bergamasco, C.; Horie, L.M.; Torrinhas, R.S.; Waitzberg, D.L. High-Fiber Orange Juice as a Nutrition Supplement in Women. J. Perenter. Enteral. Nutr. 2015, 39, 941–947. [Google Scholar] [CrossRef] [PubMed]





| Parameter | Young Adults (n = 52) | Elderly (n = 48) | ||||
|---|---|---|---|---|---|---|
| Pectin (n = 25) | Placebo (n = 27) | p-Value | Pectin (n = 24) | Placebo (n = 24) | p-Value | |
| Age (years, mean ± SD) | 23.4 ± 4.5 | 22.8 ± 4.1 | 0.613 | 69.5 ± 3.1 | 69.8 ± 2.4 | 0.723 |
| Sex (% female) | 68.0 | 48.1 | 0.148 | 37.5 | 50.0 | 0.383 |
| BMI (kg/m2, mean ± SD) | 23.2 ± 2.7 | 22.6 ± 2.7 | 0.444 | 25.5 ± 2.6 | 26.2 ± 2.8 | 0.334 |
| Serum CRP (mg/L, mean ± SD) | 1.7 ± 2.5 | 1.0 ± 1.2 | 0.161 | 1.1 ± 1.3 | 1.8 ± 2.1 | 0.203 |
| Medication (%) | N.A. | N.A. | N.A. | |||
| PPI | 12.5 | 12.5 | 1.000 | |||
| Statins | 4.2 | 4.2 | 1.000 | |||
| Antihypertensives | 12.5 | 8.3 | 0.637 | |||
| Alcohol consumption (units/week, mean ± SD) | 3.5 ± 3.2 | 5.3 ± 5.4 | 0.165 | 8.4 ± 6.9 | 9.3 ± 7.1 | 0.667 |
| Cluster | Gene Name | Young Adults | Elderly | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pectin | Placebo | p-Value | Benjamini–Hochberg p-Value | Pectin | Placebo | p-Value | Benjamini–Hochberg p-Value | ||
| Junctional complex related genes | TJP1 (ZO-1) | 1.15 ± 0.03 | 1.13 ± 0.03 | 0.313 | 0.417 | 1.15 ± 0.02 | 1.13 ± 0.02 | 0.195 | 0.260 |
| OCLN | 1.19 ± 0.02 | 1.18 ± 0.02 | 0.128 | 0.417 | 1.20 ± 0.01 | 1.19 ± 0.02 | 0.184 | 0.260 | |
| CLDN2 | 1.34 ± 0.07 | 1.36 ± 0.03 | 0.527 | 0.602 | 1.36 ± 0.05 | 1.32 ± 0.07 | 0.250 | 0.286 | |
| CLDN3 | 1.17 ± 0.02 | 1.16 ± 0.02 | 0.245 | 0.417 | 1.18 ± 0.03 | 1.16 ± 0.02 | 0.079 | 0.222 | |
| CLDN4 | 1.11 ± 0.03 | 1.10 ± 0.02 | 0.311 | 0.417 | 1.12 ± 0.02 | 1.10 ± 0.02 | 0.111 | 0.222 | |
| MLCK | 1.15 ± 0.02 | 1.15 ± 0.03 | 0.982 | 0.982 | 1.16 ± 0.02 | 1.14 ± 0.03 | 0.109 | 0.222 | |
| CDH1 | 1.17 ± 0.02 | 1.15 ± 0.01 | 0.072 | 0.417 | 1.17 ± 0.03 | 1.17 ± 0.02 | 0.852 | 0.852 | |
| CTNNB1 | 1.13 ± 0.01 | 1.12 ± 0.01 | 0.236 | 0.417 | 1.15 ± 0.02 | 1.12 ± 0.02 | 0.029 | 0.222 | |
| Defense and immune related genes | CAMP | 1.29 ± 0.05 | 1.30 ± 0.05 | 0.630 | 0.770 | 1.32 ± 0.06 | 1.28 ± 0.06 | 0.179 | 0.405 |
| DEFB1 | 1.17 ± 0.05 | 1.15 ± 0.03 | 0.468 | 0.735 | 1.18 ± 0.05 | 1.16 ± 0.03 | 0.184 | 0.405 | |
| MUC2 | 1.02 ± 0.03 | 1.01 ± 0.03 | 0.429 | 0.735 | 1.01 ± 0.03 | 1.01 ± 0.02 | 0.832 | 0.915 | |
| TFF3 | 0.99 ± 0.03 | 0.98 ± 0.04 | 0.432 | 0.735 | 0.98 ± 0.05 | 0.98 ± 0.04 | 0.832 | 0.915 | |
| IL1B | 1.32 ± 0.05 | 1.35 ± 0.05 | 0.217 | 0.597 | 1.33 ± 0.04 | 1.31 ± 0.06 | 0.232 | 0.405 | |
| IL10 | 1.25 ± 0.07 | 1.25 ± 0.03 | 0.856 | 0.856 | 1.27 ± 0.05 | 1.23 ± 0.06 | 0.141 | 0.405 | |
| TNF | 1.31 ± 0.05 | 1.35 ± 0.06 | 0.151 | 0.554 | 1.35 ± 0.04 | 1.35 ± 0.04 | 0.937 | 0.937 | |
| TLR1 | 1.15 ± 0.05 | 1.18 ± 0.04 | 0.144 | 0.554 | 1.16 ± 0.05 | 1.13 ± 0.04 | 0.153 | 0.405 | |
| TLR2 | 1.25 ± 0.06 | 1.26 ± 0.05 | 0.818 | 0.856 | 1.26 ± 0.06 | 1.23 ±.0.06 | 0.258 | 0.405 | |
| TLR4 | 1.19 ± 0.03 | 1.21 ± 0.03 | 0.056 | 0.554 | 1.21 ± 0.03 | 1.19 ± 0.03 | 0.042 | 0.405 | |
| TLR6 | 1.12 ± 0.06 | 1.29 ± 0.04 | 0.622 | 0.770 | 1.30 ± 0.07 | 1.27 ± 0.06 | 0.358 | 0.492 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilms, E.; Jonkers, D.M.A.E.; Savelkoul, H.F.J.; Elizalde, M.; Tischmann, L.; de Vos, P.; Masclee, A.A.M.; Troost, F.J. The Impact of Pectin Supplementation on Intestinal Barrier Function in Healthy Young Adults and Healthy Elderly. Nutrients 2019, 11, 1554. https://doi.org/10.3390/nu11071554
Wilms E, Jonkers DMAE, Savelkoul HFJ, Elizalde M, Tischmann L, de Vos P, Masclee AAM, Troost FJ. The Impact of Pectin Supplementation on Intestinal Barrier Function in Healthy Young Adults and Healthy Elderly. Nutrients. 2019; 11(7):1554. https://doi.org/10.3390/nu11071554
Chicago/Turabian StyleWilms, Ellen, Daisy M.A.E. Jonkers, Huub F.J. Savelkoul, Montserrat Elizalde, Lea Tischmann, Paul de Vos, Ad A.M. Masclee, and Freddy J. Troost. 2019. "The Impact of Pectin Supplementation on Intestinal Barrier Function in Healthy Young Adults and Healthy Elderly" Nutrients 11, no. 7: 1554. https://doi.org/10.3390/nu11071554
APA StyleWilms, E., Jonkers, D. M. A. E., Savelkoul, H. F. J., Elizalde, M., Tischmann, L., de Vos, P., Masclee, A. A. M., & Troost, F. J. (2019). The Impact of Pectin Supplementation on Intestinal Barrier Function in Healthy Young Adults and Healthy Elderly. Nutrients, 11(7), 1554. https://doi.org/10.3390/nu11071554






