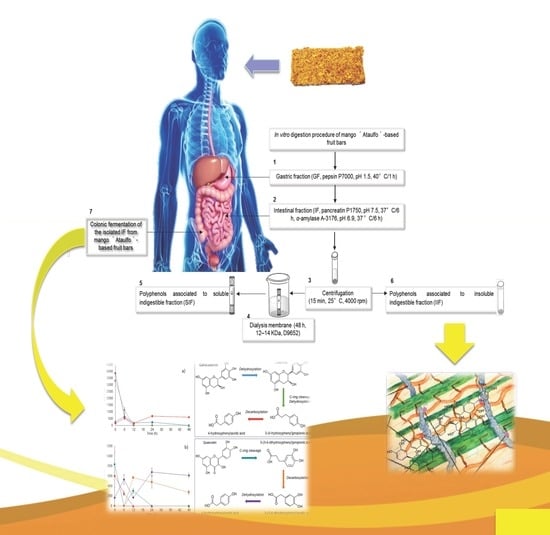
In Vitro Gastrointestinal Digestion and Colonic Fermentation of High Dietary Fiber and Antioxidant-Rich Mango (Mangifera indica L.) “Ataulfo”-Based Fruit Bars
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Mango-Based Bars
2.2. Nutritional Composition of Mango-Based Bars
2.3. Total Soluble Polyphenols (TSP), Hydrolyzable Polyphenols (HP) Content, and Antioxidant Capacity (AOX) in Mango-Based Bars
2.4. Identification of PCs by HPLC-DAD-MS Analysis of the Mango-Based Bars
2.5. In Vitro Digestion and Bioaccessibility (%) of the Mango-Based Bars
2.6. Isolation and Quantification of Indigestible Fraction (IF), and Its In Vitro Colonic Fermentation in Mango-Based Bars
2.7. SCFA Quantification by GC-MS Analysis
2.8. Statistical Evaluation
3. Results and Discussion
3.1. Nutritional Composition, PCs, and AOX in Mango-Based Bars
3.2. PCs Identified by HPLC-DAD-MS in Mango-Based Bars
3.3. Release of PCs in GasF, IntF, and %BA in Mango-Based Bars
3.4. PCs Bound to the Indigestible Fraction (IF) Isolated from Mango-Based Bars
3.5. Changes in pH and AOX during In Vitro Colonic Fermentation
3.6. Short Chain Fatty Acids (SCFA) during In Vitro Fermentation
3.7. Bioconversion of PC during In Vitro Colonic Fermentation of IF Isolated from Mango Bar
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- SIAP Atlas Agroalimentario. México Siembra Éxito. Available online: http://online.pubhtml5.com/clsi/ibhs/#p=1 (accessed on 12 November 2017).
- Instituto Mexicano de la Propiedad Industrial. Declaracion General de Protección de la Denominación de Origen Mango Ataulfo del Soconusco Chiapas. 2003. Available online: http://www.impi.gob.mx/TemasInteres/Documents/Declaratoria_Mango_Ataulfo.pdf (accessed on 25 April 2019).
- FAO. Pérdidas y desperdicios de alimentos en América Latina y el Caribe. Available online: www.fao.org/3/I4655S.pdf (accessed on 11 October 2017).
- Bo, S.; De Carli, L.; Venco, E.; Fanzola, I.; Maiandi, M.; De Michieli, F.; Durazzo, M.; Beccuti, G.; Cavallo-Perin, P.; Ghigo, E. Impact of snacking pattern on overweight and obesity risk in a cohort of 11-to 13-year-old adolescents. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.L.; French, S.A.; Harnack, L.J.; Mitchell, N.R.; Wolfson, J. Snacking behaviors, diet quality, and body mass index in a community sample of working adults. J. Acad. Nutr. Diet. 2015, 115, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.P.; Siqueira, H.H.; do Lago, R.C.; Rosell, C.M.; Vilas Boas, E.V.D.B. Developing fruit-based nutritious snack bars. J. Sci. Food Agric. 2014, 94, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Lucas-González, R.; Viuda-Martos, M.; Pérez-Alvarez, J.A.; Fernández-López, J. Antioxidant potential and quality characteristics of Mediterranean fruit-based extruded snacks. Int. J. Food Sci. Technol. 2016, 51, 2674–2681. [Google Scholar] [CrossRef]
- Hugo, P.C.; Gil-Chávez, J.; Sotelo-Mundo, R.R.; Namiesnik, J.; Gorinstein, S.; González-Aguilar, G.A. Antioxidant interactions between major phenolic compounds found in “Ataulfo” mango pulp: Chlorogenic, gallic, protocatechuic and vanillic acids. Molecules 2012, 17, 12657–12664. [Google Scholar]
- Blancas-Benitez, F.J.; Mercado-Mercado, G.; Quirós-Sauceda, A.E.; Montalvo-González, E.; Gonzalez-Aguilar, G.A.; Sayago-Ayerdi, S.G. Bioaccesibility of polyphenols associated with dietary fiber and in vitro kinetics release of polyphenols in Mexican ‘Ataulfo’ mango (Mangifera indica L.) by-products. Food Funct. 2015, 6, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef]
- Valli, V.; Taccari, A.; Di Nunzio, M.; Danesi, F.; Bordoni, A. Health benefits of ancient grains. Comparison among bread made with ancient, heritage and modern grain flours in human cultured cells. Food Res. Int. 2018, 107, 206–215. [Google Scholar] [CrossRef]
- Antognoni, F.; Mandrioli, R.; Bordoni, A.; Di Nunzio, M.; Viadel, B.; Gallego, E.; Villaba, M.P.; Tomás-Cobos, L.; Taneyo Saa, L.D.; Gianotti, A. Integrated evaluation of the potential health benefits of einkorn-based breads. Nutrients 2017, 9, 1232. [Google Scholar] [CrossRef]
- Shim, S.-M.; Ferruzzi, M.G.; Kim, Y.-C.; Janle, E.M.; Santerre, C.R. Impact of phytochemical-rich foods on bioaccessibility of mercury from fish. Food Chem. 2009, 112, 46–50. [Google Scholar] [CrossRef]
- Topping, D.L.; Lockett, T.J. Human Physiology and Health: Dietary Fiber, Short-Chain Fatty Acids, and Their Impact on Gut Physiology; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 978-0-08-100596-5. [Google Scholar]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Mañas, E.; Saura-Calixto, F. Dietary fibre analysis: Methodological error sources. Eur. J. Clin. Nutr. 1995, 49, S158. [Google Scholar] [PubMed]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz-Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Montreau, F.R. Sur le dosage des composés phénoliques totaux dans les vins par la methode Folin-Ciocalteau. Connaiss. Vigne Vin 1972, 24, 397–404. [Google Scholar]
- Hartzfeld, P.W.; Forkner, R.; Hunter, M.D.; Hagerman, A.E. Determination of hydrolyzable tannins (gallotannins and ellagitannins) after reaction with potassium iodate. J. Agric. Food Chem. 2002, 50, 1785–1790. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of ‘Antioxidant Power’: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Blancas-Benitez, F.J.; Pérez-Jiménez, J.; Montalvo-González, E.; González-Aguilar, G.A.; Sáyago-Ayerdi, S.G. In vitro evaluation of the kinetics of the release of phenolic compounds from guava (Psidium guajava L.) fruit. J. Funct. Foods 2018, 43, 139–145. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Garcia-Alonso, A.; Goni, I.; Bravo, L. In vitro determination of the indigestible fraction in foods: An alternative to dietary fiber analysis. J. Agric. Food Chem. 2000, 48, 3342–3347. [Google Scholar] [CrossRef]
- Tabernero, M.; Venema, K.; Maathuis, A.J.H.; Saura-Calixto, F.D. Metabolite production during in vitro colonic fermentation of dietary fiber: Analysis and comparison of two European diets. J. Agric. Food Chem. 2011, 59, 8968–8975. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Gasga, V.M.; Montalvo-González, E.; Loarca-Piña, G.; Vázquez-Landaverde, P.A.; Tovar, J.; Sáyago-Ayerdi, S.G. Microbial metabolites profile during in vitro human colonic fermentation of breakfast menus consumed by Mexican school children. Food Res. Int. 2017, 97, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Gasga, V.M.; Loarca-Piña, G.; Vázquez-Landaverde, P.A.; Ortiz-Basurto, R.I.; Tovar, J.; Sáyago-Ayerdi, S.G. In vitro colonic fermentation of food ingredients isolated from Agave tequilana Weber var. azul applied on granola bars. LWT Food Sci. Technol. 2015, 60, 766–772. [Google Scholar] [CrossRef]
- Parn, O.J.; Bhat, R.; Yeoh, T.K.; Al-Hassan, A.A. Development of novel fruit bars by utilizing date paste. Food Biosci. 2015, 9, 20–27. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Teoh, A.; Massarotto, C.; Wibisono, R.; Wadhwa, S. Comparative analysis of fruit-based functional snack bars. Food Chem. 2010, 119, 1369–1379. [Google Scholar] [CrossRef]
- Danalache, F.; Mata, P.; Moldão-Martins, M.; Alves, V.D. Novel mango bars using gellan gum as gelling agent: Rheological and microstructural studies. LWT Food Sci. Technol. 2015, 62, 576–583. [Google Scholar] [CrossRef]
- Ajila, C.M.; Leelavathi, K.; Prasada Rao, U.J.S. Improvement of dietary fiber content and antioxidant properties in soft dough biscuits with the incorporation of mango peel powder. J. Cereal Sci. 2008, 48, 319–326. [Google Scholar] [CrossRef]
- De Lourdes García-Magaña, M.; García, H.S.; Bello-Pérez, L.A.; Sáyago-Ayerdi, S.G.; Mata-Montes de Oca, M. Functional Properties and Dietary Fiber Characterization of Mango Processing By-products (Mangifera indica L., cv Ataulfo and Tommy Atkins). Plant Foods Hum. Nutr. 2013, 68, 254–258. [Google Scholar] [CrossRef]
- Vergara-Valencia, N.; Granados-Pérez, E.; Agama-Acevedo, E.; Tovar, J.; Ruales, J.; Bello-Pérez, L.A. Fibre concentrate from mango fruit: Characterization, associated antioxidant capacity and application as a bakery product ingredient. LWT Food Sci. Technol. 2007, 40, 722–729. [Google Scholar] [CrossRef]
- Gorinstein, S.; Poovarodom, S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Vearasilp, S.; Haruenkit, R.; Ruamsuke, P.; Katrich, E.; Tashma, Z. Antioxidant properties and bioactive constituents of some rare exotic Thai fruits and comparison with conventional fruits: In vitro and in vivo studies. Food Res. Int. 2011, 44, 2222–2232. [Google Scholar] [CrossRef]
- Sáyago-Ayerdi, S.G.; Goñi, I. Hibiscus sabdariffa L: Fuente de fibra antioxidante. Arch. Latinoam. Nutr. 2010, 60, 79–84. [Google Scholar] [PubMed]
- Saura-Calixto, F. Dietary fiber as a carrier of dietary antioxidants: An essential physiological function. J. Agric. Food Chem. 2010, 59, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Salleh, R.M.; Ying, T.L.; Mousavi, L. Development of fruit bar using sapodilla (Manilkara zapota L.). J. Food Process. Preserv. 2017, 41, e12806. [Google Scholar] [CrossRef]
- Mercado-Mercado, G.; Montalvo-González, E.; González-Aguilar, G.A.; Alvarez-Parrilla, E.; Sáyago-Ayerdi, S.G. Ultrasound-assisted extraction of carotenoids from mango (Mangifera indica L. ‘Ataulfo’) by-products on in vitro bioaccessibility. Food Biosci. 2018, 21, 125–131. [Google Scholar] [CrossRef]
- De Ancos, B.; Sánchez-Moreno, C.; Zacarías, L.; Rodrigo, M.J.; Ayerdi, S.S.; Benítez, F.J.B.; González-Aguilar, G.A. Effects of two different drying methods (freeze-drying and hot air-drying) on the phenolic and carotenoid profile of ‘Ataulfo’ mango by-products. J. Food Meas. Charact. 2018, 12, 2145–2157. [Google Scholar] [CrossRef]
- Pensamiento-Niño, C.A.; Gómez-Aldapa, C.A.; Hernández-Santos, B.; Juárez-Barrientos, J.M.; Herman-Lara, E.; Martínez-Sánchez, C.E.; Rodríguez-Miranda, J. Optimization and characterization of an extruded snack based on taro flour (Colocasia esculenta L.) enriched with mango pulp (Mangifera indica L.). J. Food Sci. Technol. 2018, 55, 4244–4255. [Google Scholar] [CrossRef] [PubMed]
- Palafox-Carlos, H.; Yahia, E.M.; González-Aguilar, G.A. Identification and quantification of major phenolic compounds from mango (Mangifera indica, cv. Ataulfo) fruit by HPLC–DAD–MS/MS-ESI and their individual contribution to the antioxidant activity during ripening. Food Chem. 2012, 135, 105–111. [Google Scholar] [CrossRef]
- Sáyago-Ayerdi, S.G.; Moreno-Hernández, C.L.; Montalvo-González, E.; García-Magaña, M.L.; de Oca, M.M.-M.; Torres, J.L.; Pérez-Jiménez, J. Mexican ‘Ataulfo’mango (Mangifera indica L.) as a source of hydrolyzable tannins. Analysis by MALDI-TOF/TOF MS. Food Res. Int. 2013, 51, 188–194. [Google Scholar] [CrossRef]
- Schieber, A.; Berardini, N.; Carle, R. Identification of flavonol and xanthone glycosides from mango (Mangifera indica L. cv. “Tommy Atkins”) peels by high-performance liquid chromatography-electrospray ionization mass spectrometry. J. Agric. Food Chem. 2003, 51, 5006–5011. [Google Scholar] [CrossRef]
- Sánchez, G.M.; Re, L.; Giuliani, A.; Nunez-Selles, A.J.; Davison, G.P.; Leon-Fernandez, O.S. Protective effects of Mangifera indica L. extract, mangiferin and selected antioxidants against TPA-induced biomolecules oxidation and peritoneal macrophage activation in mice. Pharmacol. Res. 2000, 42, 565–573. [Google Scholar] [CrossRef]
- Bhatia, H.S.; Candelario-Jalil, E.; de Oliveira, A.C.P.; Olajide, O.A.; Martínez-Sánchez, G.; Fiebich, B.L. Mangiferin inhibits cyclooxygenase-2 expression and prostaglandin E2 production in activated rat microglial cells. Arch. Biochem. Biophys. 2008, 477, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Donovan, J.L. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radic. Res. 2004, 38, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Shen, X.; Shoji, T.; Kanda, T.; Zhou, J.; Zhao, L. Characterization and activity of anthocyanins in Zijuan tea (Camellia sinensis var. kitamura). J. Agric. Food Chem. 2013, 61, 3306–3310. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Macià, A.; Romero, M.P.; Motilva, M.J.; Rubió, L. Application of in vitro gastrointestinal digestion and colonic fermentation models to pomegranate products (juice, pulp and peel extract) to study the stability and catabolism of phenolic compounds. J. Funct. Foods 2015, 14, 529–540. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.; Quirós-Sauceda, A.; Mercado-Mercado, G.; Ayala-Zavala, J.F.; Astiazarán-García, H.; Robles-Sánchez, R.M.; Wall-Medrano, A.; Sayago-Ayerdi, S.; González-Aguilar, G.A. Effect of dietary fiber on the bioaccessibility of phenolic compounds of mango, papaya and pineapple fruits by an in vitro digestion model. Food Sci. Technol. 2016, 36, 188–194. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef]
- Pekkinen, J.; Rosa, N.N.; Savolainen, O.-I.; Keski-Rahkonen, P.; Mykkänen, H.; Poutanen, K.; Micard, V.; Hanhineva, K. Disintegration of wheat aleurone structure has an impact on the bioavailability of phenolic compounds and other phytochemicals as evidenced by altered urinary metabolite profile of diet-induced obese mice. Nutr. Metab. (Lond.) 2014, 11, 1. [Google Scholar] [CrossRef]
- Ariza, M.T.; Reboredo-Rodríguez, P.; Mazzoni, L.; Forbes-Hernández, T.Y.; Giampieri, F.; Afrin, S.; Gasparrini, M.; Soria, C.; Martínez-Ferri, E.; Battino, M.; et al. Strawberry Achenes Are an Important Source of Bioactive Compounds for Human Health. Int. J. Mol. Sci. 2016, 17, 1103. [Google Scholar] [CrossRef]
- Schulz, M.; Biluca, F.C.; Gonzaga, L.V.; Borges, G.d.S.C.; Vitali, L.; Micke, G.A.; de Gois, J.S.; de Almeida, T.S.; Borges, D.L.G.; Miller, P.R.M.; et al. Bioaccessibility of bioactive compounds and antioxidant potential of juçara fruits (Euterpe edulis Martius) subjected to in vitro gastrointestinal digestion. Food Chem. 2017, 228, 447–454. [Google Scholar] [CrossRef]
- Bensadón, S.; Hervert-Hernández, D.; Sáyago-Ayerdi, S.G.; Goñi, I. By-Products of Opuntia ficus-indica as a Source of Antioxidant Dietary Fiber. Plant Foods Hum. Nutr. 2010, 65, 210–216. [Google Scholar] [CrossRef]
- Mercado-Mercado, G.; Blancas-Benitez, F.J.; Velderrain-Rodríguez, G.R.; Montalvo-González, E.; González-Aguilar, G.A.; Alvarez-Parrilla, E.; Sáyago-Ayerdi, S.G. Bioaccessibility of polyphenols released and associated to dietary fibre in calyces and decoction residues of Roselle (Hibiscus sabdariffa L.). J. Funct. Foods 2015, 18, 171–181. [Google Scholar] [CrossRef]
- Cuervo, A.; Valdés, L.; Salazar, N.; de los Reyes-Gavilán, C.G.; Ruas-Madiedo, P.; Gueimonde, M.; González, S. Pilot study of diet and microbiota: Interactive associations of fibers and polyphenols with human intestinal bacteria. J. Agric. Food Chem. 2014, 62, 5330–5336. [Google Scholar] [CrossRef] [PubMed]
- Koubala, B.B.; Kansci, G.; Mbome, L.I.; Crépeau, M.-J.; Thibault, J.-F.; Ralet, M.-C. Effect of extraction conditions on some physicochemical characteristics of pectins from “Améliorée” and “Mango” mango peels. Food Hydrocoll. 2008, 22, 1345–1351. [Google Scholar] [CrossRef]
- Siller-sánchez, A.; Alvarez-pérez, O.B.; Aguilar, C.N. Polifenoles de Cáscara de Mango (Mangifera caesia var. Ataulfo): Una Alternativa Antioxidante y Antimicrobiana. Polyphenols from Mango Peels (Mangifera caesia var. Ataulfo): An Antioxidant and Antimicrobial Alternative; Revista Científica; University Autónoma Coahuila: Saltillo, Coahuila, Mexico, 2013; Volume 1, pp. 8–11. [Google Scholar]
- Williamson, G.; Clifford, M.N. Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols. Biochem. Pharmacol. 2017, 139, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
- González-Aguilar, G.A.; Blancas-Benitez, F.J.; Sáyago-Ayerdi, S.G. Polyphenols associated with dietary fibers in plant foods: Molecular interactions and bioaccessibility. Curr. Opin. Food Sci. 2017, 13, 84–88. [Google Scholar] [CrossRef]
- Jeong, J.-J.; Jang, S.-E.; Hyam, S.R.; Han, M.J.; Kim, D.-H. Mangiferin ameliorates colitis by inhibiting IRAK1 phosphorylation in NF-κB and MAPK pathways. Eur. J. Pharmacol. 2014, 740, 652–661. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Díaz-Rubio, M.E. Polyphenols associated with dietary fibre in wine. A wine Polyphenols gap? Food Res. Int. 2007, 40, 613–619. [Google Scholar] [CrossRef]
- Wu, P.; Bhattarai, R.R.; Dhital, S.; Deng, R.; Chen, X.D.; Gidley, M.J. In vitro digestion of pectin- and mango-enriched diets using a dynamic rat stomach-duodenum model. J. Food Eng. 2017, 202, 65–78. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Ye, H.; Liu, J.; Feng, P.; Zhu, W.; Mao, S. Grain-rich diets altered the colonic fermentation and mucosa-associated bacterial communities and induced mucosal injuries in goats. Sci. Rep. 2016, 6, 20329. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Bindels, L.B.; Porporato, P.; Dewulf, E.M.; Verrax, J.; Neyrinck, A.M.; Martin, J.C.; Scott, K.P.; Calderon, P.B.; Feron, O.; Muccioli, G.G. Gut microbiota-derived propionate reduces cancer cell proliferation in the liver. Br. J. Cancer 2012, 107, 1337. [Google Scholar] [CrossRef]
- O’Keefe, S.J.D. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 691–706. [Google Scholar] [CrossRef]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef]
- Gonthier, M.-P.; Remesy, C.; Scalbert, A.; Cheynier, V.; Souquet, J.-M.; Poutanen, K.; Aura, A.-M. Microbial metabolism of caffeic acid and its esters chlorogenic and caftaric acids by human faecal microbiota in vitro. Biomed. Pharmacother. 2006, 60, 536–540. [Google Scholar] [CrossRef]
- Hügel, H.M.; Jackson, N.; May, B.; Zhang, A.L.; Xue, C.C. Polyphenol protection and treatment of hypertension. Phytomedicine 2016, 23, 220–231. [Google Scholar] [CrossRef]
- Li, J.; Liu, M.; Yu, H.; Wang, W.; Han, L.; Chen, Q.; Ruan, J.; Wen, S.; Zhang, Y.; Wang, T. Mangiferin improves hepatic lipid metabolism mainly through its metabolite-norathyriol by modulating SIRT-1/AMPK/SREBP-1c signaling. Front. Pharmacol. 2018, 9, 201. [Google Scholar] [CrossRef]
- Lin, H.; Tu, C.; Niu, Y.; Li, F.; Yuan, L.; Li, N.; Xu, A.; Gao, L.; Li, L. Dual actions of norathyriol as a new candidate hypouricaemic agent: Uricosuric effects and xanthine oxidase inhibition. Eur. J. Pharmacol. 2019, 853, 371–380. [Google Scholar] [CrossRef]
- Low, D.Y.; Hodson, M.P.; Williams, B.A.; D’Arcy, B.R.; Gidley, M.J. Microbial biotransformation of polyphenols during in vitro colonic fermentation of masticated mango and banana. Food Chem. 2016, 207, 214–222. [Google Scholar] [CrossRef]
- Serra, A.; MacIà, A.; Romero, M.P.; Reguant, J.; Ortega, N.; Motilva, M.J. Metabolic pathways of the colonic metabolism of flavonoids (flavonols, flavones and flavanones) and phenolic acids. Food Chem. 2012, 130, 383–393. [Google Scholar] [CrossRef]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.S.; Moore, K.P.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef]
- Verzelloni, E.; Pellacani, C.; Tagliazucchi, D.; Tagliaferri, S.; Calani, L.; Costa, L.G.; Brighenti, F.; Borges, G.; Crozier, A.; Conte, A.; et al. Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites. Mol. Nutr. Food Res. 2011, 55, 35–43. [Google Scholar] [CrossRef]




| Nutritional Composition (g/100 g DW) | |
|---|---|
| Moisture | 8.33 ± 0.22 |
| Protein 2 | 1.69 ± 0.13 |
| Fat | 0.45 ± 0.01 |
| Ash | 2.95 ± 0.09 |
| TSC 3 | 51.98 ± 0.61 |
| TDF 4 | 31.85 ± 0.22 |
| SDF | 14.38 ± 0.15 |
| IDF | 16.94 ± 0.11 |
| TSP (g GAE/100 g sample DW) | 14.35 ± 0.70 |
| AOX (μmol TE/g sample DW) | |
| ABTS | 314.00 ± 1.43 |
| FRAP | 201.03 ± 20.1 |
| HP (g GAE/g sample DW) | 5.43 ± 0.26 |
| Compound | RT (min) | M/Z (-) | Relative Abundance (%) 2 |
|---|---|---|---|
| Phenolic acids | |||
| Gallic acid | 3.74 | 169 | 0.46 |
| Cinnamic acids | |||
| Coumaric acid | 3.839 | 168 | 2.10 |
| Ferulic acid | 4.043 | 193 | 9.72 |
| Caffeic acid | 4.153 | 179 | 0.61 |
| Flavonoids | |||
| Quercetin | 3.914 | 301 | 2.71 |
| Xanthones | |||
| Mangiferin gallate | 4.287 | 573 | 3.27 |
| Mangiferin | 14.269 | 421 | 81.13 |
| g/100 g DW | |
|---|---|
| GasF (g/100 g DW) | |
| TSP (g GAE/100 g DW) | 16.79 ± 0.03 a |
| PCs profile (MS area) 1 | |
| Gallic acid | 1.90 |
| 2-Hydroxycinnamic acid | 0.30 |
| Ferulic acid | 2.33 |
| Caffeic acid | 0.10 |
| Mangiferin | 8.67 |
| Kaempferol | 84.30 |
| p-Coumaric acid | 1.82 |
| Quercetin | 0.58 |
| AOX (μmol TE/100 g DW) | |
| ABTS | 470.77 ± 0.02 c |
| FRAP | 22.73 ± 0.08 d |
| IntF (g/100 g DW) | |
| TSP (g GAE/100 g DW) | 15.32 ± 0.19 b |
| PCs (relative abundance% 1) | |
| Gallic acid | 5.57 |
| 2-Hydroxycinnamic acid | 21.72 |
| Ferulic acid | 1.08 |
| Caffeic acid | 0.65 |
| Mangiferin | 44.16 |
| Kaempferol | 18.59 |
| p-Coumaric acid | 5.18 |
| Quercetin | 3.05 |
| AOX (μmol TE/100 g DW) | |
| ABTS | 469.98 ± 0.01 c |
| FRAP | 14.54 ± 0.08 d |
| Bioaccessibility of PCs (%) | 53.78 ± 0.03 |
| TIF (g/100 g DW) | 38.72 ± 2.18 |
| SIF (g/100 g DW) | 24.44 ± 0.85 |
| TSP (g GAE/100 g DW) | 41.86 ± 0.10 a |
| PCs profile (relative abundance% 1) | |
| Gallic acid | 9.51 |
| 2-Hydroxycinnamic acid | 19.98 |
| Ferulic acid | ND |
| Caffeic acid | 0.32 |
| Mangiferin | 16.78 |
| Kaempferol | 50.73 |
| p-Coumaric acid | 1.25 |
| Quercetin | 1.43 |
| AOX (μmol TE/100 g DW) | |
| ABTS | 117.50 ± 0.02 a |
| FRAP | 6.15 ± 0.06 a |
| IIF (g/100 g DW) | 14.28 ± 1.35 |
| TSP (g GAE/100 g DW) | 60.98 ± 0.14 b |
| PCs profile (relative abundance% 1) | |
| Gallic acid | ND |
| 2-Hydroxycinnamic acid | ND |
| Ferulic acid | 5.65 |
| Caffeic acid | ND |
| Mangiferin | 4.86 |
| Kaempferol | 85.34 |
| p-Coumaric acid | 1.61 |
| Quercetin | 2.54 |
| AOX (μmol TE/100 g DW) | |
| ABTS | 118.21 ± 0.01 b |
| FRAP | 11.87 ± 0.01 b |
| Non-bioaccessible PCs fraction (%) | 46.22 ± 0.03 |
| SCFA | Time (h) | Raffinose mmol/L | TIF-MB mmol/L |
|---|---|---|---|
| Acetic acid | 6 | 62.60 ± 4.31 a,D | 19.75 ± 4.78 a,B |
| 12 | 193.84 ± 30.74 a,C | 25.68 ± 1.45 b,B | |
| 24 | 465.54 ± 48.85 a,B | 146.62 ± 52.87 b,A | |
| 48 | 1295.40 ± 170.86 a,A | 144.82 ± 25.61 b,A | |
| Propionic acid | 6 | ND a,D | ND a,C |
| 12 | 3.21 ± 0.84 a,C | 2.38 ± 0.89 a,B | |
| 24 | 27.05 ± 4.82 a,B | 11.10 ± 2.71 b,A | |
| 48 | 52.31 ± 1.05 a,A | 5.13 ± 1.50 b,A,B | |
| Butyric acid | 6 | 0.12 ± 0.00 a,C | ND a,D |
| 12 | 1.62 ± 0.24 a,B | 0.50 ± 0.03 b,C | |
| 24 | 4.38 ± 0.70 a,A | 3.04 ± 0.06 a,B | |
| 48 | 5.24 ± 1.94 a,A | 9.71 ± 1.32 a,A |
| Compound | RT (min) | m/z (-) | Relative Abundance 1 | |||
|---|---|---|---|---|---|---|
| 6 h | 12 h | 24 h | 48 h | |||
| Hydroxybenzoic acids | ||||||
| Gallic acid | 6.33 | 169 | 77.7 a | 18.1 b | 3.0 c | 1.2 c |
| Hydroxycinnamic acids | ||||||
| Ferulic acid | 3.91 | 193 | 93.3 a | 6.7 b | ND | ND |
| Coumaric acid | 4.33 | 163 | ND | 100.0 | ND | ND |
| Chlorogenic acid | 4.38 | 353 | 79.4 a | 20.6 b | ND | ND |
| Flavonoids | ||||||
| Quercetin | 19.64 | 301 | 100.0 | ND | ND | ND |
| Catechin | 4.59 | 289 | 61.7 a | 16.3 b | 22.0 b | ND |
| Galocatechin | 3.94 | 305 | 100.0 a | ND | ND | ND |
| Galocatechin galate | 12.41 | 457 | 93.4 a | 6.6 b | ND | ND |
| Xanthones | ||||||
| Mangiferin | 14.39 | 421 | 68.5 a | 31.5 b | ND | ND |
| Norathyriol | 11.34 | 259 | 87.7 a | 12.3 b | ND | ND |
| Hydroxyphenolic acids | ||||||
| 3-(3,4)-Dihydroxyphenylpropionic acid | 5.25 | 181 | ND | 100.0 | ND | ND |
| 3-(4-Hydroxyphenyl)propionic acid | 4.01 | 165 | 100.0 | ND | ND | ND |
| 3,4-Dihydroxyphenylacetic acid | 10.81 | 167 | ND | 43.2 a | 36.3 a | 20.5 a |
| 4-Hydroxyphenylacetic acid | 12.23 | 151 | 27.5 a | 8.8 b | 33.8 a | 29.9 a |
| 4-Hydroxybenzoic acid | 11.29 | 137 | 24.5 a | 38.4 a | 37.1 a | ND |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Maldonado, L.M.; Blancas-Benítez, F.J.; Zamora-Gasga, V.M.; Cárdenas-Castro, A.P.; Tovar, J.; Sáyago-Ayerdi, S.G. In Vitro Gastrointestinal Digestion and Colonic Fermentation of High Dietary Fiber and Antioxidant-Rich Mango (Mangifera indica L.) “Ataulfo”-Based Fruit Bars. Nutrients 2019, 11, 1564. https://doi.org/10.3390/nu11071564
Hernández-Maldonado LM, Blancas-Benítez FJ, Zamora-Gasga VM, Cárdenas-Castro AP, Tovar J, Sáyago-Ayerdi SG. In Vitro Gastrointestinal Digestion and Colonic Fermentation of High Dietary Fiber and Antioxidant-Rich Mango (Mangifera indica L.) “Ataulfo”-Based Fruit Bars. Nutrients. 2019; 11(7):1564. https://doi.org/10.3390/nu11071564
Chicago/Turabian StyleHernández-Maldonado, Luz M., Francisco J. Blancas-Benítez, Victor M. Zamora-Gasga, Alicia P. Cárdenas-Castro, Juscelino Tovar, and Sonia G. Sáyago-Ayerdi. 2019. "In Vitro Gastrointestinal Digestion and Colonic Fermentation of High Dietary Fiber and Antioxidant-Rich Mango (Mangifera indica L.) “Ataulfo”-Based Fruit Bars" Nutrients 11, no. 7: 1564. https://doi.org/10.3390/nu11071564
APA StyleHernández-Maldonado, L. M., Blancas-Benítez, F. J., Zamora-Gasga, V. M., Cárdenas-Castro, A. P., Tovar, J., & Sáyago-Ayerdi, S. G. (2019). In Vitro Gastrointestinal Digestion and Colonic Fermentation of High Dietary Fiber and Antioxidant-Rich Mango (Mangifera indica L.) “Ataulfo”-Based Fruit Bars. Nutrients, 11(7), 1564. https://doi.org/10.3390/nu11071564