Exercise Alleviates Cognitive Functions by Enhancing Hippocampal Insulin Signaling and Neuroplasticity in High-Fat Diet-Induced Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. Exercise Protocol
2.3. Glucose Tolerance Test
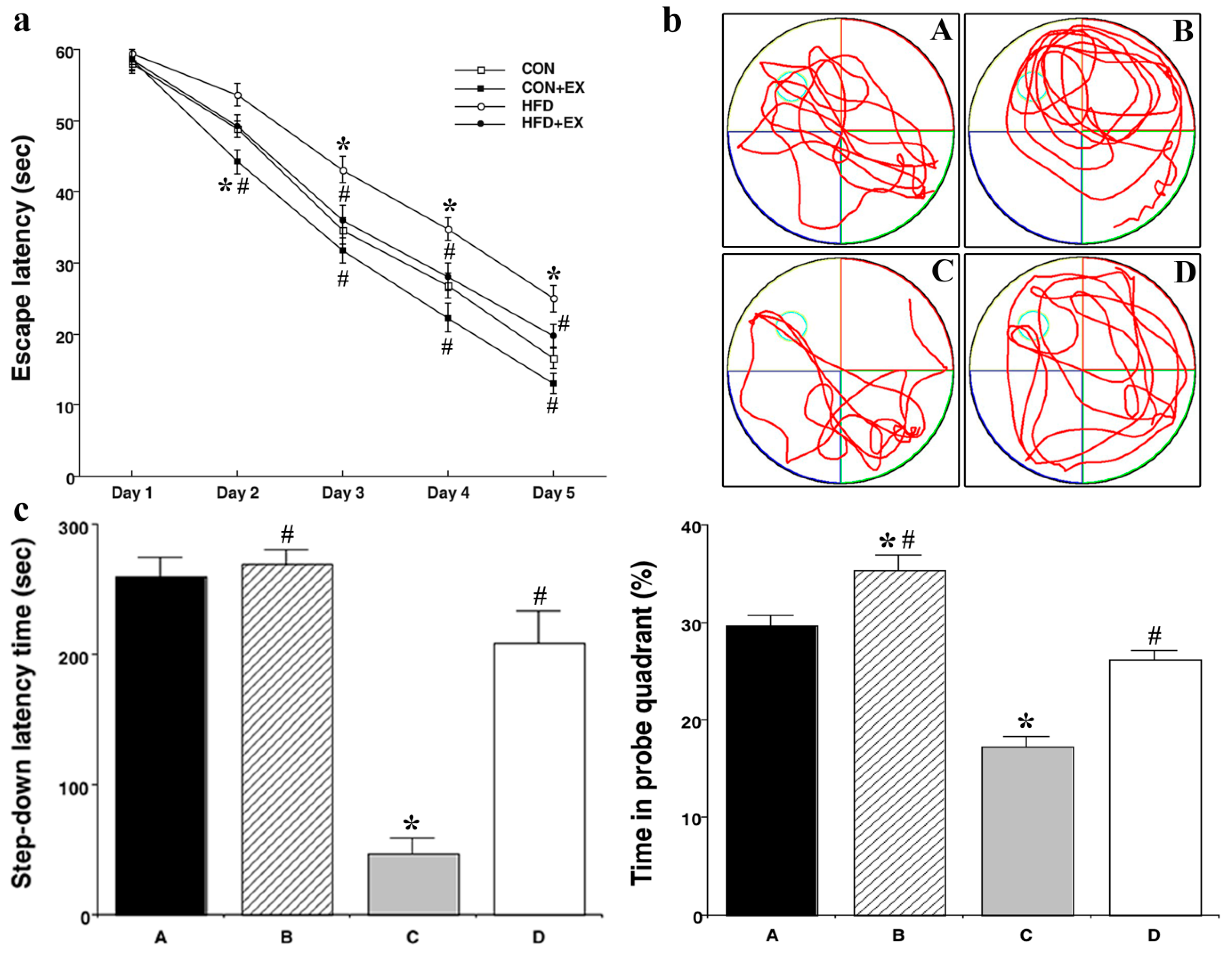
2.4. Step-Down Avoidance Task
2.5. Morris Water Maze Task
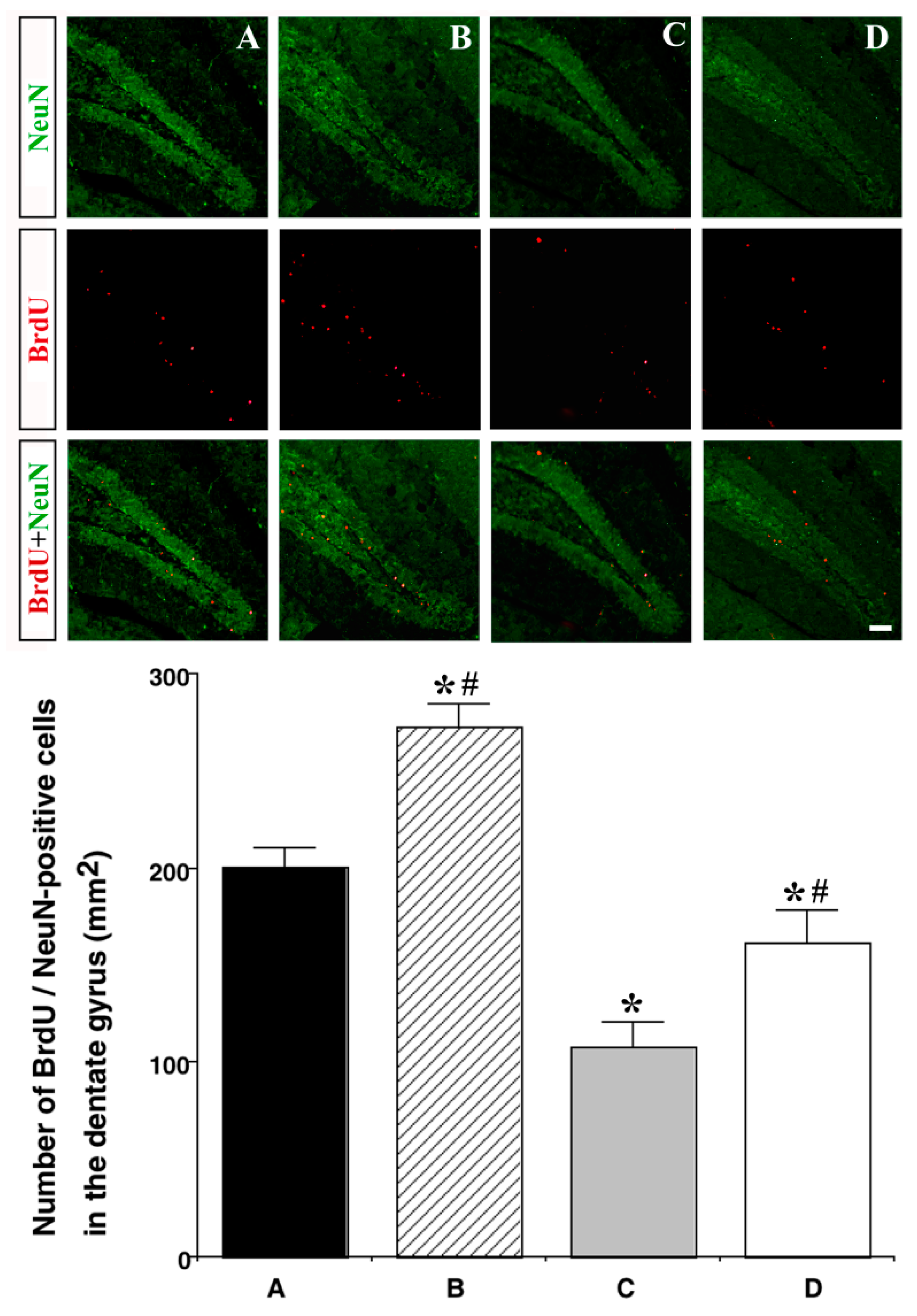
2.6. Immunofluorescence for 5-Bromo-2’-Deoxyuridine (BrdU)- and Neuronal Nucleic (NeuN)
2.7. Immunohistochemistry for Doublecortin (DCX)
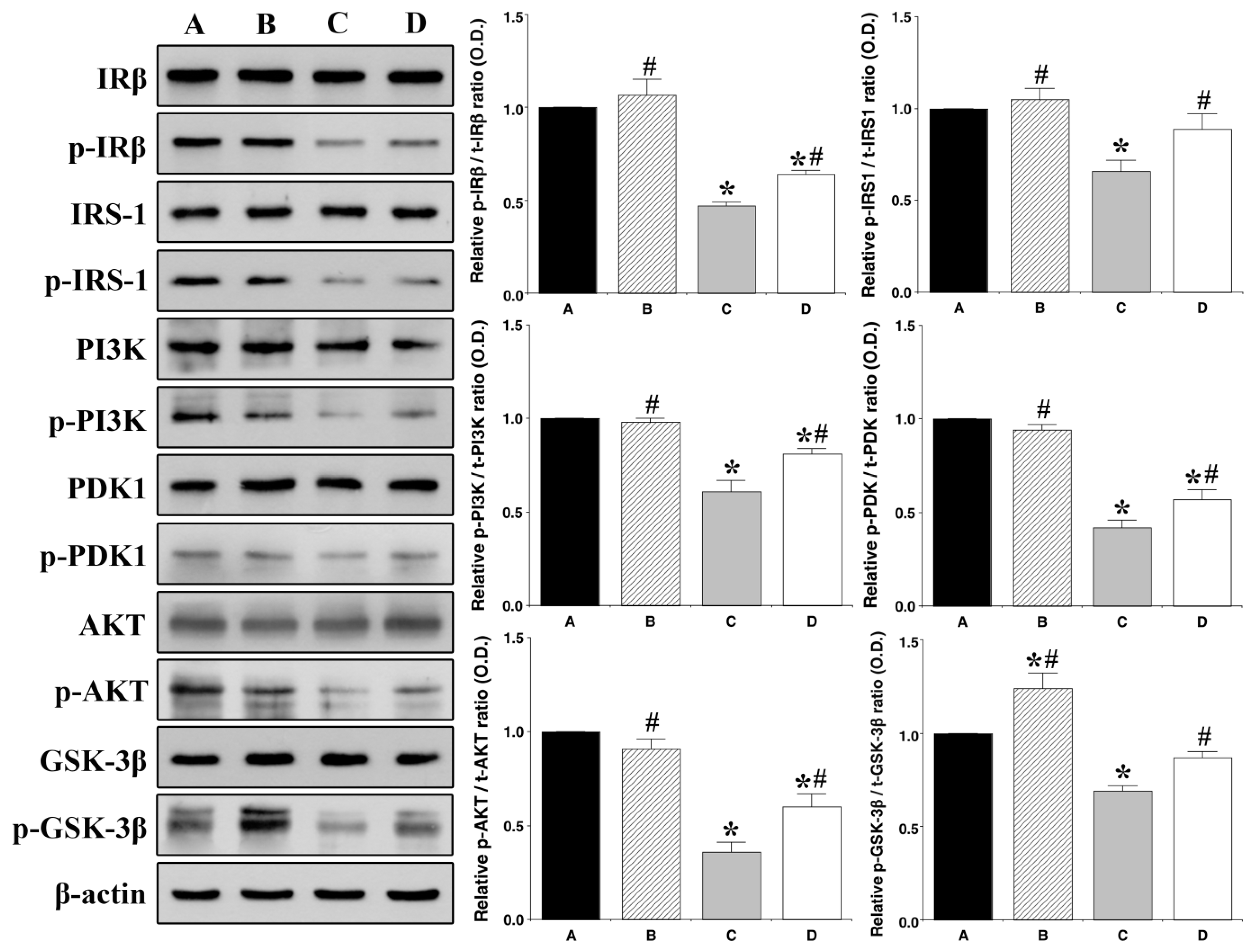
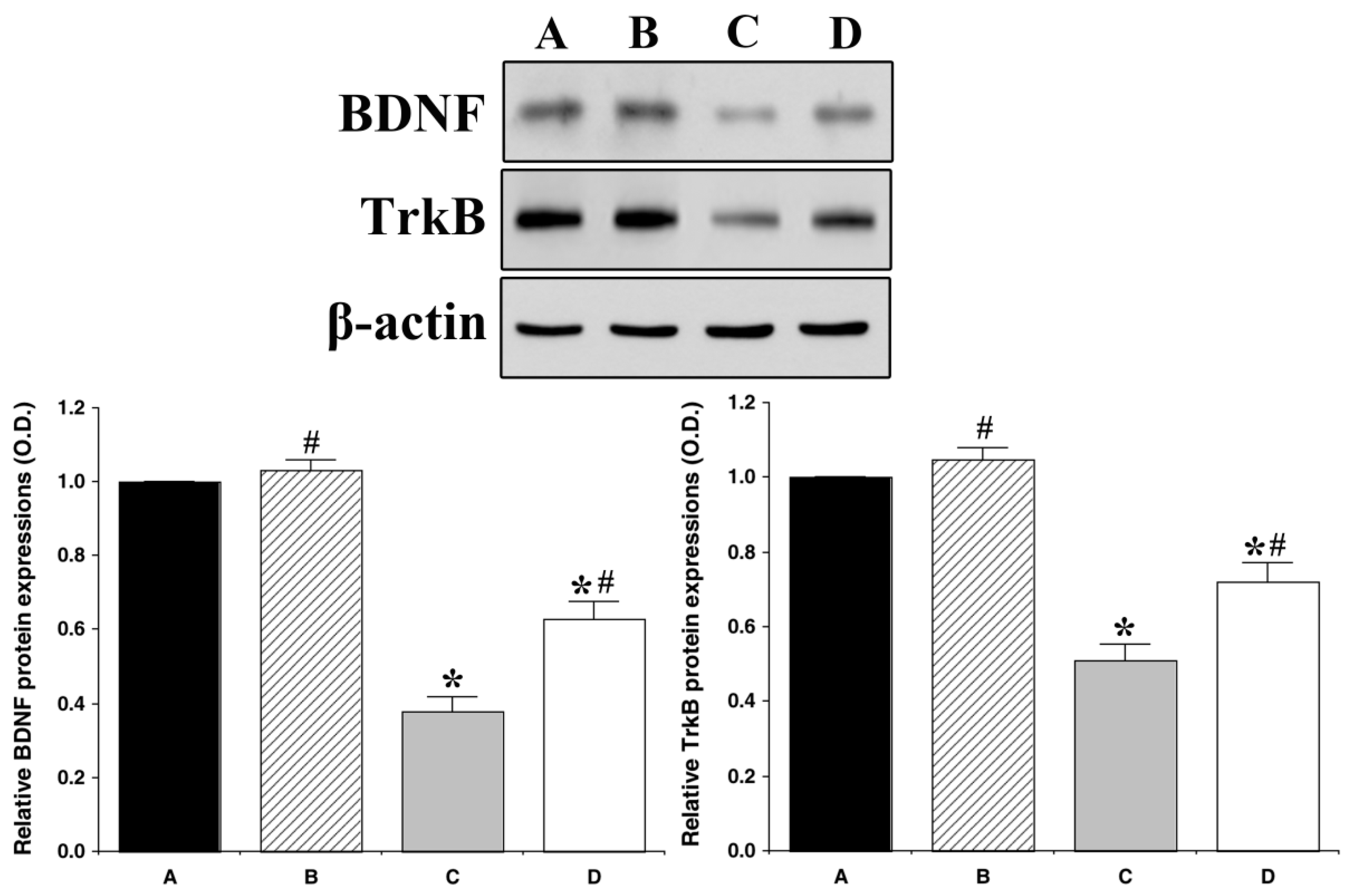
2.8. Western Blot for BDNF, Tyrosine Protein Kinase B, and Insulin Signaling
2.9. Data Analysis
3. Results
3.1. Effects of High-Fat Feeding and Exercise on Body Weight and Blood Glucose Level
3.2. Effect of Treadmill Exercise on Memory
3.3. Effect of Treadmill Exercise on Hippocampal Insulin Signaling
3.4. Effect of Treadmill Exercise on BDNF and TrkB Expression in the Hippocampus
3.5. Effect of Treadmill Exercise on Neurogenesis in the Hippocampal Dentate Gyrus
3.6. Effect of Treadmill Exercise on the Expression of DCX in the Hippocampal Dentate Gyrus
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sriram, K.; Benkovic, S.A.; Miller, D.B.; O’Callaghan, J.P. Obesity exacerbates chemically induced neurodegeneration. Neuroscience 2002, 115, 1335–1346. [Google Scholar] [CrossRef]
- Elias, M.F.; Elias, P.K.; Sullivan, L.M.; Wolf, P.A.; D’Agostino, R.B. Obesity, diabetes and cognitive deficit: The Framingham Heart Study. Neurobiol. Aging 2005, 1, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.A.; Beiser, A.; Elias, M.F.; Au, R.; Vasan, R.S.; Seshadri, S. Relation of obesity to cognitive function: Importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr. Alzheimer Res. 2007, 4, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Raji, C.A.; Ho, A.J.; Parikshak, N.N.; Becker, J.T.; Lopez, O.L.; Kuller, H.; Hua, X.; Leow, A.D.; Toga, A.W.; Thompson, P.M. Brain structure and obesity. Hum. Brain Mapp. 2010, 31, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Whitmer, R.A.; Gunderson, E.P.; Barrett-Connor, E.; Quesenberry, C.P.; Yaffe, K., Jr. Obesity in middle age and future risk of dementia: A 27 year longitudinal population based study. BMJ 2005, 330, 1360–1364. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Q.; Chen, H.; Quon, M.J.; Alkon, D.L. Insulin and the insulin receptor in experimental models of learning and memory. Eur. J. Pharmacol. 2004, 490, 71–81. [Google Scholar] [CrossRef]
- Schubert, M.; Gautam, D.; Surjo, D.; Ueki, K.; Baudler, S.; Schubert, D.; Kondo, T.; Alber, J.; Galldiks, N.; Küstermann, E.; et al. Role for neuronal insulin resistance in neurodegenerative diseases. Proc. Natl. Acad. Sci. USA 2004, 101, 3100–3105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruehl, H.; Wolf, O.T.; Sweat, V.; Tirsi, A.; Richardson, S.; Convit, A. Modifiers of cognitive function and brain structure in middle-aged and elderly individuals with type 2 diabetes mellitus. Brain Res. 2009, 1280, 186–194. [Google Scholar] [CrossRef] [Green Version]
- McNay, E.C.; Ong, C.; McCrimmon, R.J.; Cresswell, J.; Bogan, J.S.; Sherwin, R.S. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol. Learn. Mem. 2010, 93, 546–553. [Google Scholar] [CrossRef] [Green Version]
- Molteni, R.; Barnard, R.J.; Ying, Z.; Roberts, C.K.; Gómez-Pinilla, F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 2002, 112, 803–814. [Google Scholar] [CrossRef] [Green Version]
- Lindqvist, A.; Mohapel, P.; Bouter, B.; Frielingsdorf, H.; Pizzo, D.; Brundin, P.; Erlanson-Albertsson, C. High-fat diet impairs hippocampal neurogenesis in male rats. Eur. J. Neurol. 2006, 13, 1385–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms of functional implications of adult neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Bramham, C.R.; Messaoudi, E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005, 76, 99–125. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Tsuchida, A.; Itakura, Y.; Nonomura, T.; Ono, M.; Hirota, F.; Inoue, T.; Nakayama, C.; Taiji, M.; Noquchi, H. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes 2000, 49, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Reagan, L.P. Hippocampal insulin resistance and cognitive dysfunction. Nat. Rev. Neurosci. 2015, 16, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, J.A. Adiposity, hyperinsulinemia, diabetes and Alzheimer’s disease: An epidemiological perspective. Eur. J. Pharmacol. 2008, 5, 9–29. [Google Scholar]
- Norton, S.; Matthews, F.E.; Barnes, D.E.; Yaffe, K.; Brayne, C. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol. 2014, 13, 788–794. [Google Scholar] [CrossRef]
- Kiliaan, A.J.; Arnoldussen, I.A.; Gustafson, D.R. Adipokines: A link between obesity and dementia? Lancet Neurol. 2014, 13, 913–923. [Google Scholar] [CrossRef]
- Tschop, M.; Heiman, M.L. Rodent obesity models: An overview. Exp. Clin. Endocrinnol. Diabetes 2001, 109, 307–319. [Google Scholar] [CrossRef]
- Cournot, M.; Marquié, J.C.; Ansiau, D.; Martinaud, C.; Fonds, H.; Ferrières, J.; Ruidavets, J.B. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology 2006, 67, 1208–1214. [Google Scholar] [CrossRef]
- Valladolid-Acebes, I.; Stucchi, P.; Cano, V.; Fernández-Alfonso, M.S.; Merino, B.; Gil-Ortega, M.; Fole, A.; Morales, L.; Ruiz-Gayo, M.; Del Olmo, N. High-fat diets impair spatial learning in the radial-arm maze in mice. Neurobiol. Learn. Mem. 2011, 95, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Gerozissis, K. Brain insulin, energy and glucose homeostasis; genes, environment and metabolic pathologies. Eur. J. Pharmacol. 2008, 585, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Craft, S. Insulin resistance syndrome and Alzheimer’s disease: Age- and obesity-related effects on memory, amyloid, and inflammation. Neurobiol. Aging 2005, 1, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Van der Heide, L.P.; Ramakers, G.M.; Smidt, M.P. Insulin signaling in the central nervous system: Learning to survive. Prog. Neurobiol. 2006, 79, 205–221. [Google Scholar] [CrossRef]
- Ketterer, C.; Tschritter, O.; Preissl, H.; Heni, M.; Haring, H.U.; Fritsche, A. Insulin sensitivity of the human brain. Diabetes Res. Clin. Pract. 2011, 1, S47–S51. [Google Scholar] [CrossRef]
- Pratchayasakul, W.; Kerdphoo, S.; Petsophonsakul, P.; Pongchaidecha, A.; Chattipakorn, N.; Chattipakorn, S.C. Effects of high-fat diet on insulin receptor function in rat hippocampus and the level of neuronal corticosterone. Life Sci. 2011, 88, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, F.; Grundke-Iqbal, I.; Iqbal, K.; Gong, C.X. Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J. Pathol. 2011, 225, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Špolcová, A.; Mikulášková, B.; Kršková, K.; Gajdošechová, L.; Zórad, Š.; Olszanecki, R.; Suski, M.; Bujak-Giżycka, B.; Železná, B.; Maletínská, L. Deficient hippocampal insulin signaling and augmented Tau phosphorylation is related to obesity-and age-induced peripheral insulin resistance: A study in Zucker rats. BMC Neurosci. 2014, 15, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Deng, J.; Zuo, Z. High-fat diet reduces neuroprotection of isoflurane post-treatment: Role of carboxyl-terminal modulator protein-Akt signaling. Obesity (Silver Spring) 2014, 22, 2396–2405. [Google Scholar] [CrossRef] [Green Version]
- Izumi, Y.; Yamada, K.A.; Matsukawam, M.; Zorumski, C.F. Effects of insulin on long-term potentiation in hippocampal slices from diabetic rats. Diabetologia 2003, 46, 1007–1012. [Google Scholar] [CrossRef] [Green Version]
- Park, H.R.; Park, M.; Choi, J.; Park, K.Y.; Chung, H.Y.; Lee, J. A high-fat diet impairs neurogenesis: Involvement of lipid peroxidation and brain-derived neurotrophic factor. Neurosci. Lett. 2010, 482, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Shonesy, B.C.; Thiruchelvam, K.; Parameshwaran, K.; Rahman, E.A.; Karuppagounder, S.S.; Huggins, K.W.; Pinkert, C.A.; Amin, R.; Dhanasekaran, M.; Suppiramaniam, V. Central insulin resistance and synaptic dysfunction in intracerebroventricular-streptozotocin injected rodents. Neurobiol. Aging 2012, 33, e5–e18. [Google Scholar] [CrossRef] [PubMed]
- Krafft, C.E.; Schwarz, N.F.; Chi, L.; Weinberger, A.L.; Schaeffer, D.J.; Pierce, J.E.; Rodrigue, A.L.; Yanasak, N.E.; Miller, P.H.; Tomporowski, P.D.; et al. An 8-month randomized controlled exercise trial alters brain activation during cognitive tasks in overweight children. Obesity (Silver Spring) 2014, 22, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Ko, I.G.; Kim, S.E.; Hwang, L.K.; Jin, J.J.; Kim, C.J.; Kim, B.K.; Kim, H. Late starting treadmill exercise improves spatial leaning ability through suppressing CREP/BDNF/TrkB signaling pathway following traumatic brain injury in rats. J. Exerc. Rehabil. 2018, 14, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Ji, E.S.; Kim, T.W.; Kim, C.J.; Shin, M.S.; Lim, B.V.; Chung, Y.R.; Cho, Y.S. Treadmill exercise improves memory function by inhibiting hippocampal apoptosis in pilocarpine-induced epileptic rats. J. Exerc. Rehabil. 2018, 14, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.P.; Zimmer, E.R.; Kalinine, E.; Haas, C.B.; Oses, J.P.; Martimbianco de Assis, A.; Galina, A.; Souza, D.O.; Portela, L.V. Physical exercise exacerbates memory deficits induced by intracerebroventricular STZ but improves insulin regulation of H2O2 production in mice synaptosomes. J. Alzheimers Dis. 2012, 30, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.P.; Gnoatto, J.; Moreira, J.D.; Zimmer, E.R.; Haas, C.B.; Lulhier, F.; Perry, M.L.; Souza, D.O.; Torres-Aleman, I.; Portela, L.V. Exercise increases insulin signaling in the hippocampus: Physiological effects and pharmacological impact of intracerebroventricular insulin administration in mice. Hippocampus 2011, 21, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Sui, L.; Wang, J.; Li, B.M. Role of the phosphoinositide 3-kinase-Akt-mammalian target of the rapamycin signaling pathway in long-term potentiation and trace fear conditioning memory in rat medial prefrontal cortex. Learn. Mem. 2008, 15, 762–776. [Google Scholar] [CrossRef] [Green Version]
- Peineau, S.; Taghibiglou, C.; Bradley, C.; Wong, T.P.; Liu, L.; Lu, J.; Lo, E.; Wu, D.; Saule, E.; Bouschet, T.; et al. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron 2007, 53, 703–717. [Google Scholar] [CrossRef]
- Um, H.S.; Kang, E.B.; Koo, J.H.; Kim, H.T.; Kim, E.J.; Yang, C.H.; An, G.Y.; Cho, I.H.; Cho, J.Y. Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer’s disease. Neurosci. Res. 2011, 69, 161–173. [Google Scholar] [CrossRef]
- Zang, J.; Liu, Y.; Li, W.; Xiao, D.; Zhang, Y.; Luo, Y.; Liang, W.; Liu, F.; Wei, W. Voluntary exercise increases adult hippocampal neurogenesis by increasing GSK-3β activity in mice. Neuroscience 2017, 354, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Kleinridders, A.; Ferris, H.A.; Cai, W.; Kahn, C.R. Insulin action in brain regulates systemic metabolism and brain function. Diabetes 2014, 63, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Takach, O.; Gill, T.B.; Silverman, M.A. Modulation of insulin signaling rescues BDNF transport defects independent of tau in amyloid-β oligomer-treated hippocampal neurons. Neurobiol. Aging 2015, 36, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Shin, K.O.; Park, S.Y.; Jang, K.S.; Kang, S. Effects of exercise and diet change on cognition function and synaptic plasticity in high fat diet induced obese rats. Lipids Health Dis. 2013, 12, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.S.; Hwang, I.K.; Yoo, K.Y.; Park, O.K.; Yu, J.; Yan, B.; Kim, I.Y.; Kim, Y.N.; Pai, T.; Song, W.; et al. Effects of treadmill exercise on cell proliferation and differentiation in the subgranular zone of the dentate gyrus in a rat model of type II diabetes. Neurochem. Res. 2009, 34, 1039–1046. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-S.; Park, S.-S.; Kim, C.-J.; Shin, M.-S.; Kim, T.-W. Exercise Alleviates Cognitive Functions by Enhancing Hippocampal Insulin Signaling and Neuroplasticity in High-Fat Diet-Induced Obesity. Nutrients 2019, 11, 1603. https://doi.org/10.3390/nu11071603
Park H-S, Park S-S, Kim C-J, Shin M-S, Kim T-W. Exercise Alleviates Cognitive Functions by Enhancing Hippocampal Insulin Signaling and Neuroplasticity in High-Fat Diet-Induced Obesity. Nutrients. 2019; 11(7):1603. https://doi.org/10.3390/nu11071603
Chicago/Turabian StylePark, Hye-Sang, Sang-Seo Park, Chang-Ju Kim, Mal-Soon Shin, and Tae-Woon Kim. 2019. "Exercise Alleviates Cognitive Functions by Enhancing Hippocampal Insulin Signaling and Neuroplasticity in High-Fat Diet-Induced Obesity" Nutrients 11, no. 7: 1603. https://doi.org/10.3390/nu11071603
APA StylePark, H. -S., Park, S. -S., Kim, C. -J., Shin, M. -S., & Kim, T. -W. (2019). Exercise Alleviates Cognitive Functions by Enhancing Hippocampal Insulin Signaling and Neuroplasticity in High-Fat Diet-Induced Obesity. Nutrients, 11(7), 1603. https://doi.org/10.3390/nu11071603





