Selenium-Binding Protein 1 Indicates Myocardial Stress and Risk for Adverse Outcome in Cardiac Surgery
Abstract
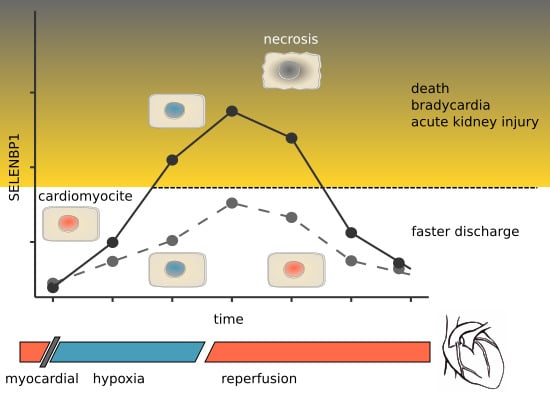
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Examination, Sample Collection and Analysis
2.3. Statistical Analysis
3. Results
3.1. Preoperative Status of SELENBP1 Follows a Normal Distribution
3.2. Intraoperative and Postoperative Kinetics of SELENBP1 Indicate a Transient Increase
3.3. Serum SELENBP1 Concentrations Correlate to the Duration of Ischemia
3.4. Elevated Serum SELENBP1 Concentrations Predict Myocardial Damage
3.5. Elevated Serum SELENBP1 Concentrations Are Indicative of Adverse Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hondal, R.J.; Marino, S.M.; Gladyshev, V.N. Selenocysteine in thiol/disulfide-like exchange reactions. Antioxid. Redox Signal. 2013, 18, 1675–1689. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Flohé, L. Selenium and redox signaling. Arch. Biochem. Biophys. 2017, 617, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Méplan, C. Selenium and chronic diseases: A nutritional genomics perspective. Nutrients 2015, 7, 3621–3651. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E. Regulation of Selenium Metabolism and Transport. Ann. Rev. Nutr. 2015, 35, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L.; Schweizer, U. Hierarchical regulation of selenoprotein expression and sex-specific effects of selenium. Biochim. Biophys. Acta 2009, 1790, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L.; Riese, C.; Renko, K.; Schweizer, U. Effect of age on sexually dimorphic selenoprotein expression in mice. Biol. Chem. 2007, 388, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Bansal, M.P.; Oborn, C.J.; Danielson, K.G.; Medina, D. Evidence for two selenium-binding proteins distinct from glutathione peroxidase in mouse liver. Carcinogenesis 1989, 10, 541–546. [Google Scholar] [CrossRef]
- Elhodaky, M.; Diamond, A.M. Selenium-Binding Protein 1 in Human Health and Disease. Int. J. Mol. Sci. 2018, 19, 3437. [Google Scholar] [CrossRef]
- Chang, P.W.; Tsui, S.K.; Liew, C.; Lee, C.C.; Waye, M.M.; Fung, K.P. Isolation, characterization, and chromosomal mapping of a novel cDNA clone encoding human selenium binding protein. J. Cell Biochem. 1997, 64, 217–224. [Google Scholar] [CrossRef]
- Lanfear, J.; Fleming, J.; Walker, M.; Harrison, P. Different patterns of regulation of the genes encoding the closely related 56 kDa selenium- and acetaminophen-binding proteins in normal tissues and during carcinogenesis. Carcinogenesis 1993, 14, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Sytkowski, A.J. Differential expression and androgen regulation of the human selenium-binding protein gene hSP56 in prostate cancer cells. Cancer Res. 1998, 58, 3150–3153. [Google Scholar] [PubMed]
- Fang, W.; Goldberg, M.L.; Pohl, N.M.; Bi, X.; Tong, C.; Xiong, B.; Koh, T.J.; Diamond, A.M.; Yang, W. Functional and physical interaction between the selenium-binding protein 1 (SBP1) and the glutathione peroxidase 1 selenoprotein. Carcinogenesis 2010, 31, 1360–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.J.; Ma, Y.Y.; He, X.J.; Wang, H.J.; Ye, Z.Y.; Tao, H.Q. Suppression of selenium-binding protein 1 in gastric cancer is associated with poor survival. Hum. Pathol. 2011, 42, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, F.; Younes, M.; Liu, H.; Chen, C.; Yao, Q. Reduced selenium-binding protein 1 in breast cancer correlates with poor survival and resistance to the anti-proliferative effects of selenium. PLoS ONE 2013, 8, e63702. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.S.; Lee, G.T.; Kim, Y.H.; Kwon, S.Y.; Choi, S.H.; Kim, T.H.; Kwon, T.G.; Yun, S.J.; Kim, I.Y.; Kim, W.J. Decreased selenium-binding protein 1 mRNA expression is associated with poor prognosis in renal cell carcinoma. World J. Surg. Oncol. 2014, 12, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burk, R.F.; Hill, K.E.; Motley, A.K. Plasma selenium in specific and non-specific forms. BioFactors 2001, 14, 107–114. [Google Scholar] [CrossRef]
- Bierl, C.; Voetsch, B.; Jin, R.C.; Handy, D.E.; Loscalzo, J. Determinants of human plasma glutathione peroxidase (GPx-3) expression. J. Biol. Chem. 2004, 279, 26839–26845. [Google Scholar] [CrossRef]
- Burk, R.F.; Olson, G.E.; Winfrey, V.P.; Hill, K.E.; Yin, D. Glutathione peroxidase-3 produced by the kidney binds to a population of basement membranes in the gastrointestinal tract and in other tissues. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G32–G38. [Google Scholar] [CrossRef] [Green Version]
- Schomburg, L.; Schweizer, U.; Holtmann, B.; Flohé, L.; Sendtner, M.; Köhrle, J. Gene disruption discloses role of selenoprotein P in selenium delivery to target tissues. Biochem. J. 2003, 370, 397–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, K.E.; Zhou, J.; McMahan, W.J.; Motley, A.K.; Atkins, J.F.; Gesteland, R.F.; Burk, R.F. Deletion of selenoprotein P alters distribution of selenium in the mouse. J. Biol. Chem. 2003, 278, 13640–13646. [Google Scholar] [CrossRef] [PubMed]
- Hollenbach, B.; Morgenthaler, N.G.; Struck, J.; Alonso, C.; Bergmann, A.; Köhrle, J.; Schomburg, L. New assay for the measurement of selenoprotein P as a sepsis biomarker from serum. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2008, 22, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Barrett, C.W.; Short, S.P.; Williams, C.S. Selenoproteins and oxidative stress-induced inflammatory tumorigenesis in the gut. Cell. Mol. Life Sci. CMLS 2017, 74, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mavrouli, M.; Katsinelos, P.; Doulberis, M.; Gavana, E.; Duntas, L. Selenoprotein P in Patients with Nonalcoholic Fatty Liver Disease. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2019. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.A.; Endermann, T.; Stephan, C.; Stoedter, M.; Behrends, T.; Wolff, I.; Jung, K.; Schomburg, L. Selenoprotein P status correlates to cancer-specific mortality in renal cancer patients. PLoS ONE 2012, 7, e46644. [Google Scholar] [CrossRef] [PubMed]
- Forceville, X.; Mostert, V.; Pierantoni, A.; Vitoux, D.; Le Toumelin, P.; Plouvier, E.; Dehoux, M.; Thuillier, F.; Combes, A. Selenoprotein P, rather than glutathione peroxidase, as a potential marker of septic shock and related syndromes. Eur. Surg. Res. Eur. Chir. Forsch. Rech. Chir. Eur. 2009, 43, 338–347. [Google Scholar] [CrossRef]
- Braunstein, M.; Kusmenkov, T.; Zuck, C.; Angstwurm, M.; Becker, N.P.; Bocker, W.; Schomburg, L.; Bogner-Flatz, V. Selenium and Selenoprotein P Deficiency Correlates with Complications and Adverse Outcome After Major Trauma. Shock (AugustaGa) 2019. [Google Scholar] [CrossRef]
- Kim, K.S.; Yang, H.Y.; Song, H.; Kang, Y.R.; Kwon, J.; An, J.; Son, J.Y.; Kwack, S.J.; Kim, Y.M.; Bae, O.N.; et al. Identification of a sensitive urinary biomarker, selenium-binding protein 1, for early detection of acute kidney injury. J. Toxicol. Environ. Health Part A 2017, 80, 453–464. [Google Scholar] [CrossRef]
- Kühn, E.C.; Slagman, A.; Kühn-Heid, E.C.D.; Seelig, J.; Schwiebert, C.; Minich, W.B.; Stoppe, C.; Möckel, M.; Schomburg, L. Circulating levels of selenium-binding protein 1 (SELENBP1) are associated with risk for major adverse cardiac events and death. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2019, 52, 247–253. [Google Scholar] [CrossRef]
- Suadicani, P.; Hein, H.O.; Gyntelberg, F. Serum selenium concentration and risk of ischaemic heart disease in a prospective cohort study of 3000 males. Atherosclerosis 1992, 96, 33–42. [Google Scholar] [CrossRef]
- Wendt, S.; Schomburg, L.; Manzanares, W.; Stoppe, C. Selenium in Cardiac Surgery. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2019, 34, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.; Roques, F.; Michel, P.; Gauducheau, E.; Lemeshow, S.; Salamon, R. European system for cardiac operative risk evaluation (EuroSCORE). Eur. J. Cardio Thorac. Surg. 1999, 16, 9–13. [Google Scholar] [CrossRef]
- Ying, Q.; Ansong, E.; Diamond, A.M.; Yang, W. A Critical Role for Cysteine 57 in the Biological Functions of Selenium Binding Protein-1. Int. J. Mol. Sci. 2015, 16, 27599–27608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, R.M.; Frazier, D.P.; Thompson, J.W.; Haliko, S.; Li, H.; Wasserlauf, B.J.; Spiga, M.G.; Bishopric, N.H.; Webster, K.A. A unique pathway of cardiac myocyte death caused by hypoxia-acidosis. J. Exp. Biol. 2004, 207, 3189–3200. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Shin, Y.J.; Park, E.Y.; Kim, N.D.; Moon, A.; Kwack, S.J.; Son, J.Y.; Kacew, S.; Lee, B.M.; Bae, O.N.; et al. Selenium-binding protein 1: A sensitive urinary biomarker to detect heavy metal-induced nephrotoxicity. Arch. Toxicol. 2017, 91, 1635–1648. [Google Scholar] [CrossRef] [PubMed]
- Boucher, F.R.; Jouan, M.G.; Moro, C.; Rakotovao, A.N.; Tanguy, S.; de Leiris, J. Does selenium exert cardioprotective effects against oxidative stress in myocardial ischemia? Acta Physiol. Hung. 2008, 95, 187–194. [Google Scholar] [CrossRef]
- Alehagen, U.; Aaseth, J.; Johansson, P. Reduced Cardiovascular Mortality 10 Years after Supplementation with Selenium and Coenzyme Q10 for Four Years: Follow-Up Results of a Prospective Randomized Double-Blind Placebo-Controlled Trial in Elderly Citizens. PLoS ONE 2015, 10, e0141641. [Google Scholar] [CrossRef]
- Canto, J.G.; Rogers, W.J.; Goldberg, R.J.; Peterson, E.D.; Wenger, N.K.; Vaccarino, V.; Kiefe, C.I.; Frederick, P.D.; Sopko, G.; Zheng, Z.J.; et al. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA 2012, 307, 813–822. [Google Scholar] [CrossRef]







| Parameter | Total (n = 66) | SELENBP1-Positive * (n = 18) | SELENBP1-Negative (n = 48) |
|---|---|---|---|
| Age (years) | 65 (58–75) | 65 (60–75) | 66 (56–75) |
| Female sex (n) | 23% (15) | 8% (5) | 15% (10) |
| Male sex (n) | 77% (51) | 20% (13) | 58% (38) |
| BMI (kg/m2) | 27 (25–31) | 27 (25–32) | 28 (25–30) |
| EUROscore 1 | 4 (2–6) | 6 (5–8) | 3 (1–6) |
| Duration of intervention (min) | 271 (207–330) | 282 (225–317) | 265 (200–331) |
| Duration of CPB (min) | 120 (85–149) | 148 (126–176) | 100 (83–134) |
| Duration of ischemia (min) | 72 (58–107) | 112 (93–134) | 63 (58–84) |
| Stay in hospital (days) | 9 (7–11) | 11 (8–14) | 9 (7–10) |
| Bradycardia (n) | 6% (4) | 5% (3) | 2% (1) |
| Exitus letalis (n) | 5% (3) | 5% (3) | 0% (0) |
| Pneumonia (n) | 5% (3) | 5% (3) | 0% (0) |
| Acute kidney failure (n) | 5% (3) | 3% (2) | 2% (1) |
| Wound infection (n) | 2% (1) | 2% (1) | 0% (0) |
| Cerebral ischemia (n) | 2% (1) | 2% (1) | 0% (0) |
| Parameter | Threshold [nM] | OR | CI | p | PPV | NPV | AUC | n |
|---|---|---|---|---|---|---|---|---|
| 15 min after CPB | ||||||||
| combined endpoint 1 * | 0.988 | 22 | 3–194 | 0.0005 | 0.333 | 0.978 | 0.84 | 8 |
| combined endpoint 2 * | 0.820 | 18 | 2–152 | 0.0011 | 0.310 | 0.976 | 0.81 | 9 |
| 1 h ICU | ||||||||
| death | 2.238 | 124 | 6.2777 | 0.0042 | 0.667 | 0.984 | 0.92 | 2 |
| acute kidney injury | 0.889 | 20 | 1–411 | 0.029 | 0.158 | 1.000 | 0.83 | 4 |
| combined endpoint 1 * | 0.956 | 30 | 3–269 | 0.0003 | 0.389 | 0.979 | 0.84 | 7 |
| combined endpoint 2 * | 0.889 | 33 | 4–296 | <0.0001 | 0.421 | 0.979 | 0.84 | 7 |
| 6 h ICU | ||||||||
| death | 1.292 | 63 | 4–1018 | 0.0077 | 0.500 | 0.984 | 0.88 | 3 |
| maximal levels | ||||||||
| bradycardia | 0.988 | 23 | 1–439 | 0.014 | 0.167 | 1.000 | 0.81 | 5 |
| cerebral ischemia | 1.396 | 25 | 1–544 | 0.034 | 0.143 | 1.000 | 0.90 | 3 |
| combined endpoint 1 * | 0.988 | 25 | 3–215 | 0.0003 | 0.333 | 0.980 | 0.88 | 8 |
| combined endpoint 2 * | 0.968 | 24 | 3–199 | 0.0003 | 0.333 | 0.979 | 0.86 | 9 |
| stay in ICU for >40 day | 0.870 | 4 | 1–10 | 0.013 | 0.484 | 0.795 | 0.70 | 14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kühn-Heid, E.C.D.; Kühn, E.C.; Ney, J.; Wendt, S.; Seelig, J.; Schwiebert, C.; Minich, W.B.; Stoppe, C.; Schomburg, L. Selenium-Binding Protein 1 Indicates Myocardial Stress and Risk for Adverse Outcome in Cardiac Surgery. Nutrients 2019, 11, 2005. https://doi.org/10.3390/nu11092005
Kühn-Heid ECD, Kühn EC, Ney J, Wendt S, Seelig J, Schwiebert C, Minich WB, Stoppe C, Schomburg L. Selenium-Binding Protein 1 Indicates Myocardial Stress and Risk for Adverse Outcome in Cardiac Surgery. Nutrients. 2019; 11(9):2005. https://doi.org/10.3390/nu11092005
Chicago/Turabian StyleKühn-Heid, Ellen C. D., Eike C. Kühn, Julia Ney, Sebastian Wendt, Julian Seelig, Christian Schwiebert, Waldemar B. Minich, Christian Stoppe, and Lutz Schomburg. 2019. "Selenium-Binding Protein 1 Indicates Myocardial Stress and Risk for Adverse Outcome in Cardiac Surgery" Nutrients 11, no. 9: 2005. https://doi.org/10.3390/nu11092005
APA StyleKühn-Heid, E. C. D., Kühn, E. C., Ney, J., Wendt, S., Seelig, J., Schwiebert, C., Minich, W. B., Stoppe, C., & Schomburg, L. (2019). Selenium-Binding Protein 1 Indicates Myocardial Stress and Risk for Adverse Outcome in Cardiac Surgery. Nutrients, 11(9), 2005. https://doi.org/10.3390/nu11092005