Raw Cow’s Milk Reduces Allergic Symptoms in a Murine Model for Food Allergy—A Potential Role for Epigenetic Modifications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
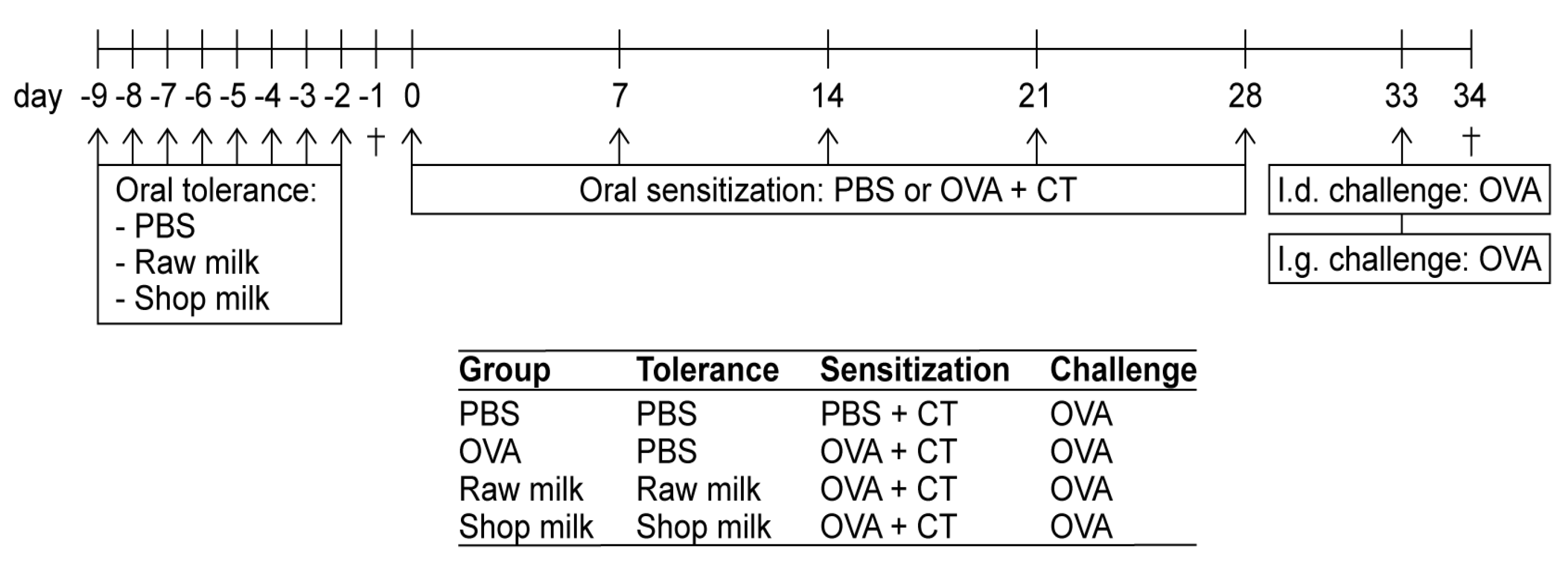
2.2. Experimental Design—Tolerance Induction, Sensitization and Challenges
2.3. Assessment of the Acute Allergic Response
2.4. Detection of OVA-Specific IgE and mMCP-1 in Serum
2.5. Ex Vivo OVA-Specific Stimulation of Splenocytes for Cytokine Measurements
2.6. Chromatin Immunoprecipitation to Determine Histone Acetylation Status in Splenocyte-Derived CD4+ T Cells and Mesenteric Lymph Nodes (MLN)
2.7. Statistical Analysis
3. Results
3.1. Raw Milk Reduces OVA-Induced Allergic Symptoms
3.2. OVA-Specific IgE Levels and Mucosal Mast Cell Degranulation Are Not Affected by Raw Milk Exposure
3.3. Raw Milk Treatment Initially Increases Histone Acetylation of Several T Cell Subset Genes, While after Both Challenges It Specifically Reduces Th2-Related Gene Acetylation
3.4. Systemically Observed Acetylation Profile of Th2-Related Genes Induced by Raw Milk also Visible Locally
3.5. Cytokine Production by OVA-Stimulated Splenocytes Corresponds to Histone Acetylation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Allergy Organisation (WAO) White Book on Allergy: Update 2013. Available online: https://www.worldallergy.org/UserFiles/file/WhiteBook2-2013-v8.pdf (accessed on 4 February 2019).
- Zuberbier, T.; Lotvall, J.; Simoens, S.; Subramanian, S.V.; Church, M.K. Economic burden of inadequate management of allergic diseases in the European Union: A GA(2) LEN review. Allergy 2014, 69, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Braun-Fahrlander, C.; Gassner, M.; Grize, L.; Neu, U.; Sennhauser, F.H.; Varonier, H.S.; Vuille, J.C.; Wuthrich, B. Prevalence of hay fever and allergic sensitization in farmer’s children and their peers living in the same rural community. SCARPOL team. Swiss Study on Childhood Allergy and Respiratory Symptoms with Respect to Air Pollution. Clin. Exp. Allergy 1999, 29, 28–34. [Google Scholar] [CrossRef]
- Kilpelainen, M.; Terho, E.O.; Helenius, H.; Koskenvuo, M. Farm environment in childhood prevents the development of allergies. Clin. Exp. Allergy 2000, 30, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Von Ehrenstein, O.S.; Von Mutius, E.; Illi, S.; Baumann, L.; Bohm, O.; von Kries, R. Reduced risk of hay fever and asthma among children of farmers. Clin. Exp. Allergy 2000, 30, 187–193. [Google Scholar] [CrossRef]
- Riedler, J.; Eder, W.; Oberfeld, G.; Schreuer, M. Austrian children living on a farm have less hay fever, asthma and allergic sensitization. Clin. Exp. Allergy 2000, 30, 194–200. [Google Scholar] [CrossRef]
- Alfven, T.; Braun-Fahrlander, C.; Brunekreef, B.; von Mutius, E.; Riedler, J.; Scheynius, A.; van Hage, M.; Wickman, M.; Benz, M.R.; Budde, J.; et al. Allergic diseases and atopic sensitization in children related to farming and anthroposophic lifestyle--the PARSIFAL study. Allergy 2006, 61, 414–421. [Google Scholar] [CrossRef]
- Von Mutius, E.; Vercelli, D. Farm living: Effects on childhood asthma and allergy. Nat. Rev. Immunol. 2010, 10, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Riedler, J.; Braun-Fahrlander, C.; Eder, W.; Schreuer, M.; Waser, M.; Maisch, S.; Carr, D.; Schierl, R.; Nowak, D.; von Mutius, E.; et al. Exposure to farming in early life and development of asthma and allergy: A cross-sectional survey. Lancet 2001, 358, 1129–1133. [Google Scholar] [CrossRef]
- Waser, M.; Michels, K.B.; Bieli, C.; Floistrup, H.; Pershagen, G.; von Mutius, E.; Ege, M.; Riedler, J.; Schram-Bijkerk, D.; Brunekreef, B.; et al. Inverse association of farm milk consumption with asthma and allergy in rural and suburban populations across Europe. Clin. Exp. Allergy 2007, 37, 661–670. [Google Scholar] [CrossRef]
- Ege, M.J.; Frei, R.; Bieli, C.; Schram-Bijkerk, D.; Waser, M.; Benz, M.R.; Weiss, G.; Nyberg, F.; van Hage, M.; Pershagen, G.; et al. Not all farming environments protect against the development of asthma and wheeze in children. J. Allergy Clin. Immunol. 2007, 119, 1140–1147. [Google Scholar] [CrossRef]
- Perkin, M.R.; Strachan, D.P. Which aspects of the farming lifestyle explain the inverse association with childhood allergy? J. Allergy Clin. Immunol. 2006, 117, 1374–1381. [Google Scholar] [CrossRef]
- Loss, G.; Apprich, S.; Waser, M.; Kneifel, W.; Genuneit, J.; Buchele, G.; Weber, J.; Sozanska, B.; Danielewicz, H.; Horak, E.; et al. The protective effect of farm milk consumption on childhood asthma and atopy: The GABRIELA study. J. Allergy Clin. Immunol. 2011, 128, 766–773 e4. [Google Scholar] [CrossRef]
- Abbring, S.; Verheijden, K.A.T.; Diks, M.A.P.; Leusink-Muis, A.; Hols, G.; Baars, T.; Garssen, J.; van Esch, B. Raw Cow’s Milk Prevents the Development of Airway Inflammation in a Murine House Dust Mite-Induced Asthma Model. Front. Immunol. 2017, 8, 1045. [Google Scholar] [CrossRef]
- Brick, T.; Schober, Y.; Bocking, C.; Pekkanen, J.; Genuneit, J.; Loss, G.; Dalphin, J.C.; Riedler, J.; Lauener, R.; Nockher, W.A.; et al. Omega-3 fatty acids contribute to the asthma-protective effect of unprocessed cow’s milk. J. Allergy Clin. Immunol. 2016, 137, 1699–1706 e13. [Google Scholar] [CrossRef]
- Brick, T.; Ege, M.; Boeren, S.; Bock, A.; von Mutius, E.; Vervoort, J.; Hettinga, K. Effect of Processing Intensity on Immunologically Active Bovine Milk Serum Proteins. Nutrients 2017, 9, 963. [Google Scholar] [CrossRef]
- Abbring, S.; Hols, G.; Garssen, J.; van Esch, B. Raw cow’s milk consumption and allergic diseases—The potential role of bioactive whey proteins. Eur. J. Pharmacol. 2019, 843, 55–65. [Google Scholar] [CrossRef]
- Van Neerven, R.J.; Knol, E.F.; Heck, J.M.; Savelkoul, H.F. Which factors in raw cow’s milk contribute to protection against allergies? J. Allergy Clin. Immunol. 2012, 130, 853–858. [Google Scholar] [CrossRef]
- Potaczek, D.P.; Harb, H.; Michel, S.; Alhamwe, B.A.; Renz, H.; Tost, J. Epigenetics and allergy: From basic mechanisms to clinical applications. Epigenomics 2017, 9, 539–571. [Google Scholar] [CrossRef]
- Alhamwe, B.A.; Khalaila, R.; Wolf, J.; von Bulow, V.; Harb, H.; Alhamdan, F.; Hii, C.S.; Prescott, S.L.; Ferrante, A.; Renz, H.; et al. Histone modifications and their role in epigenetics of atopy and allergic diseases. Allergy Asthma Clin. Immunol. 2018, 14, 39. [Google Scholar] [CrossRef]
- Acevedo, N.; Frumento, P.; Harb, H.; Alashkar Alhamwe, B.; Johansson, C.; Eick, L.; Alm, J.; Renz, H.; Scheynius, A.; Potaczek, D.P. Histone Acetylation of Immune Regulatory Genes in Human Placenta in Association with Maternal Intake of Olive Oil and Fish Consumption. Int. J. Mol. Sci. 2019, 20, 1060. [Google Scholar] [CrossRef]
- Verordnung über die Güteprüfung und Bezahlung der Anlieferungsmilch (Milch-Güteverordnung). Available online: http://www.gesetze-im-internet.de/milchg_v/index.html (accessed on 9 January 2019).
- Li, X.M.; Schofield, B.H.; Huang, C.K.; Kleiner, G.I.; Sampson, H.A. A murine model of IgE-mediated cow’s milk hypersensitivity. J. Allergy Clin. Immunol. 1999, 103, 206–214. [Google Scholar] [CrossRef]
- Abbring, S.; Ryan, J.T.; Diks, M.A.P.; Hols, G.; Garssen, J.; van Esch, B. Suppression of Food Allergic Symptoms by Raw Cow’s Milk in Mice is Retained after Skimming but Abolished after Heating the Milk-A Promising Contribution of Alkaline Phosphatase. Nutrients 2019, 11, 1499. [Google Scholar] [CrossRef]
- Abbring, S.; Kusche, D.; Roos, T.C.; Diks, M.A.P.; Hols, G.; Garssen, J.; Baars, T.; van Esch, B. Milk processing increases the allergenicity of cow’s milk-Preclinical evidence supported by a human proof-of-concept provocation pilot. Clin. Exp. Allergy 2019, 49, 1013–1025. [Google Scholar] [CrossRef]
- Harb, H.; Amarasekera, M.; Ashley, S.; Tulic, M.K.; Pfefferle, P.I.; Potaczek, D.P.; Martino, D.; Kesper, D.A.; Prescott, S.L.; Renz, H. Epigenetic Regulation in Early Childhood: A Miniaturized and Validated Method to Assess Histone Acetylation. Int. Arch. Allergy Immunol. 2015, 168, 173–181. [Google Scholar] [CrossRef]
- Haring, M.; Offermann, S.; Danker, T.; Horst, I.; Peterhansel, C.; Stam, M. Chromatin immunoprecipitation: Optimization, quantitative analysis and data normalization. Plant Methods 2007, 3, 11. [Google Scholar] [CrossRef]
- Chinthrajah, R.S.; Hernandez, J.D.; Boyd, S.D.; Galli, S.J.; Nadeau, K.C. Molecular and cellular mechanisms of food allergy and food tolerance. J. Allergy Clin. Immunol. 2016, 137, 984–997. [Google Scholar] [CrossRef] [Green Version]
- Pabst, O.; Mowat, A.M. Oral tolerance to food protein. Mucosal. Immunol. 2012, 5, 232–239. [Google Scholar] [CrossRef]
- Palmer, D.J.; Prescott, S.L. Does early feeding promote development of oral tolerance? Curr. Allergy Asthma Rep. 2012, 12, 321–331. [Google Scholar] [CrossRef]
- Kostadinova, A.I.; Willemsen, L.E.; Knippels, L.M.; Garssen, J. Immunotherapy - risk/benefit in food allergy. Pediatr. Allergy Immunol. 2013, 24, 633–644. [Google Scholar] [CrossRef]
- Bellach, J.; Schwarz, V.; Ahrens, B.; Trendelenburg, V.; Aksunger, O.; Kalb, B.; Niggemann, B.; Keil, T.; Beyer, K. Randomized placebo-controlled trial of hen’s egg consumption for primary prevention in infants. J. Allergy Clin. Immunol. 2017, 139, 1591–1599 e2. [Google Scholar] [CrossRef]
- Palmer, D.J.; Metcalfe, J.; Makrides, M.; Gold, M.S.; Quinn, P.; West, C.E.; Loh, R.; Prescott, S.L. Early regular egg exposure in infants with eczema: A randomized controlled trial. J. Allergy Clin. Immunol. 2013, 132, 387–392 e1. [Google Scholar] [CrossRef]
- Gourbeyre, P.; Denery, S.; Bodinier, M. Probiotics, prebiotics, and synbiotics: Impact on the gut immune system and allergic reactions. J. Leukoc. Biol. 2011, 89, 685–695. [Google Scholar] [CrossRef]
- Vonk, M.; Kostadinova, A.; Kopp, M.; Van Esch, B.; Willemsen, L.; Knippels, L.; Garssen, J. Dietary Interventions in Infancy. Allergy Immun. Toler. Early Child. First Steps Atopic March 2015, 261, 261–284. [Google Scholar]
- Michalski, M.C.; Januel, C. Does homogenization affect the human health properties of cow’s milk? Trends Food Sci. Technol. 2006, 17, 423–437. [Google Scholar] [CrossRef]
- Suarez-Alvarez, B.; Rodriguez, R.M.; Fraga, M.F.; Lopez-Larrea, C. DNA methylation: A promising landscape for immune system-related diseases. Trends Genet. 2012, 28, 506–514. [Google Scholar] [CrossRef]
- Harb, H.; Raedler, D.; Ballenberger, N.; Bock, A.; Kesper, D.A.; Renz, H.; Schaub, B. Childhood allergic asthma is associated with increased IL-13 and FOXP3 histone acetylation. J. Allergy Clin. Immunol. 2015, 136, 200–202. [Google Scholar] [CrossRef]
- Gandhi, N.A.; Bennett, B.L.; Graham, N.M.; Pirozzi, G.; Stahl, N.; Yancopoulos, G.D. Targeting key proximal drivers of type 2 inflammation in disease. Nat. Rev. Drug Discov. 2016, 15, 35–50. [Google Scholar] [CrossRef]
- Polukort, S.H.; Rovatti, J.; Carlson, L.; Thompson, C.; Ser-Dolansky, J.; Kinney, S.R.; Schneider, S.S.; Mathias, C.B. IL-10 Enhances IgE-Mediated Mast Cell Responses and Is Essential for the Development of Experimental Food Allergy in IL-10-Deficient Mice. J. Immunol. 2016, 196, 4865–4876. [Google Scholar] [CrossRef]
- Paparo, L.; Nocerino, R.; Cosenza, L.; Aitoro, R.; D’Argenio, V.; Del Monaco, V.; Di Scala, C.; Amoroso, A.; Di Costanzo, M.; Salvatore, F.; et al. Epigenetic features of FoxP3 in children with cow’s milk allergy. Clin. Epigenetics 2016, 8, 86. [Google Scholar] [CrossRef]
- Lluis, A.; Depner, M.; Gaugler, B.; Saas, P.; Casaca, V.I.; Raedler, D.; Michel, S.; Tost, J.; Liu, J.; Genuneit, J.; et al. Increased regulatory T-cell numbers are associated with farm milk exposure and lower atopic sensitization and asthma in childhood. J. Allergy Clin. Immunol. 2014, 133, 551–559. [Google Scholar] [CrossRef]
- Weiner, H.L. Oral tolerance: Immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect. 2001, 3, 947–954. [Google Scholar] [CrossRef]
- Brand, S.; Teich, R.; Dicke, T.; Harb, H.; Yildirim, A.O.; Tost, J.; Schneider-Stock, R.; Waterland, R.A.; Bauer, U.M.; von Mutius, E.; et al. Epigenetic regulation in murine offspring as a novel mechanism for transmaternal asthma protection induced by microbes. J. Allergy Clin. Immunol. 2011, 128, 618–625 e1-7. [Google Scholar] [CrossRef]
- D’Vaz, N.; Meldrum, S.J.; Dunstan, J.A.; Lee-Pullen, T.F.; Metcalfe, J.; Holt, B.J.; Serralha, M.; Tulic, M.K.; Mori, T.A.; Prescott, S.L. Fish oil supplementation in early infancy modulates developing infant immune responses. Clin. Exp. Allergy 2012, 42, 1206–1216. [Google Scholar] [CrossRef]
- Harb, H.; Irvine, J.; Amarasekera, M.; Hii, C.S.; Kesper, D.A.; Ma, Y.; D’Vaz, N.; Renz, H.; Potaczek, D.P.; Prescott, S.L.; et al. The role of PKCzeta in cord blood T-cell maturation towards Th1 cytokine profile and its epigenetic regulation by fish oil. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef]
- Rajani, P.S.; Seppo, A.E.; Jarvinen, K.M. Immunologically Active Components in Human Milk and Development of Atopic Disease, With Emphasis on Food Allergy, in the Pediatric Population. Front Pediatr. 2018, 6, 218. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbring, S.; Wolf, J.; Ayechu-Muruzabal, V.; Diks, M.A.P.; Alashkar Alhamwe, B.; Alhamdan, F.; Harb, H.; Renz, H.; Garn, H.; Garssen, J.; et al. Raw Cow’s Milk Reduces Allergic Symptoms in a Murine Model for Food Allergy—A Potential Role for Epigenetic Modifications. Nutrients 2019, 11, 1721. https://doi.org/10.3390/nu11081721
Abbring S, Wolf J, Ayechu-Muruzabal V, Diks MAP, Alashkar Alhamwe B, Alhamdan F, Harb H, Renz H, Garn H, Garssen J, et al. Raw Cow’s Milk Reduces Allergic Symptoms in a Murine Model for Food Allergy—A Potential Role for Epigenetic Modifications. Nutrients. 2019; 11(8):1721. https://doi.org/10.3390/nu11081721
Chicago/Turabian StyleAbbring, Suzanne, Johanna Wolf, Veronica Ayechu-Muruzabal, Mara A.P. Diks, Bilal Alashkar Alhamwe, Fahd Alhamdan, Hani Harb, Harald Renz, Holger Garn, Johan Garssen, and et al. 2019. "Raw Cow’s Milk Reduces Allergic Symptoms in a Murine Model for Food Allergy—A Potential Role for Epigenetic Modifications" Nutrients 11, no. 8: 1721. https://doi.org/10.3390/nu11081721
APA StyleAbbring, S., Wolf, J., Ayechu-Muruzabal, V., Diks, M. A. P., Alashkar Alhamwe, B., Alhamdan, F., Harb, H., Renz, H., Garn, H., Garssen, J., Potaczek, D. P., & van Esch, B. C. A. M. (2019). Raw Cow’s Milk Reduces Allergic Symptoms in a Murine Model for Food Allergy—A Potential Role for Epigenetic Modifications. Nutrients, 11(8), 1721. https://doi.org/10.3390/nu11081721







