Natural Hydrogen Sulfide Donors from Allium sp. as a Nutraceutical Approach in Type 2 Diabetes Prevention and Therapy
Abstract
:1. Introduction
2. Therapeutic Potential of Nutraceuticals Consumed in Type 2 DM
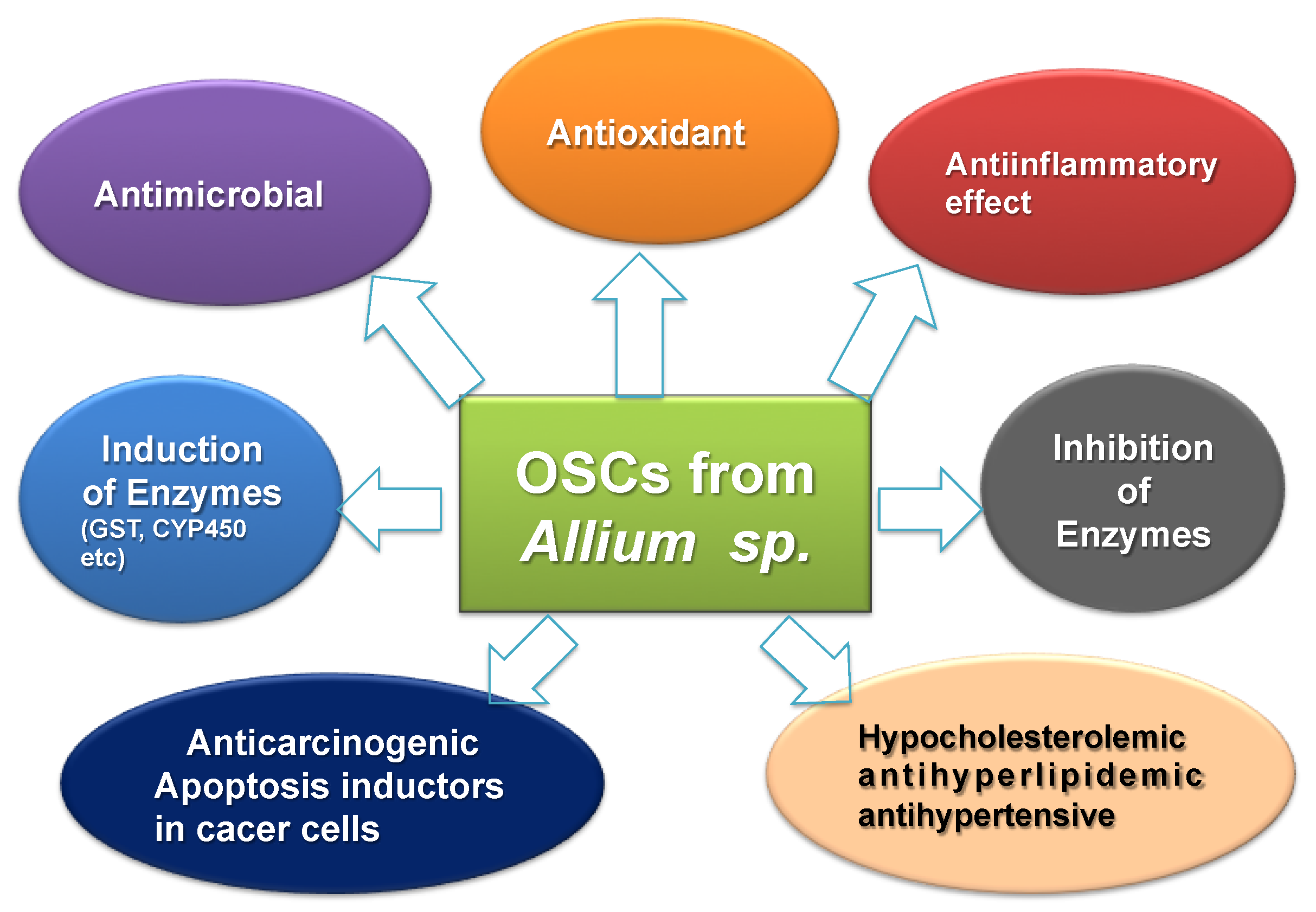
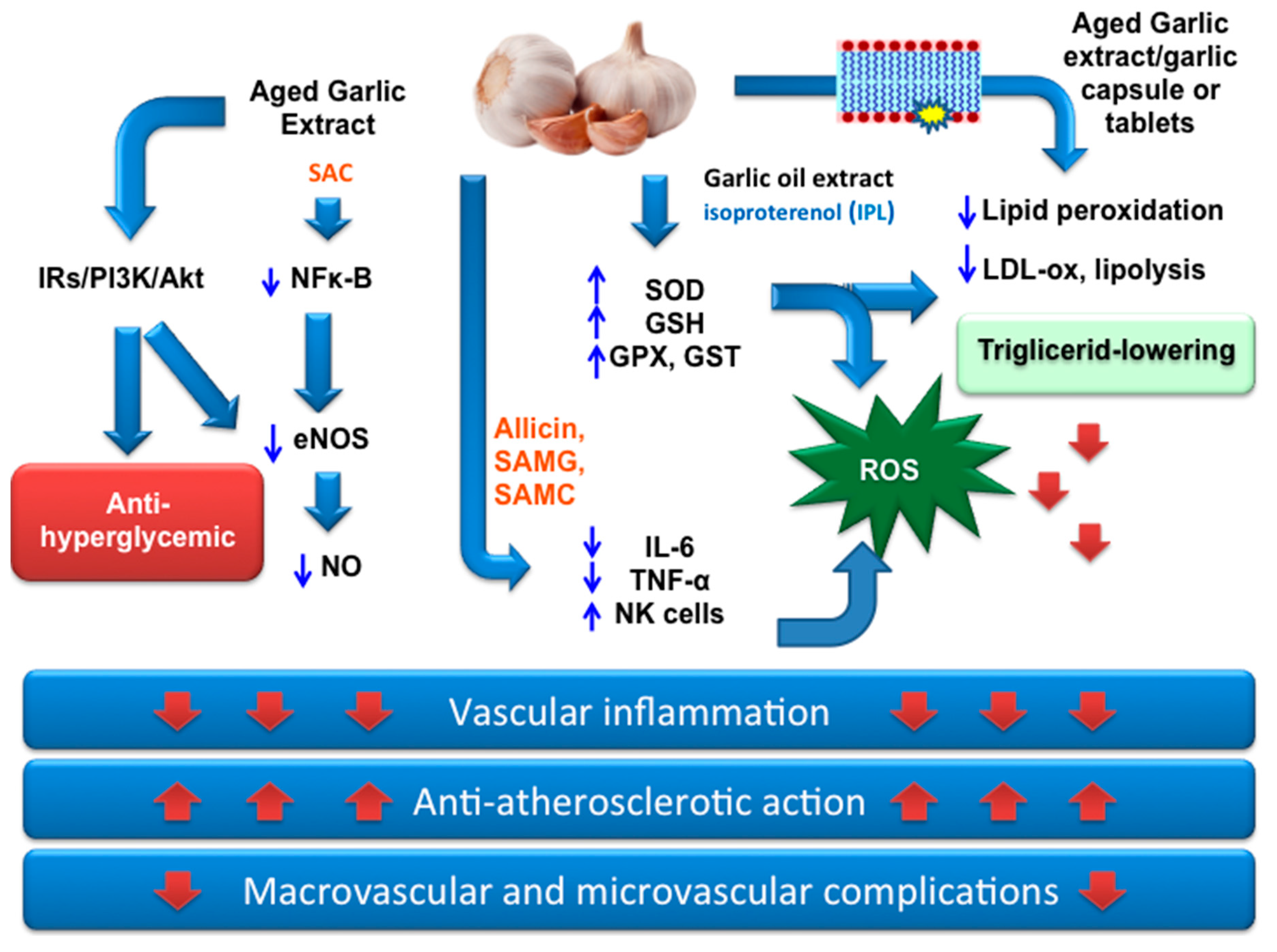
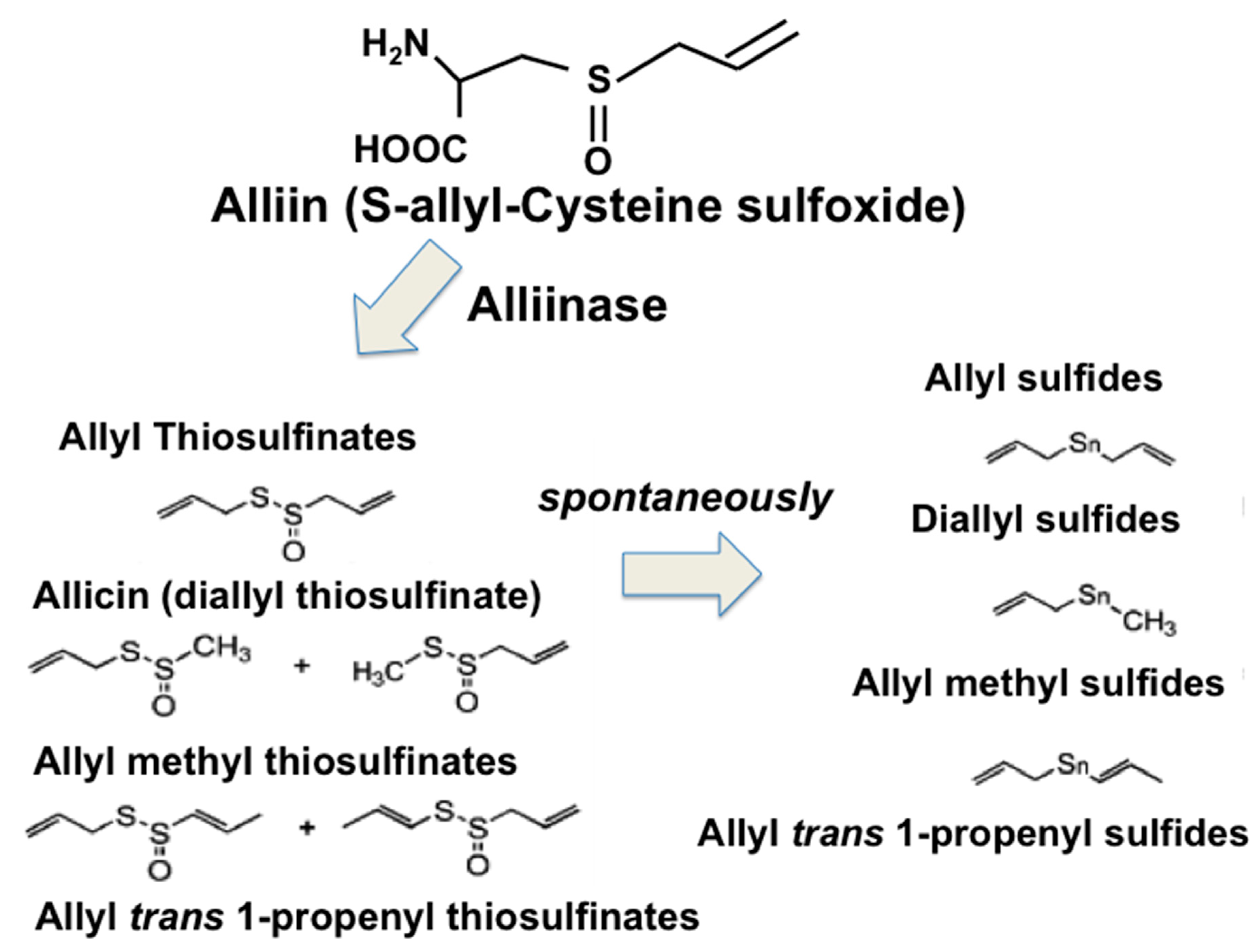
3. OSCs from Garlic as Nutraceuticals for Prevention and Therapy in Type 2 DM
4. H2S-Releasing Agents for Prevention and a Therapeutic Approach in Type 2 DM
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-MP: 3-mercaptopyruvate | CD95: Cluster of differentiation 95 |
| ADA: American Diabetes Association | CPSSA: S-allyl-mercapto-captopril |
| AMS: allyl methyl sulfide | CRP: C-reactive protein |
| Ang-1: angiopoietin-1 | CSE: cystathionine γ-lyase |
| ARE: antioxidant response elements | CTGF: connective tissue growth factor; |
| Bcl2: B-cell lymphoma 2 | DADS: diallyl disulfide; |
| BMI: Body mass index | DAS: diallyl monosulfide; |
| CBS: cystathionine β-synthase | FDPS: Finnish Diabetes Prevention Study |
| DATES: diallyl tetrasulfide | GIP: Glucose-dependent insulinotropic polypeptide |
| DATS: diallyl trisulfide | GK: glucokinase |
| DM: diabetes mellitus | GLUT2: Glucose transporter-2 |
| DPP: Diabetes Prevention Program | GLUT4: Glucose transporter-4 |
| ECs: vascular endothelial cells | GPx: Glutathione peroxidase |
| ECGC: epigallocatechin-3-gallate | GSH: reduced Glutathione |
| eNOS: endothelial nitric oxide synthase | GSSH: glutathione persulfide |
| EPC: endothelial progenitor cell | GST: Glutathione-S-transferase |
| FBG: Fast blood glucose | H2S: Hydrogen sulfide |
| HbA1c: Glycated hemoglobin | MSNs: mesoporous silica nanoparticles |
| HDL: Hight density lipoprotein | MST: 3-mercaptopyruvate sulfurtransferase |
| ICa-L: L-type calcium current | NK cells: Natural killer cells |
| IK1: inward rectifier potassium current | NO: Nitric oxide |
| IL-6: Interleukin 6 | TGF- β1: transforming growth factor β1 |
| INS-1E: Insulinoma cell line 1E | Nrf2: nuclear factor erythroid 2-related factor 2 |
| IRs: Insulin Receptors | OSCs: organosulfur compounds |
| LDL: Light density lipoprotein | PUFAs: Polyunsaturated fatty acids |
| MIN6: mouse insulinoma cell line 6 | ROS: Reactive oxygen species |
| p38 MAPK: p38 mitogen-activated protein kinases | SAC: S-allyl cysteine |
| PFM: polylactic fibrous membranes | SAMC: S-allylmercaptocysteine |
| PLP: pyridoxal 5’-phosphate | SAMG: S-allylmercaptoglutatione |
| PPARs: peroxisome proliferator-activated receptors | TNF-α: Tumor necrosis factor |
| PPBG: Postprandial blood glucose | VEGF: Vascular endothelial growth factor |
| SGLT1: Sodium glucose transporter protein 1 | VLDL: Very low density lipoprotein |
| SOD: Superoxide dismutase | WHO: World Health Organization. |
| TG: Triglyceride | |
| AMPK: activating 5-adenosine monophosphate-activated protein kinase | |
| P13k/Akt: phosphoinositide-3-kinase/ Protein Kinase B | |
| p-ERK1/2: phosphorylated extracellular signal–regulated kinases 1/2 | |
| NF-κB: nuclear factor kappa light chain enhancer of activated B cells | |
| EASD: European Association for the Study of Diabetes | |
| NOX: nicotinamide adenine dinucleotide phosphate oxidase | |
| HIT-T15: insulin release from a cloned hamster B-cell line | |
References
- Akkati, S.; Sam, K.G.; Tungha, G. Emergence of promising therapies in diabetes mellitus. J. Clin. Pharmacol. 2011, 51, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Aronson, D. Hyperglycemia and the pathobiology of diabetic complications. Adv. Cardiol. 2008, 45, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Baynes, J.W.; Thorpe, S.R. Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes 1999, 48, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B., 3rd. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef] [PubMed]
- López-Candales, A. Metabolic syndrome X: A comprehensive review of the pathophysiology and recommended therapy. J. Med. 2001, 32, 283–300. [Google Scholar] [PubMed]
- Ritz, E.; Rychlík, I.; Locatelli, F.; Halimi, S. End-stage renal failure in type 2 diabetes: A medical catastrophe of worldwide dimensions. Am. J. Kidney Dis. 1999, 34, 795–808. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Gakidou, E.; Mallinger, L.; Abbott-Klafter, J.; Guerrero, R.; Villalpando, S.; Ridaura, R.L.; Aekplakorn, W.; Naghavi, M.; Lim, S.; Lozano, R.; et al. Management of diabetes and associated cardiovascular risk factors in seven countries: A comparison of data from national health examination surveys. Bull. World Health Organ. 2011, 89, 172–183. [Google Scholar] [CrossRef]
- Golbidi, S.; Badran, M.; Laher, I. Antioxidant and Anti-Inflammatory Effects of Exercise in Diabetic Patients. Exp. Diabetes Res. 2012, 2012, 1–16. [Google Scholar] [CrossRef]
- Eriksson, K.F.; Lindgarde, F. Prevention of Type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise. The 6-year Malmo feasibility study. Diabetologia 1991, 34, 891–898. [Google Scholar] [CrossRef]
- Pan, X.; Li, G.; Hu, Y.; Wang, J.X.; Yang, W.Y.; An, Z.X.; Hu, Z.X.; Lin, J.; Xiao, J.Z.; Cao, H.B.; et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Tuomilehto, J.; Lindstrom, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of Type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Reduction in the incidence of Type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Snehalatha, C.; Mary, S.; Mukesh, B.; Bhaskar, A.D.; Vijay, V. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent Type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006, 49, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Orozco, L.J.; Buchleitner, A.M.; Gimenez-Perez, G.; Roque, I.F.M.; Richter, B.; Mauricio, D. Exercise or exercise and diet for preventing Type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2008, 16. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of Hyperglycemia in Type 2 Diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef] [PubMed]
- Marín-Peñalver, J.J.; Martín-Timón, I.; Sevillano-Collantes, C.; Cañizo-Gómez, F.J. Update on the treatment of type 2 diabetes mellitus. World J. Diabetes 2016, 7, 354–395. [Google Scholar] [CrossRef] [PubMed]
- Kar, A.; Choudhary, B.K.; Bandyopadhyay, N.G. Comparative evaluation of hypoglycaemic activity of some Indian medicinal plants in alloxan diabetic rats. J. Ethnopharmacol. 2003, 84, 105–108. [Google Scholar] [CrossRef]
- Heinrich, M.; Barnes, J.; Gibbons, S.; Williamson, E. Fundamentals of Pharmacognosy and Phytotherapy, 2nd ed.; Elsevier: Atlanta, GA, USA, 2012; ISBN 9780702033889. [Google Scholar]
- Evans, W.C. Trease and Evans Pharmacognosy, 16th ed.; Saunders: Philadelphia, PA, USA, 2009; ISBN-13: 978-0702029332. [Google Scholar]
- Cusi, K.; DeFronzo, R.A. Metformin: A review of its metabolic effects. Diabetes Rev. 1998, 6, 89–131. [Google Scholar]
- Evans, J.L.; Bahng, M.K. Non-pharmaceutical Intervention Options for type 2 Diabetes: Diets and Dietary Supplements (Botanicals, Antioxidants, and Minerals). In Diabetes Mellitus and Carbohydrate Metabolism; MDText.com, Inc.: South Dartmouth, MA, USA, 2014; Volume 16, pp. 1–13. [Google Scholar]
- Modak, M.; Dixit, P.; Londhe, J.; Ghaskadbi, S.; Devasagayam, T.P. Indian herbs and herbal drugs used for the treatment of diabetes. J. Clin. Biochem. Nutr. 2007, 40, 163–173. [Google Scholar] [CrossRef]
- Marles, R.J.; Farnsworth, N.R. Antidiabetic plants and their active constituents. Phytomedicine 1995, 2, 137–189. [Google Scholar] [CrossRef]
- Ota, A.; Ulrih, N.P. An Overview of Herbal Products and Secondary Metabolites Used for Management of Type Two Diabetes. Front. Pharmacol. 2017, 8, 436. [Google Scholar] [CrossRef] [PubMed]
- Governa, P.; Baini, G.; Borgonetti, V.; Cettolin, G.; Giachetti, D.; Rosa Magnano, A.; Miraldi, E.; Biagi, M. Phytotherapy in the Management of Diabetes: A Review. Molecules 2018, 23, 105. [Google Scholar] [CrossRef] [PubMed]
- Cefalu, W.T.; Ye, J.; Zuberi, A.; Ribnicky, D.M.; Raskin, I.; Liu, Z.; Wang, Z.Q.; Brantley, P.J.; Howard, L.; Lefevre, M. Botanicals and the metabolic syndrome. Am. J. Clin. Nutr. 2008, 87, 481S–487S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghorbani, A. Best herbs for managing diabetes: A review of clinical studies. Braz. J. Pharm. Sci. 2013, 49, 413–422. [Google Scholar] [CrossRef]
- Cefalu, W.T.; Stephens, J.M.; Ribnicky, D.M. Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; p. 9. [Google Scholar]
- Rios, J.L.; Francini, F.; Schinella, G.R. Natural products for the treatment of type 2 Diabetes mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Hua, C.K.; Mun, C.S.; Jing, J.K.; Kong, L.; Ern, L.Y.; Ashraf, N.A.; Kit, S.W.; Yee, T.S.; et al. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J. Tradit. Complement. Med. 2018, 8, 361–376. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef]
- Han, X.; Loa, T. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Song, Y.; Manson, J.E.; Buring, J.E.; Sesso, H.D.; Liu, S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: A prospective study and cross-sectional analysis. J. Am. Coll. Nutr. 2005, 24, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.B.; Högger, P. Dietary polyphenols and Type 2 diabetes: Current insights and future perspectives. Curr. Med. Chem. 2015, 22, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Sales, P.M.; Souza, P.M.; Simeoni, L.A.; Silveira, D. α-Amylase inhibitors: A review of raw material and isolated compounds from plant source. J. Pharm. Pharm. Sci. 2012, 15, 141–183. [Google Scholar] [CrossRef] [PubMed]
- Shori, A.B. Screening of antidiabetic and antioxidant activities of medicinal plants. J. Integr. Med. 2015, 13, 297–305. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and Glycemic Control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Solayman, M.; Ali, Y.; Alam, F.; Islam, M.A.; Alam, N.; Khalil, M.I.; Gan, S.H. Polyphenols: Potential Future Arsenals in the Treatment of Diabetes. Curr. Pharm. Des. 2016, 22, 549–565. [Google Scholar] [CrossRef]
- Yin, P.; Zhao, S.; Chen, S.; Liu, J.; Shi, L.; Wang, X.; Liu, Y.; Ma, C. Hypoglycemic and hypolipidemic effects of polyphenols from burs of Castanea mollissima Blume. Molecules 2011, 16, 9764–9774. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, M.; Xie, Q.; Xu, B.; Sun, C.; Chen, K.; Wu, Y. Anthocyanins from Chinese bayberry extract protect β cells from oxidative stress-mediated injury via HO-1 upregulation. J. Agric. Food Chem. 2011, 59, 537–545. [Google Scholar] [CrossRef]
- Liu, Z.M.; Chen, Y.M.; Ho, S.C. Effects of soy intake on glycemic control: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2011, 93, 1092–1101. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, W.; Zhen, W.; Lum, H.; Nadler, J.; Bassaganya-Riera, J.; Jia, Z.; Wang, Y.; Misra, H.; Liu, D.; et al. Genistein induces pancreatic beta-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology 2010, 151, 3026–3037. [Google Scholar] [CrossRef] [PubMed]
- Priscilla, D.H.; Roy, D.; Suresh, A.; Kumar, V.; Thirumurugan, K. Naringenin inhibits a-glucosidase activity: A promising strategy for the regulation of postprandial hyperglycemia in high fat diet fed streptozotocin induced diabetic rats. Chem. Biol. Interact. 2014, 210, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Su, A.; Yuan, S.; Zhao, H.; Tan, S.; Hu, C.; Deng, H.; Guo, Y. Evaluation of total flavonoids, myricetin, and quercetin from Hovenia dulcis Thunb. as inhibitors of α-amylase and α-glucosidase. Plant Foods Hum. Nutr. 2016, 71, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Ahmed, F.; Banerjee, S.; Saha, U. Naringenin ameliorates streptozotocin-induced diabetic rat renal impairment by downregulation of TGF-b1 and IL-1 via modulation of oxidative stress correlates with decreased apoptotic events. Pharm. Biol. 2016, 54, 1616–1627. [Google Scholar] [CrossRef]
- Jung, U.J.; Lee, M.K.; Park, Y.B.; Kang, M.A.; Choi, M.S. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mrna levels in type-2 diabetic mice. Int. J. Biochem. Cell Biol. 2006, 38, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.H.; Kruhlak, M.; Levine, M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007, 21, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Coskun, O.; Kanter, M.; Korkmaz, A.; Oter, S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and b-cell damage in rat pancreas. Pharmacol. Res. 2005, 51, 117–123. [Google Scholar] [CrossRef]
- Li, B.Y.; Cheng, M.; Gao, H.Q.; Ma, Y.B.; Xu, L.; Li, X.H.; Li, X.L.; You, B.A. Back-regulation of six oxidative stress proteins with grape seed proanthocyanidin extracts in rat diabetic nephropathy. J. Cell. Biochem. 2008, 104, 668–679. [Google Scholar] [CrossRef]
- Cui, X.P.; Li, B.Y.; Gao, H.Q.; Wei, N.; Wang, W.L.; Lu, M. Effects of grape seed proanthocyanidin extracts on peripheral nerves in streptozocin-induced diabetic rats. J. Nutr. Sci. Vitaminol. 2008, 54, 321–328. [Google Scholar] [CrossRef]
- Ortsäter, H.; Grankvist, N.; Wolfram, S.; Kuehn, N.; Sjöholm, A. Diet supplementation with green tea extract epigallocatechin gallate prevents progression to glucose intolerance in db/db mice. Nutr. Metab. 2012, 14, 9–11. [Google Scholar] [CrossRef]
- Wang, C.T.; Chang, H.H.; Hsiao, C.H.; Lee, M.J.; Ku, H.C.; Hu, Y.J.; Kao, Y.H. The effects of green tea (−)-epigallocatechin-3-gallate on reactive oxygen species in 3T3-L1 preadipocytes and adipocytes depend on the glutathione and 67 kDa laminin receptor pathways. Mol. Nutr. Food Res. 2009, 53, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Suzuki, M.; Satsu, H.; Arai, S.; Hara, Y.; Suzuki, K. Green tea polyphenols inhibit the sodium-dependent glucose transporter of intestinal epithelial cells by a competitive mechanism. J. Agric. Food Chem. 2000, 48, 5618–5623. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Bae, J.H.; Im, S.S.; Song, D.K. Green tea and type 2 diabetes. Integr. Med. Res. 2014, 3, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Honma, K.; Yoshinari, O.; Nanjo, F.; Hara, Y. Effects of dietary catechins on glucose tolerance, blood pressure and oxidative status in Goto-Kakizaki rats. J. Nutr. Sci. Vitaminol. 2007, 53, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jin, J.Y.; Baek, W.K.; Park, S.H.; Sung, H.Y.; Kim, Y.K.; Lee, J.; Song, D.K. Ambivalent role of gallated catechins in glucose tolerance in humans: A novel insight into non-absorbable gallated catechin-derived inhibitors of glucose absorption. J. Physiol. Pharmacol. 2009, 60, 101–109. [Google Scholar] [PubMed]
- Ismail, M.Y.M. Clinical evaluation of antidiabetic activity of Trigonella seeds and Aegle marmelos leaves. World Appl. Sci. J. 2009, 7, 1231–1234. [Google Scholar]
- Ismail, M.Y.M. Clinical Evaluation of Antidiabetic Activity of Bael Leaves. World Appl. Sci. J. 2009, 6, 1518–1520. [Google Scholar]
- Sankhla, A.; Sharma, S.; Sharma, N. Hypoglycemic effect of bael leaves (Aegle marmelos) in NIDDM patients. J. Dairy. Food HS 2009, 28, 233–236. [Google Scholar]
- Mathew, P.T.; Augusti, K.T. Hypoglycaemic effects of onion, Allium cepa Linn. on diabetes mellitus—A preliminary report. Indian J. Physiol. Pharmacol. 1975, 19, 213–217. [Google Scholar]
- Eldin, I.M.T.; Ahmed, E.M.; Elwahab, H.M.A. Preliminary Study of the Clinical Hypoglycemic Effects of Allium cepa (Red Onion) in Type 1 and Type 2 Diabetic Patients. Environ. Health Insights 2010, 4, 71–77. [Google Scholar] [CrossRef]
- Bayan, L.; Koulivand, H.P.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar] [PubMed]
- Gautam, S.; Pal, S.; Maurya, R.; Srivastava, A.K. Ethanolic extract of Allium cepa stimulates glucose transporter typ 4-mediated glucose uptake by the activation of insulin signaling. Planta Med. 2015, 81, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Padiya, R.; Banerjee, S.K. Garlic as an anti-diabetic agent: Recent progress and patent reviews. Recent Pat. Food Nutr. Agric. 2013, 5, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Del Villar, M.; Puebla-Pérez, A.M.; Sánchez-Peña, M.J.; González-Ortiz, L.J.; Martínez-Abundis, E.; González-Ortiz, M. Effect of Artemisia dracunculus administration on glycemic control, insulin sensitivity, and insulin secretion in patients with impaired glucose tolerance. J. Med. Food 2016, 19, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.X.; Xu, Y.L.; Li, S.H.; Hui, R.; Wu, Y.J.; Huang, X.H. Effects of green tea catechins with or without caffeine on glycemic control in adults: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2013, 97, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Van Dieren, S.; Uiterwaal, C.S.P.M.; van der Schouw, Y.T.; van der A, D.L.; Boer, J.M.; Spijkerman, A.; Grobbee, D.E.; Beulens, J.W. Coffee and tea consumption and risk of type 2 diabetes. Diabetologia 2009, 52, 2561–2569. [Google Scholar] [CrossRef] [Green Version]
- Iso, H.; Date, C.; Wakai, K.; Fukui, M.; Tamakoshi, A. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann. Intern. Med. 2006, 144, 554–562. [Google Scholar] [CrossRef]
- Khan, A.; Safdar, M.; Ali Khan, M.M.; Khattak, K.N.; Anderson, R.A. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care 2003, 26, 3215–3218. [Google Scholar] [CrossRef]
- Akilen, R.; Tsiami, A.; Devendra, D.; Robinson, N. Cinnamon in glycemic control: Systematic review and meta analysis. Clin. Nutr. 2012, 31, 609–615. [Google Scholar] [CrossRef]
- Subash Babu, P.; Prabuseenivasan, S.; Ignacimuthu, S. Cinnamaldehyde-A potential antidiabetic agent. Phytomedicine 2007, 14, 15–22. [Google Scholar] [CrossRef]
- Munasinghe, M.A.A.K.; Abeysena, C.; Yaddehige, I.S.; Vidanapathirana, T.; Piyumal, K.P.B. Blood sugar lowering effect of Coccinia grandis (L.) J.Voigt: Path for a new drug for diabetes mellitus. Exp. Diabetes Res. 2011, 2011, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kuriyan, R.; Rajendran, R.; Bantwal, G.; Kurpad, A.V. Effect of supplementation of Coccinia cordifolia extract on newly detected diabetic patients. Diabetes Care 2008, 31, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Ludvik, B.; Neuffer, B.; Pacini, G. Efficacy of Ipomoea batatas (Caiapo) on diabetes control in type 2 diabetic subjects treated with diet. Diabetes Care 2004, 27, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Ludvik, B.; Hanefeld, M.; Pacini, G. Improved metabolic control by Ipomoea batatas (Caiapo) is associated with increased adiponectin and decreased fibrinogen levels in type 2 diabetic subjects. Diabetes Obes. Metab. 2008, 10, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Mishra, B.N.; Sangwan, N.S. Phytochemical and Pharmacological Properties of Gymnema sylvestre: An Important Medicinal Plant. Biomed. Res. Int. 2014, 2014, 1–18. [Google Scholar] [CrossRef]
- Kumar, S.N.; Mani, U.V.; Mani, I. An open label study on the supplementation of Gymnema sylvestre in type 2 diabetics. J. Diet. Suppl. 2010, 7, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Kathori, S.; Upadhyay, B. Effect of Gurmar (Gymnema sylvestre) powder intervention on the blood glucose levels among diabetics. Stud. Ethno-Med. 2009, 3, 133–135. [Google Scholar] [CrossRef]
- Al-Romaiyan, A.; Liu, B.; Asare-Anane, H.; Maity, C.R.; Chatterjee, S.K.; Koley, N.; Biswas, T.; Chatterji, A.K.; Huang, G.-C.; Amiel, S.A.; et al. A novel Gymnema sylvestre extract stimulates insulin secretion from human islets in vivo and in vitro. Phytother. Res. 2010, 24, 1370–1376. [Google Scholar] [CrossRef]
- Mani, U.V.; Mani, I.; Biswas, M.; Kumar, S.N. An open-label study on the effect of flax seed powder (Linum usitatissimum) supplementation in the management of diabetes mellitus. J. Diet. Suppl. 2011, 8, 257–265. [Google Scholar] [CrossRef]
- Thakur, G.; Mitra, A.; Pal, K.; Rousseau, D. Effect of flaxseed gum on reduction of blood glucose and cholesterol in type 2 diabetic patients. Int. J. Food Sci. Nutr. 2009, 60, 126–136. [Google Scholar] [CrossRef]
- Rahman, I.; Malik, S.A.; Bashir, M.; Khan, R.; Iqbal, M. Serum sialic acid changes in noninsulin dependant diabetes mellitus (NIDDM) patients following bitter melon (Momordica charantia) and rosiglitazone (Avandia) treatment. Phytomedicine 2009, 16, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Fuangchan, A.; Sonthisombat, P.; Seubnukarn, T.; Chanouan, R.; Chotchaisuwat, P.; Sirigulsatien, V.; Ingkaninan, K.; Plianbangchang, P.; Haines, S.T. Hypoglycemic effect of bitter melon compared with metformin in newly diagnosed type 2 diabetes patients. J. Ethnopharmacol. 2011, 134, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Grover, J.K.; Yadav, S.P. Pharmacological actions and potential uses of Momordica charantia: A review. J. Ethnopharmacol. 2004, 93, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.-J.; Ye, J.-M.; Turner, N.; Hohnen-Behrens, C.; Ke, C.-Q.; Tang, C.-P.; Chen, T.; Weiss, H.-C.; Gesing, E.-R.; Rowland, A.; et al. Antidiabetic activities of triterpenoids isolated from bitter melon associated with activation of the AMPK pathway. Chem. Biol. 2008, 15, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.L.; Huang, H.K.; Chang, C.I.; Tsai, C.P.; Chou, C.H. A cell-based screening identifies compounds from the stem of Momordica charantia that overcome insulin resistance and activate AMP-activated protein kinase. J. Agric. Food Chem. 2008, 56, 6835–6843. [Google Scholar] [CrossRef]
- Rodrigues, E.L.; Marcelino, G.; Silva, G.T.; Figueiredo, P.S.; Garcez, W.S.; Corsino, J.; Guimarães, R.C.A.; Freitas, K.C. Review Nutraceutical and Medicinal Potential of the Morus Species in Metabolic Dysfunctions. Int. J. Mol. Sci. 2019, 20, 301. [Google Scholar] [CrossRef]
- Hwang, S.H.; Li, H.M.; Lim, S.S.; Wang, Z.; Hong, J.S.; Huang, B. Evaluation of a Standardized Extract from Morus alba against α-Glucosidase Inhibitory Effect and Postprandial Antihyperglycemic in Patients with Impaired Glucose Tolerance: A Randomized Double-Blind Clinical Trial. Evid. Based Complement. Altern. Med. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Choi, K.H.; Lee, H.A.; Park, M.H.; Han, J.S. Mulberry (Morus alba L.) Fruit Extract Containing Anthocyanins Improves Glycemic Control and Insulin Sensitivity via Activation of AMP-Activated Protein Kinase in Diabetic C57BL/Ksj-db/db Mice. J. Med. Food 2016, 19, 737–745. [Google Scholar] [CrossRef]
- Agrawali, P.; Rai, V.; Singh, R.B. Randomized placebo-controlled, single blind trial of holy basil leaves in patients with noninsulin-dependent diabetes mellitus. Int. J. Clin. Pharmacol. Ther. 1996, 34, 406–409. [Google Scholar]
- Rai, V.; Mani, U.V.; Iyer, U.M. Effect of Ocimum sanctum leaf powder in blood lipoproteins, glycated proteins and total amino acids in patients with non-insulin-dependent diabetes mellitus. J. Nutr. Environ. Med. 1997, 7, 113–118. [Google Scholar] [CrossRef]
- Kochhar, A.; Sharma, N.; Schdeva, R. Effect of supplementation of tulsi (Ocimum sanctum) and neem (Azadirachta indica) leaf powder on diabetic symptoms, anthropometric parameters and blood pressure of non insulin dependent male diabetics. Stud. Ethno-Med. 2009, 3, 5–9. [Google Scholar] [CrossRef]
- Satapathy, S.; Das, N.; Bandyopadhyay, D.; Mahapatra, S.C.; Sahu, D.S.; Meda, M. Effect of Tulsi (Ocimum sanctum Linn.) Supplementation on Metabolic Parameters and Liver Enzymes in Young Overweight and Obese Subjects. Indian J. Clin. Biochem. 2017, 32, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Leem, K.H.; Kim, M.G.; Hahm, Y.T.; Kim, H.K. Hypoglycemic Effect of Opuntia ficus-indica var. saboten is Due to Enhanced Peripheral Glucose Uptake through Activation of AMPK/p38 MAPK Pathway. Nutrients 2016, 8, 800. [Google Scholar] [CrossRef]
- López-Romero, P.; Pichardo-Ontiveros, E.; Avila-Nava, A.; Vázquez-Manjarrez, N.; Tovar, A.R.; Pedraza-Chaverri, J.; Torrez, N. The effect of nopal (Opuntia ficus indica) on postprandial blood glucose, incretins, and antioxidant activity in Mexican patients with type 2 diabetes after consumption of two different composition breakfasts. J. Acad. Nutr. Diet. 2014, 114, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Frati, A.C.; Gordillo, B.E.; Altamirano, P.; Ariza, C.R.; Cortés-Franco, R.; Chavez-Negrete, A. Acute hypoglycemic effect of Opuntia streptacantha Lemaire in NIDDM. Diabetes Care 1990, 13, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Vuksan, V.; Stavro, M.P.; Sievenpiper, J.L.; Beljan-Zdravkovic, U.; Leiter, L.A.; Josse, R.G.; XU, Z. Similar postprandial glycemic reductions with escalation of dose and administration time of American ginseng in type 2 diabetes mellitus. Diabetes Care 2000, 23, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Sotaniemi, E.A.; Haapakoski, E.; Rautio, A. Ginseng therapy in non-insulin-dependent diabetic patients. Diabetes Care 1995, 18, 1373–1375. [Google Scholar] [CrossRef]
- Jiang, S.; Ren, D.; Li, J.; Yuan, G.; Li, H.; Xu, G.; Han, X.; Du, P.; An, L. Effects of compound K on hyperglycemia and insulin resistance in rats with type 2 diabetes mellitus. Fitoterapia 2014, 95, 58–64. [Google Scholar] [CrossRef]
- Kajimoto, O.K.S.; Shimoda, H.; Kawahara, Y.; Hirata, H.; Takahashi, T. Effects of a diet containing Salacia reticulata on mild type 2 diabetes in humans. A placebo controlled, cross over trial. J. Jpn. Soc. Food Sci. 2000, 53, 199–205. [Google Scholar] [CrossRef]
- Shivaprasad, H.N.; Bhanumathy, M.; Sushma, G.; Midhun, T.; Raveendra, K.R.; Sushma, K.R.; Venkateshwarlu, K. Salacia reticulata improves serum lipid profiles and glycemic control in patients with prediabetes and mild to moderate hyperlipidemia: A double-blind, placebo-controlled, randomized trial. J. Med. Food 2013, 16, 564–568. [Google Scholar] [CrossRef]
- Stohs, S.J.; Ray, S. Anti-diabetic and Anti-hyperlipidemic Effects and Safety of Salacia reticulata and Related Species. Phytother. Res. 2015, 29, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Huseini, H.F.; Larijani, B.; Heshmat, R.; Fakhrzadeh, H.; Radjabipour, B.; Toliat, T.; Raza, M. The efficacy of Silybum marianum (L.) Gaertn. (silymarin) in the treatment of type II diabetes: A randomized, double-blind, placebo-controlled, clinical trial. Phytother. Res. 2006, 20, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Lirussi, F.; Beccarello, A.; Zanette, G.; De Monte, A.; Donadon, V.; Velussi, M.; Crepaldi, G. Silybin-beta-cyclodextrin in the treatment of patients with diabetes mellitus and alcoholic liver disease. Efficacy study of a new preparation of an anti-oxidant agent. Diabetes Nutr. Metab. 2002, 15, 222–231. [Google Scholar] [PubMed]
- Velussi, M.; Cernigoi, A.M.; De Monte, A.; Dapas, F.; Caffau, C.; Zilli, M. Long-term (12 months) treatment with an anti-oxidant drug (silymarin) is effective on hyperinsulinemia, exogenous insulin need and malondialdehyde levels in cirrhotic diabetic patients. J. Hepatol. 1997, 26, 871–879. [Google Scholar] [CrossRef]
- Ebrahimpour Koujan, S.; Gargari, B.P.; Mobasseri, M.; Valizadeh, H.; Asghari-Jafarabadi, M. Effects of Silybum marianum (L.) Gaertn. (silymarin) extract supplementation on antioxidant status and hs-CRP in patients with type 2 diabetes mellitus: A randomized, triple-blind, placebo-controlled clinical trial. Phytomedicine 2015, 22, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Hannan, J.M.A.; Ali, L.; Rokeya, B.; Khaleque, J.; Akhter, M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Soluble dietary fibre fraction of Trigonella foenum-graecum (fenugreek) seed improves glucose homeostasis in animal models of type 1 and type 2 diabetes by delaying carbohydrate digestion and absorption, and enhancing insulin action. Br. J. Nutr. 2007, 97, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, N.; Narayanan, M.; De Souza, R.J.; Van Dam, R.M. Effect of fenugreek (Trigonellafoenum-graecum L.) intake on glycemia: A meta-analysis of clinical trials. Nutr. J. 2014, 13, 7. [Google Scholar] [CrossRef]
- Shidfar, F.; Rajab, A.; Rahideh, T.; Khandouzi, N.; Hosseini, S.; Shidfar, S. The effect of ginger (Zingiber officinale) on glycemic markers in patients with type 2 diabetes. J. Complement. Integr. Med. 2015, 12, 165–170. [Google Scholar] [CrossRef]
- Munday, R. Harmful and beneficial effects of organic monosulfides, disulfides, and polysulfides in animals and humans. Chem. Res. Toxicol. 2012, 25, 47–60. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Potential efficacy of broccoli sprouts as a unique supplement for management of type 2 diabetes and its complications. J. Med. Food 2013, 16, 375–382. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Derosa, G.; Gaddi, A. What do herbalists suggest to diabetic patients in order to improve glycemic control? Evaluation of scientific evidence and potential risks. Acta Diabetol. 2004, 41, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M.; Al-Qattan, K.K.; Divya, J.S.; Ali, M. Anti-diabetic and anti-oxidant potential of aged garlic extract (AGE) in streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2016, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Kwiecieñ, I.; Włodek, L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ. Mol. Mutagen. 2009, 50, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Dirsch, V.M.; Gerbes, A.L.; Vollmar, A.M. Ajoene, a compound of garlic, induces apoptosis in human promyeloleukemic cells, accompanied by generation of reactive oxygen species and activation of nuclear factor kappaB. Mol. Pharmacol. 1998, 53, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Knowles, L.M.; Milner, J.A. Allyl sulfides modify cell growth. Drug Metabol. Drug Interact. 2000, 17, 81–107. [Google Scholar] [CrossRef] [PubMed]
- Lea, M.A. Organosulfur compounds and cancer. Adv. Exp. Med. Biol. 1996, 401, 147–154. [Google Scholar] [PubMed]
- Lea, M.A.; Randolph, V.M.; Patel, M. Increased acetylation of histones induced by diallyl disulfide and structurally related molecules. Int. J. Oncol. 1999, 15, 347–352. [Google Scholar] [CrossRef]
- Li, G.; Qiao, C.; Lin, R.; Pinto, J.; Osborne, M.; Tiwari, R. Antiproliferative effects of garlic constituents in cultured human breast-cancer cells. Oncol. Rep. 1995, 2, 787–791. [Google Scholar] [CrossRef]
- Pinto, J.T.; Qiao, C.; Xing, J.; Rivlin, R.S.; Protomastro, M.L.; Weissler, M.L.; Tao, Y.; Thaler, H.; Heston, W.D. Effects of garlic thioallyl derivatives on growth, glutathione concentration, and polyamine formation of human prostate carcinoma cells in culture. Am. J. Clin. Nutr. 1997, 66, 398–405. [Google Scholar] [CrossRef] [Green Version]
- Pinto, J.T.; Rivlin, R.S. Antiproliferative effects of allium derivatives from garlic. J. Nutr. 2001, 131, 1058S–1060S. [Google Scholar] [CrossRef]
- Sakamoto, K.; Lawson, L.D.; Milner, J.A. Allyl sulfides from garlic suppress the in vitro proliferation of human A549 lung tumor cells. Nutr. Cancer 1997, 29, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Scharfenberg, K.; Wagner, R.; Wagner, K.G. The cytotoxic effect of ajoene, a natural product from garlic, investigated with different cell lines. Cancer Lett. 1990, 53, 103–108. [Google Scholar] [CrossRef]
- Scharfenberg, K.; Ryll, T.; Wagner, R.; Wagner, K.G. Injuries to cultivated BJA-B cells by ajoene, a garlic-derived natural compound: Cell viability, glutathione metabolism, and pools of acidic amino acids. J. Cell. Physiol. 1994, 158, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Sigounas, G.; Hooker, J.L.; Li, W.; Anagnostou, A.; Steiner, M. S-allylmercaptocysteine, a stable thioallyl compound, induces apoptosis in erythroleukemia cell lines. Nutr. Cancer 1997, 28, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, S.G.; Milner, J.A. Impact of organosulfur compounds in garlic on canine mammary tumor cells in culture. Cancer Lett. 1993, 74, 85–90. [Google Scholar] [CrossRef]
- Sundaram, S.G.; Milner, J.A. Diallyl disulfide induces apoptosis of human colon tumor cells. Carcinogenesis 1996, 17, 669–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeyama, H.; Hoon, D.S.; Saxton, R.E.; Morton, D.L.; Irie, R.F. Growth inhibition and modulation of cell markers of melanoma by S-allyl cysteine. Oncology 1993, 50, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.; Wuarin, L.; Sidell, N. Antiproliferative effect of the garlic compound S-allyl cysteine on human neuroblastoma cells in vitro. Cancer Lett. 1992, 63, 211–219. [Google Scholar] [CrossRef]
- Nian, H.; Delage, B.; Pinto, J.T.; Dashwood, R.H. Allyl mercaptan, a garlic-derived organosulfur compound, inhibits histone deacetylase and enhances Sp3 binding on the P21WAF1 promoter. Carcinogenesis 2008, 29, 1816–1824. [Google Scholar] [CrossRef] [Green Version]
- Padiya, R.; Khatua, T.N.; Bagul, P.K.; Kuncha, M.; Banerjee, S.K. Garlic improves insulin sensitivity and associated metabolic syndromes in fructse fed rats. Nutr. Metab. 2011, 8, 53. [Google Scholar] [CrossRef]
- Shiju, T.M.; Rajkumar, R.; Rajesh, N.G.; Viswanathan, P. Aqueous extract of Allium sativum L bulbs offer nephroprotection by attenuating vascular endothelial growth factor and extracellular signal-regulated kinase-1 expression in diabetic rats. Indian J. Exp. Biol. 2013, 51, 139–158. [Google Scholar] [PubMed]
- Al-Qattan, K.K.; Thomson, M.; Jayasree, D.; Ali, M. Garlic Attenuates Plasma and Kidney ACE-1 and AngII Modulations in Early Streptozotocin-Induced Diabetic Rats: Renal Clearance and Blood Pressure Implications. Evid. Based Complement. Altern. Med. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathibabu Uddandrao, V.V.; Brahmanaidu, P.; Saravanan, G. Therapeutical Perspectives of S-Allylcysteine: Effect on diabetes and other disorders in Animal Models. Cardiovasc. Hematol. Agents Med. Chem. 2018, 15, 71–77. [Google Scholar] [CrossRef]
- Ashraf, R.; Khan, R.A.; Ashraf, I. Effects of garlic on blood glucose levels and HbA1c in patients with type 2 diabetes mellitus. J. Med. Plants Res. 2011, 5, 2922–2928. [Google Scholar]
- Atkin, M.; Laight, D.; Cummings, M.H. The effects of garlic extract upon endothelial function, vascular inflammation, oxidative stress and insulin resistance in adults with type 2 diabetes at high cardiovascular risk. A pilot double blind randomized placebo controlled trial. J. Diabetes Complicat. 2016, 30, 723–727. [Google Scholar] [CrossRef] [Green Version]
- Sheela, C.G.; Kumud, K.; Augusti, K.T. Anti-diabetic effect of onion and garlic sulfoxide amino acids in rats. Planta Med. 1995, 61, 356–357. [Google Scholar] [CrossRef]
- Lee, C.W.; Lee, H.S.; Cha, Y.J.; Joo, W.H.; Kang, D.O.; Moon, J.Y. In vivo investigation of anti-diabetic properties of ripe onion juice in normal and streptozotocin-induced diabetic rats. Prev. Nutr. Food Sci. 2013, 18, 169–174. [Google Scholar] [CrossRef]
- Padiya, R.; Chowdhury, D.; Borkar, R.; Srinivas, R.; Pal Bhadra, M.; Banerjee, S.K. Garlic attenuates cardiac oxidative stress via activation of PI3K/AKT/Nrf2-Keap1 pathway in fructose-fed diabetic rat. PLoS ONE 2014, 9, e94228. [Google Scholar] [CrossRef]
- Yang, J.; Wang, T.; Yang, J.; Rao, K.; Zhan, Y.; Chen, R.B.; Liu, Z.; Li, M.C.; Zhuan, L.; Zang, G.H.; et al. S-allyl cysteine restores erectile function through inhibition of reactive oxygen species generation in diabetic rats. Andrology 2013, 1, 487–494. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Kiasalari, Z.; Afshin-Majd, S.; Ghasemi, Z.; Roghani, M. S-allyl cysteine ameliorates cognitive deficits in streptozotocin-diabetic rats via suppression of oxidative stress, inflammation, and acetylcholinesterase. Eur. J. Pharmacol. 2017, 794, 69–76. [Google Scholar] [CrossRef]
- Zarezadeh, M.; Baluchnejadmojarad, T.; Kiasalari, Z.; Afshin-Majd, S.; Roghani, M. Garlic active constituent s-allyl cysteine protects against lipopolysaccharide-induced cognitive deficits in the rat: Possible involved mechanisms. Eur. J. Pharmacol. 2017, 795, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Mirunalini, S.; Krishnaveni, M.; Ambily, V. Effects of raw garlic (Allium sativum) on hyperglycemia in patients with type 2 diabetes mellitus. Pharmacologyonline 2011, 2, 968–974. [Google Scholar] [CrossRef]
- Eidi, A.; Eidi, M.; Esmaeili, E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine 2006, 13, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.T.; Wong, P.L.; Lii, C.K.; Hse, H.; Sheen, L.Y. Antidiabetic effect of garlic oil but not diallyl disulfide in rats with streptozotocin-induced diabetes. Food Chem. Toxicol. 2006, 44, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Mao, P.; Wang, J.; Wang, T.; Xie, C.H. Allicin protects PC12 cells against 6OHDA-induced oxidative stress and mitochondrial dysfunction via regulating mitochondrial dynamics. Cell. Physiol. Biochem. 2015, 36, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Chhatwal, S.; Sahiba Arora, S.; Sharma, S.; Singh, J.; Singh, N.; Bhandari, V.; Khurana, A. Antihyperglycemic, antihyperlipidemic, anti-inflammatory and adenosine deaminase–lowering effects of garlic in patients with type 2 diabetes mellitus with obesity. Diabetes Metab. Syndr. Obes. 2013, 6, 49–56. [Google Scholar] [CrossRef]
- Sobenin, I.A.; Nedosugova, L.V.; Filatova, L.V.; Balabolkin, M.I.; Gorchakova, T.V.; Orekhov, A.N. Metabolic effects of time-released garlic powder tablets in type 2 diabetes mellitus: The results of double-blinded placebo-controlled study. Acta Diabetol. 2008, 45, 1–6. [Google Scholar] [CrossRef]
- Locatelli, D.A.; Nazareno, M.A.; Fusari, C.M.; Camargo, A.B. Cooked garlic and antioxidant activity: Correlation with organosulfur compound composition. Food Chem. 2017, 220, 219–224. [Google Scholar] [CrossRef]
- Zhao, N.N.; Zhang, H.; Zhang, X.C.; Luan, X.B.; Zhou, C.; Liu, Q.Z.; Shi, W.P.; Liu, Z.L. Evaluation of acute toxicity of essential oil of garlic (Allium sativum) and its selected major constituent compounds against overwintering Cacopsylla chinensis (Hemiptera: Psyllidae). J. Econ. Entomol. 2013, 106, 1349–1354. [Google Scholar] [CrossRef]
- Augusti, K.T.; Jose, R.; Sajitha, G.R.; Augustine, P. A rethinking on the benefits and drawbacks of common antioxidants and a proposal to look for the antioxidants in allium products as ideal agents: A review. Indian J. Clin. Biochem. 2012, 27, 6–20. [Google Scholar] [CrossRef]
- Liu, H.; May, K. Disulfide bond structures of IgG molecules: Structural variations, chemical modifications and possible impacts to stability and biological function. MABS 2012, 4, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, Y.; Cao, Y.G.; Qi, H.P.; Li, L.; Bai, B.; Liu, Y.; Sun, H.L. Antiarrhythmic effects and ionic mechanisms of allicin on myocardial injury of diabetic rats induced by streptozotocin. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2013, 386, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jiang, Y.; Mao, G.; Yuan, F.; Zheng, H.; Ruan, Y.; Wu, T. Protective effects of allicin on streptozotocin- induced diabetic nephropathy in rats. J. Sci. Food Agric. 2017, 97, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Younis, F.; Mirelman, D.; Rabinkov, A.; Rosenthal, T. S-Allyl-Mercapto-Captopril: A novel compound in the treatment of cohen-rosenthal diabetic hypertensive rats. J. Clin. Hypertens. 2010, 12, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pang, S.; Lin, J.; Xia, J.; Wang, Y. Allicin prevents oxidized lowdensity lipoprotein-induced endothelial cell injury by inhibiting apoptosis and oxidative stress pathway. BMC Complement. Altern. Med. 2016, 16, 133. [Google Scholar] [CrossRef] [PubMed]
- Asaba, K.; Tojo, A.; Onozato, M.L.; Goto, A.; Quinn, M.T.; Fujita, T.; Wilcox, C.S. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int. 2005, 67, 1890–1898. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Chen, S.; Li, S.; Deng, L.; Li, Y.; Li, H. Effect of allicin against ischemia/ hypoxia-induced H9c2 myoblast apoptosis via eNOS/NO pathway-mediated antioxidant activity. Evid. Based Complement. Altern. Med. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Becker, P.M.; Van Wikselaar, P.G.; Mul, M.F.; Pol, A.; Engel, B.; Wijdenes, J.W.; Vander Peet-Schwering, C.M.; Wisselink, H.J.; Stockhofe-Zurwieden, N. Actinobacillus pleuropneumoniae is impaired by the garlic volatile allyl methyl sulfide (AMS) in vitro and in-feed garlic alleviates pleuropneumonia in a pig model. Vet. Microbiol. 2012, 154, 316–324. [Google Scholar] [CrossRef]
- Yin, M.C.; Hwang, S.W.; Chan, K.C. Nonenzymatic antioxidant activity of four organosulfur compounds derived from garlic. J. Agric. Food Chem. 2003, 50, 6143–6147. [Google Scholar] [CrossRef]
- Wargovich, M.J. Diallylsulfide and Allyl methyl sulfide are uniquely effective among organosulfur compounds in inhibiting CYP2E1 protein in animal models. J. Nutr. 2006, 136, 832–834. [Google Scholar] [CrossRef]
- Sujithraa, K.; Srinivasan, S.; Indumathi, D.; Vinothkumar, V. Allyl methyl sulfide, an organosulfur compound alleviates hyperglycemia mediated hepatic oxidative stress and inflammation in streptozotocin -ninduced experimental rats. Biomed. Pharmacother. 2018, 107, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Jayachandran, M.; Xu, B.A. Critical review on hepatoprotective effects of bioactive food components. Crit. Rev. Food Sci. Nutr. 2018, 7, 1165–1229. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, A.I.; Papajani, V.T.; Paci, M.; Melino, S. Glutathione-garlic sulfur conjugates: Slow hydrogen sulfide releasing agents for therapeutic applications. Molecules 2015, 20, 1731–1750. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Testai, L.; Breschi, M.C.; Blandizzi, C.; Virdis, A.; Taddei, S.; Calderone, V. Hydrogen sulphide: Novel opportunity for drug discovery. Med. Res. Rev. 2012, 32, 1093–1130. [Google Scholar] [CrossRef] [PubMed]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, J.L.; Wang, R. Hydrogen sulfide-based therapeutics: Exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 2015, 14, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Untereiner, A.; Wu, L. Hydrogen sulfide and glucose homeostasis: A tale of sweet and the stink. Antioxid. Redox Signal. 2018, 28, 1463–1482. [Google Scholar] [CrossRef]
- Whiteman, M.; Armstrong, J.S.; Chu, S.H.; Siau, J.-L.; Wong, B.S.; Cheung, N.S.; Halliwell, B.; Moore, P.K. The novel neuromodulator hydrogen sulfide: An endogenous peroxynitrite ‘scavenger’? J. Neurochem. 2004, 90, 765–768. [Google Scholar] [CrossRef]
- Mitsuhashi, H.; Yamashita, S.; Ikeuchi, H.; Kuroiwa, T.; Kaneko, Y.; Hiromura, K.; Ueki, K.; Nojima, Y. Oxidative stress-dependent conversion of hydrogen sulfide to sulfite by activated neutrophils. Shock 2005, 24, 529–534. [Google Scholar] [CrossRef]
- Geng, B.; Chang, L.; Pan, C.; Qi, Y.; Zhao, J.; Pang, Y.; Du, J.; Tang, C. Hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem. Biophys. Res. Commun. 2004, 318, 756–763. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Grieshaber, M.K. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008, 275, 3352–3361. [Google Scholar] [CrossRef] [PubMed]
- Tiranti, V.; Viscomi, C.; Hildebrandt, T.; Di Meo, I.; Mineri, R.; Tiveron, C.; Levitt, M.D.; Prelle, A.; Fagiolari, G.; Rimoldi, M.; et al. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat. Med. 2009, 15, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Viscomi, C.; Burlina, A.B.; Dweikat, I.; Savoiardo, M.; Lamperti, C.; Hildebrandt, T.; Tiranti, V.; Zeviani, M. Combined treatment with oral metronidazole and Nacetylcysteine is effective in ethylmalonic encephalopathy. Nat. Med. 2010, 16, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Koike, S.; Ogasawara, Y.; Shibuya, N.; Kimura, H.; Ishii, K. Polysulfide exerts a protective effect against cytotoxicity caused by t-butylhydroperoxide through Nrf2 signaling in neuroblastoma cells. FEBS Lett. 2013, 587, 3548–3555. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Wu, L.; Liang, W.; Wang, R. Direct stimulation of K(ATP) channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol. Pharmacol. 2005, 68, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Bucci, M.; Papapetropoulos, A.; Vellecco, V.; Zhou, Z.; Pyriochou, A.; Roussos, C.; Roviezzo, F.; Brancaleone, V.; Cirino, G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Sen, U.; Mishra, P.K.; Tyagi, N.; Tyagi, S.C. Homocysteine to hydrogen sulfide or hypertension. Cell Biochem. Biophys. 2010, 57, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Dawe, G.S.; Han, S.P.; Bian, J.S.; Moore, P.K. Hydrogen sulphide in the hypothalamus causes an ATP-sensitive K+ channel-dependent decrease in blood pressure in freely moving rats. Neuroscience 2008, 152, 169–177. [Google Scholar] [CrossRef]
- Kimura, H. Signaling of hydrogen sulfide and polysulfides. Antioxid. Redox Signal. 2015, 22, 347–349. [Google Scholar] [CrossRef]
- Eto, K.; Asada, T.; Arima, K.; Makifuchi, T.; Kimura, H. Brain hydrogen sulfide is severely decreased in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2002, 293, 1485–1488. [Google Scholar] [CrossRef]
- Wallace, J.L. Physiological and pathophysiological roles of hydrogen sulfide in the gastrointestinal tract. Antioxid. Redox Signal. 2010, 12, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Mard, S.A.; Neisi, N.; Solgi, G.; Hassanpour, M.; Darbor, M.; Maleki, M. Gastroprotective effect of NaHS against mucosal lesions induced by ischemia-reperfusion injury in rat. Dig. Dis. Sci. 2012, 57, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Kimura, T.; Taniguchi, S.; Souma, M.; Kojima, Y.; Kimura, Y.; Kimura, H.; Niki, I. Glucose-induced production of hydrogen sulfide may protect the pancreatic beta-cells from apoptotic cell death by high glucose. FEBS Lett. 2009, 583, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, S.; Niki, I. Significance of hydrogen sulfide production in the pancreatic beta-cell. J. Pharmacol. Sci. 2011, 116, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y. Targeting inflammation in the treatment of type 2 diabetes: Time to start. Nat. Rev. Drug Discov. 2014, 13, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y. Multiple benefits of targeting inflammation in the treatment of type 2 diabetes. Diabetologia 2016, 59, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Esser, N.; Paquot, N.; Scheen, A.J. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin. Investig. Drugs 2015, 24, 283–307. [Google Scholar] [CrossRef]
- Taniguchi, S.; Kang, L.; Kimura, T.; Niki, I. Hydrogen sulphide protects mouse pancreatic beta-cells from cell death induced by oxidative stress, but not by endoplasmic reticulum stress. Br. J. Pharmacol. 2011, 162, 1171–1178. [Google Scholar] [CrossRef]
- Okamoto, M.; Yamaoka, M.; Takei, M.; Ando, T.; Taniguchi, S.; Ishii, I.; Tohya, K.; Ishizaki, T.; Niki, I.; Kimura, T. Endogenous hydrogen sulfide protects pancreatic beta-cells from a high-fat diet-induced glucotoxicity and prevents the development of type 2 diabetes. Biochem. Biophys. Res. Commun. 2013, 442, 227–233. [Google Scholar] [CrossRef]
- Yang, G.; Yang, W.; Wu, L.; Wang, R. H2S, endoplasmic reticulum stress, and apoptosis of insulin-secreting beta cells. J. Biol. Chem. 2007, 282, 16567–16576. [Google Scholar] [CrossRef]
- Yang, W.; Yang, G.; Jia, X.; Wu, L.; Wang, R. Activation of KATP channels by H2S in rat insulin-secreting cells and the underlying mechanisms. J. Physiol. 2005, 569, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Zhang, L.; Yang, G.; Wu, L.; Wang, R. Hydrogen sulfideinduced inhibition of L-type Ca2C channels and insulin secretion in mouse pancreatic beta cells. Diabetologia 2013, 56, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Lipson, K.L.; Fonseca, S.G.; Urano, F. Endoplasmic reticulum stress-induced apoptosis and auto-immunity in diabetes. Curr. Mol. Med. 2006, 6, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Kwong Huat, B.T.; Hsu, A.; Whiteman, M.; Bhatia, M.; Moore, P.K. Streptozotocin-induced diabetes in the rat is associated with enhanced tissue hydrogen sulfide biosynthesis. Biochem. Biophys. Res. Commun. 2005, 333, 1146–1152. [Google Scholar] [CrossRef]
- Ali, M.Y.; Whiteman, M.; Low, C.M.; Moore, P.K. Hydrogen sulphide reduces insulin secretion from HIT-T15 cells by a KATP channel-dependent pathway. J. Endocrinol. 2007, 195, 105–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Yang, W.; Jia, X.; Yang, G.; Duridanova, D.; Cao, K.; Wang, R. Pancreatic islet overproduction of H2S and suppressed insulin release in Zucker diabetic rats. Lab. Investig. 2009, 89, 59–67. [Google Scholar] [CrossRef]
- Okamoto, M.; Ishizaki, T.; Kimura, T. Protective effect of hydrogen sulfide on pancreatic beta-cells. Nitric Oxide 2015, 46, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, J.; Sun, A.; Sun, Y.; Yu, X.; Liu, N.; Dong, S.; Yang, F.; Zhang, L.; Zhong, X.; et al. Hydrogen sulfide decreases high glucose/palmitate-induced autophagy in endothelial cells by the Nrf2-ROS-AMPK signaling pathway. Cell. Biosci. 2016, 6, 33. [Google Scholar] [CrossRef]
- Xiao, T.; Luo, J.; Wu, Z.; Li, F.; Zeng, O.; Yang, J. Effects of hydrogen sulfide on myocardial fibrosis and PI3K/AKT1-regulated autophagy in diabetic rats. Mol. Med. Rep. 2016, 13, 1765–1773. [Google Scholar] [CrossRef]
- Talaei, F.; Van Praag, V.M.; Shishavan, M.H.; Landheer, S.W.; Buikema, H.; Henning, R.H. Increased protein aggregation in Zucker diabetic fatty rat brain: Identification of key mechanistic targets and the therapeutic application of hydrogen sulfide. BMC Cell Biol. 2014, 15, 1. [Google Scholar] [CrossRef]
- Liu, F.; Chen, D.D.; Sun, X.; Xie, H.H.; Yuan, H.; Jia, W.; Chen, A.F. Hydrogen sulfide improves wound healing via restoration of endothelial progenitor cell functions and activation of angiopoietin-1 in type 2 diabetes. Diabetes 2014, 63, 1763–1778. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.T.; Zhao, Y.; Xian, M.; Li, J.H.; Dong, Q.; Bai, H.B.; Xu, J.D.; Zhang, M.F. A novel controllable hydrogen sulfide-releasing molecule protects human skin keratinocytes against methylglyoxal-induced injury and dysfunction. Cell. Physiol. Biochem. 2014, 34, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.T.; Meng, F.H.; Chen, L.; Li, X.; Cen, L.J.; Wen, Y.H.; Li, C.C.; Zhang, H. Inhibition of methylglyoxal-induced AGEs/RAGE expression contributes to dermal protection by N-acetyl-L-cysteine. Cell. Physiol. Biochem. 2017, 41, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Sidik, K.; Mahmood, A.; Salmah, I. Acceleration of Wound Healing by Aqueous Extract of Allium sativum in Combination with Honey on Cutaneous Wound Healing in Rats. Int. J. Mol. Med. Adv. Sci. 2006, 2, 231–235. [Google Scholar]
- Mauretti, A.; Neri, A.; Kossover, O.; Seliktar, D.; Nardo, P.D.; Melino, S. Design of a Novel Composite H2S-Releasing Hydrogel for Cardiac Tissue Repair. Macromol. Biosci. 2016, 16, 847–858. [Google Scholar] [CrossRef]
- Lin, W.C.; Huang, C.C.; Lin, S.J.; Li, M.J.; Chang, Y.; Lin, Y.J.; Wan, W.L.; Shih, P.C.; Sung, H.W. In situ depot comprising phase-change materials that can sustainably release a gasotransmitter H2S to treat diabetic wounds. Biomaterials 2017, 145, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, X.; Zhang, H.; Yang, C.; Liu, Y.; Yang, W.; Guo, C.; Wang, C. Controlled release hydrogen sulfide delivery system based on mesoporous silica nanoparticles protects graft endothelium from ischemia-reperfusion injury. Int. J. Nanomedicine 2016, 11, 3255–3263. [Google Scholar] [CrossRef]
- Cacciotti, I.; Ciocci, M.; Di Giovanni, E.; Nanni, F.; Melino, S. Hydrogen Sulfide-Releasing Fibrous Membranes: Potential Patches for Stimulating Human Stem Cells Proliferation and Viability under Oxidative Stress. Int. J. Mol. Sci. 2018, 19, 11. [Google Scholar] [CrossRef]
- Phadatare, A.G.; Viswanathan, V.; Mukne, A. Novel strategies for optimized delivery of select components of Allium sativum. Pharmacogn. Res. 2014, 6, 334–340. [Google Scholar] [CrossRef]
- Ciocci, M.; Iorio, E.; Carotenuto, F.; Khashoggi, H.A.; Nanni, F.; Melino, S. H2S-releasing nanoemulsions: A new formulation to inhibit tumor cells proliferation and improve tissue repair. Oncotarget 2016, 7, 84338–84358. [Google Scholar] [CrossRef]
 = allyl-thiol;
= allyl-thiol;  = S-allyl-glutathione;
= S-allyl-glutathione;  = allyl-glutathione disulfide.
= allyl-glutathione disulfide.
 = allyl-thiol;
= allyl-thiol;  = S-allyl-glutathione;
= S-allyl-glutathione;  = allyl-glutathione disulfide.
= allyl-glutathione disulfide.
 Ang-1 upregulation,
Ang-1 upregulation,  NF-kB sulfidration,
NF-kB sulfidration,  Nrf2 activation by sulfidration of Keep1
Nrf2 activation by sulfidration of Keep1  and upregulation of CBS, CSE and antioxidant enzyme (NQO1, HO1, etc.), opening
and upregulation of CBS, CSE and antioxidant enzyme (NQO1, HO1, etc.), opening  KATP channels; (B) effects of H2S on insulin
KATP channels; (B) effects of H2S on insulin  release under hyperglycemic conditions (
release under hyperglycemic conditions (  inhibition) at late stage of diabetes in beta cells: upregulation of CSE
inhibition) at late stage of diabetes in beta cells: upregulation of CSE  and CBS
and CBS  ; MST
; MST  ; closure
; closure  of L-type voltage-dependent Ca2C channels
of L-type voltage-dependent Ca2C channels  , opening of KATP channels
, opening of KATP channels  , and hyperpolarization.
, and hyperpolarization.
 Ang-1 upregulation,
Ang-1 upregulation,  NF-kB sulfidration,
NF-kB sulfidration,  Nrf2 activation by sulfidration of Keep1
Nrf2 activation by sulfidration of Keep1  and upregulation of CBS, CSE and antioxidant enzyme (NQO1, HO1, etc.), opening
and upregulation of CBS, CSE and antioxidant enzyme (NQO1, HO1, etc.), opening  KATP channels; (B) effects of H2S on insulin
KATP channels; (B) effects of H2S on insulin  release under hyperglycemic conditions (
release under hyperglycemic conditions (  inhibition) at late stage of diabetes in beta cells: upregulation of CSE
inhibition) at late stage of diabetes in beta cells: upregulation of CSE  and CBS
and CBS  ; MST
; MST  ; closure
; closure  of L-type voltage-dependent Ca2C channels
of L-type voltage-dependent Ca2C channels  , opening of KATP channels
, opening of KATP channels  , and hyperpolarization.
, and hyperpolarization.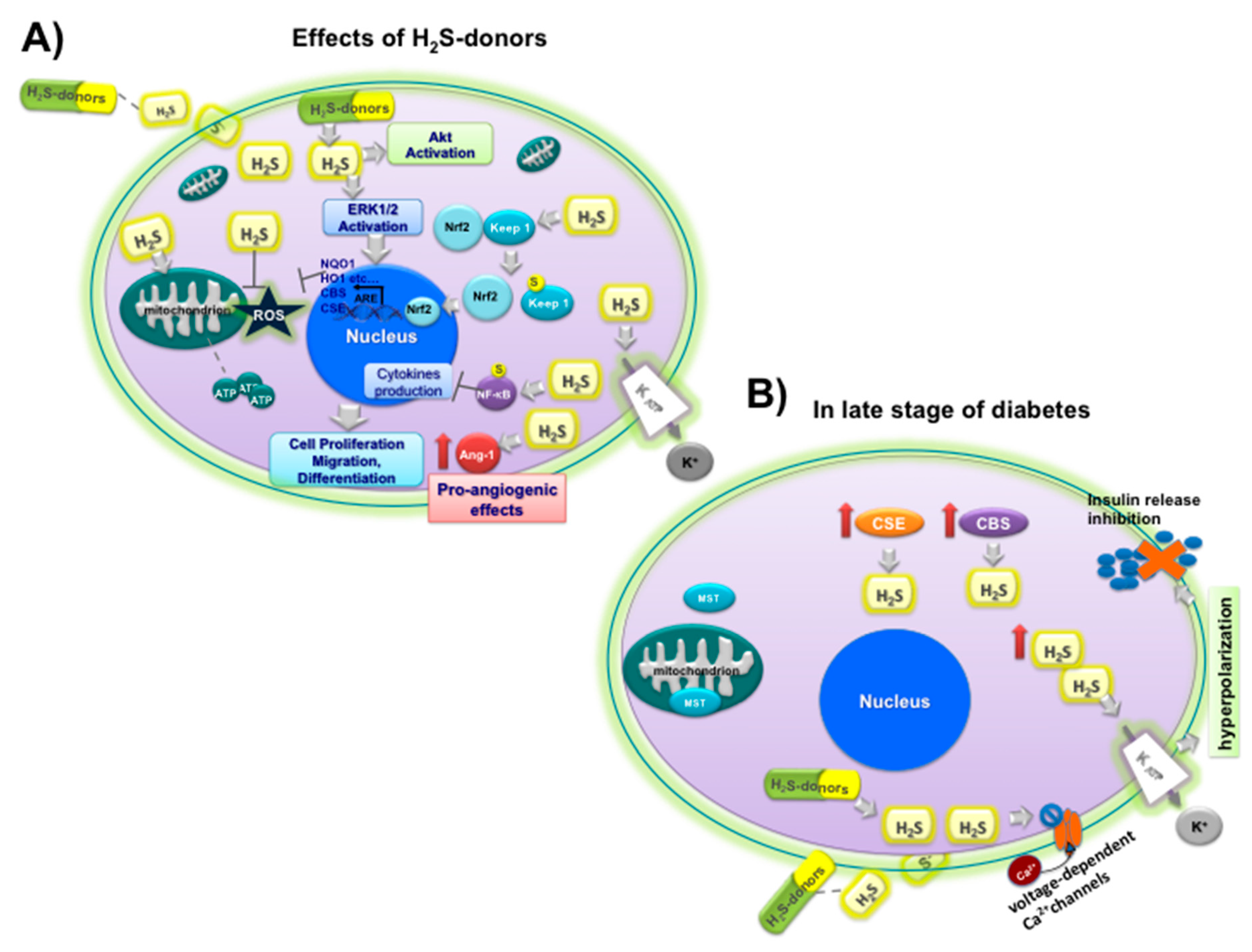
| Plants/Vegetables Species | Phytochemicals/Nutraceuticals | Effects on Type 2 DM | References |
|---|---|---|---|
| Aegle marmelos (Common name: bael) | coumarins (umbelliferone β-D-galactopyranoside) alkaloids, and steroids | ↓ PPBG and lipid peroxidation; ↑ hypoglycemic effect of standard oral drugs in type 2 DM patients and antioxidant activity | [60,61,62] |
| Allium cepa and A. sativum. (Common names: onion and garlic) | OSCs and flavonoids (quercetin and its glycosides) | ↓ FBG and intestinal glucosidase inhibition, serum cholesterol and triacylglycerol and LDL-cholesterol; ↓ blood glucose and lipid levels; ↑ GLUT-4 translocation, glucose uptake and insulin action, SOD, GPx and catalase activity | [63,64,65,66,67] |
| Artemisia dracunculus (Common name: Russian tarragon) | essential oils, coumarins, flavonoids, and phenolic acids | ↓ systolic blood pressure; ↓ HbA1c and total insulin secretion; ↑ HDL-cholesterol levels | [68] |
| Camellia sinensis (Common name: green tea) | Polyphenols: catechins like EGCG, epigallocatechin,epicatechin-3-gallate and epicatechin | ↓ FBG and blood glucose; ↑ insulin sensitivity and secretion; ↓ intestinal glucose absorption by SGLT1 inhibition and oxidative stress; ↑ immune response | [54,55,56,69,70,71] |
| Cinnamomum spp. (Common name: cinnamon) | cinnamaldehyde, procyanidin oligomers | ↓ FBG, HbA1c, triglyceride, LDL cholesterol and total cholesterol; ↑ glucose up-take (GLUT4 translocation) and insulin release | [72,73,74] |
| Coccinia indica/grandis (Common name: ivy gourd) | triterpenoid, saponin coccinioside, flavonoid glycoside | ↓ levels of the enzymes glucose-6-phosphatase, lactate dehydrogenase; ↑ lipase activity and insulin-secreting through glucose metabolism | [75,76] |
| Ipomoea batatas (Common name: caiapo) | acidic glycoprotein, coumarins, caffeic acid, and flavonoids | ↓ FBG and HbA1c; ↑ insulin sensitivity and adiponectin; ↓ fibrinogen levels | [77,78] |
| Gymnema sylvestre (Common name: gurmar) | gymnemic acids, gymnema saponins, and gurmarin dihydroxy gymnemic triacetate | ↓ FBG, PPBG and HbA1c of type 2 DM patients; ↑ insulin secretion and C-peptide; ↓ intestinal glucose absorption; ↑ plasma insulin and muscle and liver glycogen in diabetic rats; ↑ islet β cell regeneration | [79,80,81,82] |
| Linum ussitatisimum (Common name: flaxseed) | PUFAs (α-linoleic and linolenic acid), polyphenols, triterpenoids | ↓ fasting blood glucose, HbA1c, triglycerides, total and LDL cholesterol, apolipoprotein B; ↑ HDL cholesterol levels | [83,84] |
| Momordica charantia (Common name: bitter melon) | cucurbitane triterpenoids, charantin etc. polypeptide-p | ↓ FBG and PPBG levels in type 2 DM; ↓ total cholesterol; ↓ related complications (retinopathy and myocardial infarction); ↑ glucose uptake through stimulation of GLUT-4 translocation, AMPK system; ↓ α-glucosidase activity | [85,86,87,88,89] |
| Morus alba (Common name: morus) | Phenols, flavonoids, anthocyanins, alkaloids | ↑ the postprandial glycemic control; ↓ plasma glucose, α-glucosidase; ↑ AMPK and plasma membrane GLUT4 levels in skeletal muscle | [90,91,92] |
| Ocimum sanctum (Common name: holy basil) | tannins and essential oil (eugenol, methyleugenol, and caryophyllene) | ↓ FBG and PPBG; ↓ total cholesterol level; ↓ insulin resistance and normalization of serum lipid profile, body weight and BMI, diabetic symptoms, lipid peroxidation; ↑ activity of antioxidant enzymes | [93,94,95,96] |
| Opuntia spp. (Common name: nopal) | flavonoids, phenolic acids, betalains, phytosterol, PUFAs | ↓ PPBG and serum insulin, glucose absorption from the intestine and plasma GIP levels; ↑ increase antioxidant activity and glucose uptake (through the AMPK/p38 MAPK signaling pathway and GLUT4 translocation in muscle cells) | [97,98,99] |
| Panax ginseng and P. quinquefolius (Common name: Asian and American ginseng) | triterpene saponins, (ginsenosides, protopanaxadiol and protopanaxatriol- type saponins, compound K | ↓ FBG and body weight; ↑ glucose metabolism and VEGF expression; ↑ angiogenesis by eNOS activation; ↓ insulin resistance and apoptosis; ↑ fasting serum insulin and insulin sensitivity | [100,101,102] |
| Salacia reticulata (Common name: Kothala himbutu) | polyphenols (mangiferin, catechins, and tannins) | ↓ FBG, HbA1c and lipid levels (cholesterol, LDL, VLDL and triglyceride levels) | [103,104,105] |
| Silybum marianum (Common name: milk thistle) | flavonolignans (silymarin complex: silybin and isosilybin, silychristin and silydianin), the flavonol taxifolin | ↓ glucose and lipids levels, FBG, HbA1c, total cholesterol, LDL, TG and hepatic enzymes; ↓ PPBG, insulin resistance and insulin production; ↑ antioxidant system (SOD and GPx activities and total antioxidant capacity); ↓ C reactive protein | [106,107,108,109] |
| Trigonella foenum graecum (Common name: fenugreek) | steroid saponins (diosgenin, yamogenin, tigogenin), protoalkaloids, trigonelline, 4-hydroxyisoleucin, soluble fiber fraction | ↓ PPBG, FBG, HbA1c, TG, VLDL, lipid; ↓ intestinal glycosidase; ↑ lipogenic enzymes, glucose uptake, HDL level and insulin sensitivity | [110,111] |
| Zingiber officinale (Common name: ginger) | metabolites ginger oleoresin, 8-gingerol, 10-gingerol and 6-shogaol | ↓ serum glucose, HbA1c and insulin resistance; ↑ total antioxidant capacity | [112] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melino, S.; Leo, S.; Toska Papajani, V. Natural Hydrogen Sulfide Donors from Allium sp. as a Nutraceutical Approach in Type 2 Diabetes Prevention and Therapy. Nutrients 2019, 11, 1581. https://doi.org/10.3390/nu11071581
Melino S, Leo S, Toska Papajani V. Natural Hydrogen Sulfide Donors from Allium sp. as a Nutraceutical Approach in Type 2 Diabetes Prevention and Therapy. Nutrients. 2019; 11(7):1581. https://doi.org/10.3390/nu11071581
Chicago/Turabian StyleMelino, Sonia, Sara Leo, and Vilma Toska Papajani. 2019. "Natural Hydrogen Sulfide Donors from Allium sp. as a Nutraceutical Approach in Type 2 Diabetes Prevention and Therapy" Nutrients 11, no. 7: 1581. https://doi.org/10.3390/nu11071581
APA StyleMelino, S., Leo, S., & Toska Papajani, V. (2019). Natural Hydrogen Sulfide Donors from Allium sp. as a Nutraceutical Approach in Type 2 Diabetes Prevention and Therapy. Nutrients, 11(7), 1581. https://doi.org/10.3390/nu11071581






